Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor
The Working Party on von Willebrand Disease Classification is part of the von Willebrand Disease Subcommittee of the Scientific and Standardization Committee of the ISTH.
Abstract
Summary. von Willebrand disease (VWD) is a bleeding disorder caused by inherited defects in the concentration, structure, or function of von Willebrand factor (VWF). VWD is classified into three primary categories. Type 1 includes partial quantitative deficiency, type 2 includes qualitative defects, and type 3 includes virtually complete deficiency of VWF. VWD type 2 is divided into four secondary categories. Type 2A includes variants with decreased platelet adhesion caused by selective deficiency of high-molecular-weight VWF multimers. Type 2B includes variants with increased affinity for platelet glycoprotein Ib. Type 2M includes variants with markedly defective platelet adhesion despite a relatively normal size distribution of VWF multimers. Type 2N includes variants with markedly decreased affinity for factor VIII. These six categories of VWD correlate with important clinical features and therapeutic requirements. Some VWF gene mutations, alone or in combination, have complex effects and give rise to mixed VWD phenotypes. Certain VWD types, especially type 1 and type 2A, encompass several pathophysiologic mechanisms that sometimes can be distinguished by appropriate laboratory studies. The clinical significance of this heterogeneity is under investigation, which may support further subdivision of VWD type 1 or type 2A in the future.
Introduction
The Subcommittee on von Willebrand factor (VWF) published recommendations for the classification of von Willebrand disease (VWD) in 1994 [1]. This classification was intended to be simple, to rely mainly on widely available laboratory tests, and to correlate with important clinical characteristics. Subsequent research has increased our knowledge of how VWF functions, how it is metabolized, and how defects in VWF cause disease. Therefore, the classification of VWD has been reevaluated, to incorporate these advances into the conceptual framework for understanding the pathophysiology of VWD.
The purpose of the classification remains primarily clinical, to facilitate the diagnosis, treatment and counseling of patients with VWD. In practice, distinctions between certain VWD types are not always easy to make. Difficulties may arise because patient phenotypes vary over time, VWF mutations can have complex effects, certain laboratory tests are inherently imprecise, and the boundary between normal and abnormal phenotypes may not be sharply defined. These problems will be discussed in order to suggest strategies to minimize them through the application of current knowledge or through additional research.
The classification does not depend on genotypic data but emphasizes the VWF protein phenotype of the patient, because protein characteristics are accessible through commonly available laboratory tests, whereas the underlying genetic defects are not. As gene sequencing becomes easier to do, the detection of VWF mutations should provide a useful additional component for the classification of VWD.
VWF synthesis, structure, function, assembly, secretion, and catabolism will be reviewed in order to provide a foundation for discussing the rationale and criteria for each type of VWD (Table 1).
| Type | Description |
|---|---|
| 1 | Partial quantitative deficiency of VWF |
| 2 | Qualitative VWF defects |
| 2A | Decreased VWF-dependent platelet adhesion and a selective deficiency of high-molecular-weight VWF multimers |
| 2B | Increased affinity for platelet glycoprotein Ib |
| 2M | Decreased VWF-dependent platelet adhesion without a selective deficiency of high-molecular-weight VWF multimers |
| 2N | Markedly decreased binding affinity for factor VIII |
| 3 | Virtually complete deficiency of VWF |
- VWF, von Willebrand factor.
Structure and function of VWF
VWF is a multimeric plasma glycoprotein (GP) composed mostly of identical subunits of ∼250 kDa. The multimers range in size from dimers of ∼500 kDa to species of > 10 million Da that contain > 40 subunits and exceed 2 micrometers in length [2]. High-molecular-weight (HMW) VWF multimers mediate platelet adhesion at sites of vascular injury by binding to connective tissue and to platelets. VWF also binds and stabilizes blood clotting factor (F) VIII. Therefore, defects in VWF can cause bleeding with features typical of platelet dysfunction, or of mild to moderately severe hemophilia A, or of both [3,4].
Several binding functions have been localized to discrete sites in the VWF subunit (Fig. 1). Platelet GPIb interacts with domain A1, and integrin αIIbβ3 interacts with an Arg-Gly-Asp sequence in domain C1. Fibrillar collagens interact mainly with domain A3, and collagen VI appears to bind domain A1. FVIII binds the N-terminal D′D3 region [4].
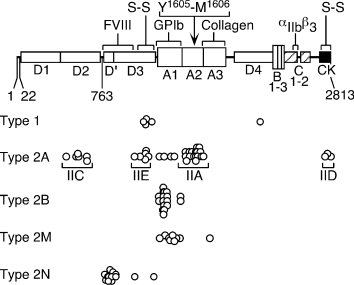
Structure of the von Willebrand factor (VWF) precursor and location of mutations in von Willebrand disease (VWD) patients. Amino acid residues are numbered by codon number. The VWF precursor consists of a signal peptide (residues 1–22), propeptide (residues 23–763), and mature subunit (residues 764–2813). Structural domains (A, B, C, D, CK), intersubunit disulfide bonds (S-S), and binding sites for factor VIII, platelet glycoprotein Ib, collagen, and platelet integrin αIIbβ3 are labeled. ADAMTS-13 cleaves the Tyr1605-Met1606 bond in domain A2 (arrow). Circles show the positions of some mutations that cause dominant VWD type 1 and variants of VWD type 2. Brackets show the location of mutations that correspond to variants of VWD type 2A with characteristic multimer patterns because of increased proteolysis (IIA) or defective multimer assembly caused by mutations in the propeptide (IIC), the cystine knot domain (IID), or the D3 domain (IIE).
Assembly and secretion of VWF multimers
Endothelial cells and megakaryocytes make proVWF subunits that dimerize ‘tail-to-tail’ in the endoplasmic reticulum through disulfide bonds between C-terminal cystine knot (CK) domains. The proVWF dimers form multimers in the Golgi through ‘head-to-head’ disulfide bonds between D3 domains. Multimer formation depends on the propeptide and on the acidic pH of the Golgi. Multimers may be secreted constitutively or stored for later regulated secretion in the Weibel–Palade bodies of endothelial cells or the α-granules of platelets [3].
Catabolism of plasma VWF
After secretion, the fate of a VWF multimer depends on its size, interactions with platelets and other cells, susceptibility to proteolysis, and the rate of clearance from circulation (Fig. 2). Under high fluid shear stress, multimers large enough to engage platelets may be stretched and expose the Tyr1605-Met1606 bond in VWF domain A2, which then can be cleaved by the ADAMTS-13 metalloprotease. By this mechanism ADAMTS-13 remodels the initial VWF multimer distribution that is secreted into the blood, converting large multimers into smaller ones and producing characteristic cleavage products. As a consequence, the electrophoretic pattern of plasma VWF displays minor or ‘satellite’ bands that flank the major multimer bands typical of endothelial cell VWF (Fig. 2). VWF is also cleared from the blood with a half-life of 12–20 h [5,6] by a process that appears to be relatively insensitive to multimer size [7].
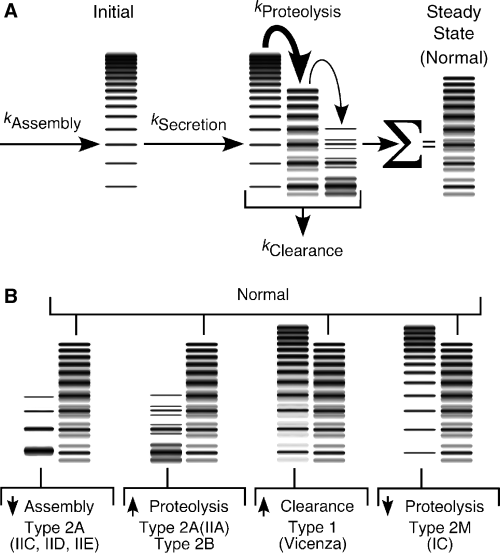
Synthesis and catabolism of von Willebrand factor (VWF) multimers. (A) The initial multimer distribution within the endothelium is determined by the rate of assembly (kAssembly). The relationships between the rates of secretion (kSecretion), clearance (kClearance), and proteolysis by ADAMTS-13 (kProteolysis) determine the plasma VWF concentration and multimer distribution. At steady-state in healthy persons, the largest plasma VWF multimers are smaller than those assembled initially, and faint satellite bands flanking the smallest multimers reflect the extent of proteolytic remodeling. (B) Changes in the rates of assembly, secretion, clearance, or proteolysis cause specific variants of von Willebrand disease and alter the steady-state plasma VWF concentration and multimer distribution. Patterns associated with specific known variants are shown adjacent to the normal multimer pattern for comparison. Mutations that affect more than one process can cause intermediate or blended phenotypes.
The concentration of plasma VWF is determined by the rates of secretion and clearance, and the multimer distribution reflects the balance between multimer assembly, clearance from circulation and proteolysis by ADAMTS-13. Mutations that affect these processes produce a variety of VWD phenotypes.
Nomenclature and abbreviations
The abbreviations for VWF and its activities [8] and conventions for describing mutations [9] adhere to recommendations in previous VWF Subcommittee reports. In particular, nucleotides of the human VWF cDNA sequence are numbered positively, with ‘+1’ assigned to the ‘A’ of the initiation codon. The encoded amino acid residues are numbered from 1 to 2813, beginning with the initiator methionine.
A database of mutations and polymorphisms in the VWF gene [10,11] is maintained at the University of Sheffield and is accessible at http://www.shef.ac.uk/vwf/index.html.
Phenotypic classification of VWD
VWD is a bleeding disorder caused by inherited defects in the concentration, structure, or function of VWF. The previous classification restricted VWD to mutations within the VWF gene [1], but this criterion has been relaxed (Table 2). No generally available method can identify or exclude VWF mutations in a significant percentage of patients, so a requirement for such a mutation can rarely be satisfied in practice. In addition, mutations in other genes could conceivably produce a disorder indistinguishable from VWD that is caused by intragenic VWF mutations. For example, the ectopic expression in endothelium of an intestinal N-acetylgalactosaminyltransferase leads to the rapid clearance of abnormally glycosylated VWF and very low plasma levels of VWF in RIIIS/J mice [12]. Although no similar human condition has been identified, locus heterogeneity cannot be excluded for VWD.
| Previous [1] | Current |
|---|---|
| VWD is caused by mutations at the VWF locus | VWD is not restricted to VWF gene mutations |
| VWD type 1 includes partial quantitative deficiency of VWF. The multimers distribution and structure of plasma VWF is indistinguishable from normal. | VWD type 1 includes partial quantitative deficiency of VWF. Plasma VWF may contain mutant subunits, but has normal functional activity relative to antigen level. The proportion of large multimers is not decreased significantly. |
- VWF, von Willebrand factor.
Acquired disorders that mimic VWD are referred to as acquired von Willebrand syndrome (AVWS). The clinical characteristics of AVWS are discussed in a VWF Subcommittee report [13].
VWD type 1 includes partial quantitative deficiency of VWF. Bleeding in VWD type 1 is attributed to a decrease in VWF concentration, not to a selective decrease in the hemostatically effective large multimers or to specific abnormalities in ligand binding sites. The key laboratory findings in VWD type 1 are that the circulating VWF has a normal ratio of functional activities compared with VWF antigen level (VWF:Ag). The proportion of high-molecular-weight multimers is not decreased significantly.
This definition of VWD type 1 is broader than that proposed in the 1994 classification [1] (Table 2). The principal change is to include patients in whom the proportion of HMW plasma VWF multimers is decreased slightly, but not enough to prevent the achievement of a hemostatically effective level of large multimers after desmopressin. In addition, the plasma VWF multimers may or may not contain mutant VWF subunits. When sensitive assay methods are used, many patients with VWD type 1 have mild abnormalities of multimer structure or distribution. For example, in the Canadian Type 1 VWD Study, 194 families were submitted to participate and 12 (6%) had abnormal multimer results or a VWF:ristocetin cofactor (RCo)/VWF:Ag ratio of < 0.6 [14]. In the European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease Study (MCMDM-1 VWD), 143 families were enrolled and 59 of them (41%) included subjects with abnormal multimers, although the abnormalities were often minimal [15].
VWD type 1 can be caused by reduced secretion of functionally normal VWF with a nearly normal multimer distribution. Reduced secretion might be caused by VWF mutations affecting gene expression, although this mechanism has been difficult to demonstrate consistently. Some linkage studies have not identified an influence of the VWF gene [16], but others have found that about 20% of the variance in VWF plasma levels is attributable to loci in the VWF gene [17]. Association studies in Canadian type O blood donors suggested that some of this variation is linked to a common polymorphic haplotype in the VWF promoter [18,19], but this was not confirmed in a study of mixed ABO blood types in the Netherlands [20].
Mutations can decrease secretion by impairing the intracellular transport of VWF subunits and cause a severe, dominantly inherited form of VWD type 1, although the phenotype is often mixed and may have features of both VWD type 1 and type 2A, as discussed below (Multiple pathophysiologic mechanisms). For example, a patient from the Netherlands had a VWF level of 10 IU dL−1 and a roughly normal distribution of VWF multimers. Two of three children had a similar phenotype. The affected subjects were heterozygous for the mutation Cys1149Arg in the D3 domain (Fig. 1). Recombinant Cys1149Arg mutant subunits were retained in the endoplasmic reticulum of transfected cells. When coexpressed with wild-type VWF, the Cys1149Arg subunits caused the intracellular retention and degradation of proVWF heterodimers composed of wild-type and mutant subunits, reducing the transport of normal VWF through the secretory pathway [21,22]. Similar behavior has been reported for two other mutations in the D3 domain, Cys1130Phe [23] and Thr1156Met [24].
Accelerated clearance also can cause dominant VWD type 1 (Fig. 2). For example, the mutations Cys1130Phe and Cys1149Arg had relatively modest effects on the secretion of VWF, suggesting that increased clearance might contribute to the low plasma VWF levels of affected patients. In fact, patients with the Cys1130Phe or Cys1149Arg mutation had a very brief response to desmopressin, with a VWF:Ag half-life of 1.5 h [25,26] compared with 6–9 h for healthy controls [26–28]. Similarly, two patients with the mutation Ser2179Phe in domain D4 had a short VWF:Ag half-life of 3–4 h after desmopressin [29]. Other patients with VWD type 1 have had increased VWF clearance after desmopressin, and the VWF:Ag half-life appears to correlate with the baseline VWF level [30,31].
VWD Vicenza may be an extreme example of increased VWF clearance. VWD Vicenza is characterized by VWF:Ag levels of 6–12 IU dL−1, normal platelet VWF, and ultra-large plasma VWF multimers [32]. Whether VWD Vicenza should be classified as type 1 or type 2M has been controversial [25,33], but when the VWF level is sufficient for precise measurement, then the levels of VWF:RCo and VWF:Ag [33] are decreased proportionately [25]. Therefore, this variant is classified under VWD type 1.
VWD Vicenza is caused by the heterozygous mutation Arg1205His in the D3 domain. Recombinant VWF Arg1205His is secreted efficiently with a normal multimer distribution, suggesting that the low VWF concentration and ultra-large multimers in patient plasma do not reflect a biosynthetic defect. Compared with healthy controls, however, the half-life of VWF Vicenza is reduced 4.4-fold after desmopressin [33], suggesting that rapid clearance accounts for the moderately severe VWF deficiency.
Increased clearance also may be sufficient to explain the ultra-large multimer distribution of VWD Vicenza (Fig. 2). Rapid clearance decreases the time during which a large, circulating VWF multimer can be cleaved by ADAMTS-13. Consequently, increased clearance should shift the plasma VWF multimer distribution toward that secreted initially from endothelial cells. Under these conditions, plasma VWF should include ultra-large species and show relatively little subunit proteolysis, and both features are characteristic of VWD Vicenza [32,34].
Increased susceptibility of VWF to proteolytic cleavage may also modulate the severity of VWD type 1. The substitution Tyr1584Cys has been identified in 3–25% of patients with VWD type 1, compared with < 2% of healthy controls [14,35–38]. This mutation does not reduce the intravascular survival of VWF [39] but slightly increases the susceptibility of VWF to cleavage by ADAMTS-13 [36], which may impair platelet plug formation. The Tyr1584Cys mutation is associated weakly with low VWF:Ag or VWF:RCo [14], which is consistent with increased intracellular retention of the recombinant 1584Cys variant [35]. The 1584Cys variant does not co-segregate consistently with a low VWF level or with bleeding symptoms in affected families [14,37,38], suggesting that it is a relatively modest risk factor for VWD type 1.
It is clear that several distinct mechanisms can cause VWD type 1, and some can be distinguished by suitable testing. For example, variants associated with rapid clearance may be identified by the characteristic response to a test dose of desmopressin. The clinical significance of this heterogeneity is under investigation, which could lead to changes in the classification of VWD.
VWD type 2 includes qualitative defects of VWF. VWD type 2 is subdivided based on specific functional and structural defects that impair platelet adhesion or FVIII binding.
VWD type 2A includes qualitative variants with decreased VWF-dependent platelet adhesion and a selective deficiency of HMW VWF multimers. A significant relative deficiency of large multimers may result either from defects in multimer assembly or from intrinsically increased sensitivity to cleavage by ADAMTS-13. In some cases, these mechanisms can be distinguished and the location of mutations can be inferred from the VWF multimer pattern. Regardless of mechanism, the loss of large multimers is associated with disproportionate decreases in VWF–platelet interactions (e.g. VWF:RCo) or VWF–connective tissue interactions (e.g. VWF:CB) [40] relative to VWF:Ag. VWD type 2A usually appears to be inherited as a dominant trait, although some variants are recessive.
Impaired multimer assembly leads to the secretion of small multimers that do not strongly bind to platelets or to other cells. As a result, they experience little proteolysis [41] and the steady-state plasma VWF multimer distribution resembles that secreted initially (Fig. 2B). Such a phenotype can be caused by mutations in at least three regions of the VWF subunit.
Firstly, defects in assembly can be produced by homozygous or compound heterozygous mutations in the VWF propeptide that prevent multimerization in the Golgi apparatus. These mutations cause a characteristically simple multimer pattern that is essentially devoid of satellite bands. This clinically recessive phenotype was initially designated ‘VWD type IIC’ [42–44].
Defects in multimer assembly can also be caused by heterozygous mutations in the C-terminal CK domain that prevent dimerization in the endoplasmic reticulum. In this case, a mixture of mutant proVWF monomers and wild-type dimers arrive in the Golgi, where the incorporation of a mutant monomer into a growing VWF multimer terminates its elongation. As a result, small multimers are secreted that contain minor species with an odd number of VWF subunits in addition to the usual major species with an even number of subunits. This phenotype was initially designated ‘VWD type IID’ [45,46].
Finally, defects in multimer assembly can be caused by heterozygous mutations in the D3 domain that interfere with intersubunit disulfide bond formation in the Golgi. Such mutations frequently occur at cysteine residues and often produce a ‘smeary’ multimer pattern that suggests a heterogeneous disulfide bond structure. This phenotype was initially designated ‘VWD type IIE’ [41,47]. In some cases, heterozygous mutations in the D3 region (e.g. Cys1099Tyr or Met1051Thr) [48] do not appear to cause aberrant disulfide bond formation and instead produce a clean pattern of small multimers indistinguishable from that caused by VWF propeptide mutations, but with a plasma VWF concentration substantially higher than normal. This phenotype was initially designated ‘VWD type IIC Miami’ [49].
Increased proteolysis can cause VWD type 2A despite normal VWF multimer assembly and secretion (Fig. 2). Variants of VWD type 2A originally designated ‘VWD type IIA’ exhibit intense subunit proteolysis [41] by ADAMTS-13 [50]. Mutations causing this phenotype lie within or near VWF domain A2 and have been divided into two groups. Group I mutations enhance proteolysis and also impair VWF multimer assembly, whereas group II mutations enhance proteolysis without decreasing the secretion of large multimers [51]. Computer modeling suggests that mutations of both groups impair the folding of VWF domain A2 and make the Tyr1605-Met1606 bond accessible to ADAMTS-13 without a need for platelet binding or high fluid shear stress. Group I mutations appear to have a more disruptive effect on A2 domain structure, which may account for their additional effect on multimer assembly [52].
VWD type 2A is heterogeneous in mechanism, but these distinctions are not currently employed to further subdivide VWD type 2A because their clinical utility has not been demonstrated. At present, the discrimination between type 2A variants requires high-resolution multimer gel electrophoresis or gene sequencing, and these techniques are not widely available.
VWD type 2B includes qualitative variants with increased affinity for platelet GPIb. This type is characterized by increased ristocetin-induced platelet aggregation (RIPA) at low concentrations of ristocetin [53], because of enhanced interaction of the mutant VWF with platelet GPIb. Patients with VWD type 2B often have variable thrombocytopenia that can be exacerbated by stress or by desmopressin [54]. Most patients with VWD type 2B have a decreased proportion of large VWF multimers and exhibit markedly increased proteolysis of VWF subunits [41,53]. VWD type 2B mutations do not impair the assembly of large VWF multimers, but after secretion the multimers bind spontaneously to platelets and become cleaved by ADAMTS-13. The resulting small multimers do not mediate platelet adhesion effectively, and also appear to bind platelets and directly inhibit their interaction with connective tissue [55].
Heterozygous mutations that cause VWD type 2B cluster within or near VWF domain A1 [10,47,56–58], which changes conformation when it binds to platelet GPIb [59]. The mutations appear to enhance platelet binding by stabilizing the bound conformation of domain A1 [59].
VWD type 2B Malmö or New York is caused by the mutation Pro1266Leu and is associated with increased RIPA at low concentrations of ristocetin [60], although RIPA has been normal in some patients with this mutation [61]. The plasma multimer distribution is normal, VWF subunit proteolysis is not increased, and desmopressin does not cause thrombocytopenia. Some people with VWD type 2B Malmö or New York have had mild bleeding, whereas others have had none [60–63]. Thus, VWF Pro1266Leu can exhibit increased sensitivity to ristocetin in vitro, but usually mediates platelet adhesion normally in vivo. These observations suggest that decreased large plasma VWF multimers and increased subunit proteolysis may correlate with the likelihood of significant bleeding for patients with RIPA values consistent with VWD type 2B.
A phenotype similar to VWD type 2B can be caused by heterozygous gain-of-function mutations in platelet GPIbα [64–67], and this disorder is referred to as platelet-type pseudo-VWD [68,69]. The mutations are thought to stabilize the bound conformation of platelet GPIbα in the VWF domain A1-GPIbα complex [59].
VWD type 2M includes qualitative variants with decreased VWF-dependent platelet adhesion without a selective deficiency of high-molecular-weight VWF multimers. The assembly and secretion of large VWF multimers is approximately normal, and a functional defect is caused by mutations that disrupt VWF binding to platelets or to subendothelium. In an example of VWD type 2M initially designated ‘VWD type IC’, multimer gels showed a decrease in satellite bands and a shift in the multimer distribution toward larger multimer sizes [70]. These findings suggest that decreased platelet binding reduces the exposure of VWF subunits to cleavage by ADAMTS-13, thereby preserving a multimer distribution like that initially secreted from endothelial cells (Fig. 2B). Additional studies would be useful to assess the relationship between the effect of VWD type 2M mutations on platelet binding, multimer distribution and subunit degradation.
Most cases of VWD type 2M have been identified based upon a value for VWF:RCo that is disproportionately low compared with VWF:Ag, and such patients usually have mutations within VWF domain A1 that impair binding to platelet GPIb [10,47,58,71]. One family has been reported in which a mother and daughter with VWD type 2M had disproportionately low VWF collagen binding capacity (VWF:CB) associated with a mutation in VWF domain A3 [72].
The detection of VWD type 2M may depend on the assays used. For example, VWF:CB is insensitive to mutations that impair platelet binding and decrease VWF:RCo. Conversely, VWF:RCo cannot detect defects in collagen binding that might impair platelet adhesion in vivo. Collagen binding defects may be uncommon, but their incidence will remain unknown until more data are available about the use of VWF:CB assays for the diagnosis of VWD.
VWD type 2N includes variants with markedly decreased binding affinity for FVIII. VWD type 2N is caused by homozygous or compound heterozygous VWF mutations that impair FVIII binding capacity (VWF:FVIIIB). Sometimes both VWF alleles have FVIII binding mutations, but often one allele has a FVIII binding mutation and the other allele expresses little or no VWF. Mutations in VWD type 2N are usually within the FVIII binding site of VWF, which lies between Ser764 and Arg1035 and spans domain D′ and part of domain D3 [10,73]. Some mutations in the D3 domain C-terminal to Arg1035 can also reduce FVIII binding [74–76]. VWD type 2N can be confused with mild hemophilia A, especially for males who do not have compelling evidence for X-linked inheritance [77].
The FVIII level is decreased disproportionately relative to VWF:Ag in VWD type 2N, and the diagnosis depends on measuring the affinity of patient VWF for FVIII (VWF:FVIIIB), usually in a solid-phase immunoassay [78]. Values of VWF:FVIIIB are usually < 0.1 for patients with VWD type 2N and cluster around 0.5 for heterozygous carriers [73,79].
The plasma FVIII level correlates with specific VWD type 2N mutations. In one study, patients with mutations that severely impair FVIII binding had FVIII levels of 8.4 ± 5.2 IU dL−1, and those with a relatively common but less severe mutation (Arg854Gln) had FVIII levels of 21.8 ± 9.8 [79]. The distinction has clinical utility because subjects with the Arg854Gln mutation may have a sustained and therapeutically useful FVIII increase after desmopressin, whereas those with more severe mutations usually do not [80–83].
VWD type 3 includes virtually complete deficiency of VWF. VWD type 3 is inherited as a recessive trait, and heterozygous relatives usually have mild or no bleeding symptoms [84–86]. In most cases, VWF:RCo, VWF:CB and VWF:Ag are < 5 IU dL−1 and FVIII levels are < 10 IU dL−1. The VWF mutations that cause VWD type 3 are usually nonsense mutations or frameshifts because of small insertions or deletions. Large deletions, splice site mutations and missense mutations are less common [10,87,88].
Virtually complete deficiency of VWF is categorized as VWD type 3, regardless of the phenotype of heterozygous relatives. The clinical course or treatment of a patient with VWD type 3 does not depend on whether other family members have another type of VWD, although this information can be relevant for genetic counseling.
The term ‘severe’ VWD has been used sometimes for VWD type 3 and sometimes for symptomatic VWD type 1 characterized by very low VWF levels, but these conditions are almost always clinically distinct. VWD type 1 caused by dominant heterozygous mutations is rarely associated with VWF levels as low as 10 IU dL−1, and such patients with dominant VWD type 1 can have therapeutically useful responses to desmopressin. VWD type 3 caused by clinically recessive mutations is usually associated with undetectable VWF levels (< 5 IU dL−1), and patients seldom have a measurable response to desmopressin.
Compound heterozygosity and compound phenotypes
The phenotype of heterozygous patients can depend on interactions between subunits encoded by both VWF alleles. If compound heterozygosity can be inferred from laboratory studies of the patient or relatives, then the compound phenotype can be represented by a separate designation for each allele, separated by a slash (/). For example, coinheritance of VWD type 2N and a non-expressing or ‘null’ VWF allele can be described as ‘VWD type 2N/3’. Recognition of compound heterozygosity can have implications for treatment and for genetic counseling.
Multiple pathophysiologic mechanisms
Single mutations may cause VWD by more than one mechanism. For example, mutations in the D3 domain can interfere with multimer assembly [23,47], reduce secretion [21,23], promote clearance from the circulation [25,33], cause aberrant and heterogeneous disulfide bond structures [47], and decrease affinity for FVIII [73], in various combinations. These effects can produce VWD type 1, type 2A, type 2N, or blended phenotypes.
A hierarchical approach to classification
There are two major levels of classification: primary (1, 2, 3) and secondary (A, B, M, N). Additional ‘tertiary’ information that is not reflected in the defined types of VWD can be appended in parentheses. Such information may include a place name that indicates a remarkable phenotype (e.g. Vicenza), the patient's mutation using standard nomenclature [9], or a VWF multimer pattern that suggests a specific disease mechanism (e.g. IIA, IIC, IID, IIE).
A defect in multimer distribution or ligand binding tends to impair responsiveness to desmopressin, whereas a moderate decrease in plasma VWF level usually does not. In most cases, therefore, a complex phenotype with features of both VWD type 2 and type 1 should be classified under ‘type 2’ in order to preserve a correlation with the response to desmopressin. A phenotype with prominent defects in more than one ‘type 2’ character simultaneously, such as multimer structure and ligand binding, can be designated as ‘type 2 (mixed phenotype)’ without further differentiation.
Issues in laboratory testing
The classification of VWD does not rely on specific laboratory testing protocols, so that changes in assay methods may be accommodated without a need for revision. Using currently available tests, however, the distinction between the primary categories of VWD can usually be made by measuring VWF:RCo (or VWF:CB), VWF:Ag, and FVIII. Concordant decreases in all levels suggest VWD type 1, disproportionate decreases in VWF:RCo (or VWF:CB) or FVIII suggest a form of VWD type 2, and virtual absence of VWF:Ag suggests VWD type 3. Discrimination among type 2 variants often requires tests that are usually performed in specialized hemostasis laboratories. Recognition of VWD type 2B currently depends on RIPA with fresh platelet-rich plasma, distinguishing between VWD type 2A and type 2M requires multimer gel electrophoresis, and recognition of VWD type 2N requires an assay of VWF:FVIIIB.
Archetypal examples of each VWD type are easy to recognize, but subtle defects can be difficult to characterize and classify. The major problems include determining whether large VWF multimers are decreased and whether the VWF is functionally abnormal.
The VWF multimer distribution and satellite bands can be evaluated quantitatively by densitometric scanning to obtain information about multimer assembly, infer their sensitivity to ADAMTS-13, and predict the location of mutations [34,89,90]. The most important problem is to distinguish normal or only subtly abnormal multimer distributions (VWD types 1, 2M, and 2N) from those with a ‘significant’ decrease in the proportion of high-molecular-weight multimers (VWD types 2A and 2B). What constitutes a functionally significant change in multimer distribution has not been established experimentally, and consequently some patients may be difficult to classify. The standardized interpretation of multimer scans would be facilitated by increased availability of reference plasmas, widespread adoption of validated analytical methods and diagnostic criteria, and additional data on VWF multimer patterns associated with specific VWD mutations.
Normal and abnormal VWF function should be distinguishable by comparisons among VWF binding activities and VWF:Ag. As a practical rule of thumb, abnormal function may be indicated by a low value for the ratio of VWF activity to antigen. For example, abnormal VWF:RCo/VWF:Ag has been defined as < 0.5 [40], < 0.6 [14,91], or < 0.7 [15,92,93] to distinguish between VWD type 1 and type 2. In one study the VWF:RCo/VWF:Ag ratio was determined for 681 healthy controls: the mean was 1.0 with a range ±2SD of 0.72 to 1.26 [94]. These data provide a foundation for defining a ratio of VWF:RCo/VWF:Ag < 0.7 as indicative of a qualitative VWF defect. However, technical limitations of most VWF:RCo assays make the VWF:RCo/VWF:Ag ratio unreliable for a basal level of VWF:Ag less than 15–20 IU dL−1. Similar criteria have been proposed for VWF:CB/VWF:Ag [40,92,93]. Lower ratios are likely to be more specific predictors of a poor response to desmopressin, although this relationship has not been investigated systematically. Further study is needed to establish the value of particular test combinations and ratios for the classification of VWD type 2 variants.
VWD type 1 vs. low VWF
VWD type 1 can be hard to diagnose with confidence because the major laboratory criterion is merely a low value for the plasma VWF concentration, but VWF levels vary widely and are continuously distributed. Bleeding risk also varies continuously with VWF level, so that no VWF threshold separates patients into groups with distinctly different clinical features.
Two limiting conditions illustrate the problem. Very low VWF levels (e.g. 5–20 IU dL−1) tend to be highly heritable, are often associated with bleeding, and are frequently caused by apparently dominant VWF mutations [21–24]. The classification of such patients under VWD type 1 seems justified in order to facilitate their clinical management. On the other hand, VWF levels at the low end of the population distribution (e.g. 35–50 IU dL−1) show very low heritability [95,96], rarely segregate with bleeding symptoms [97,98], and rarely exhibit linkage to the VWF locus [98,99]. A diagnosis of VWD type 1 is not very useful for such patients. Instead, low VWF levels in this range may be managed as a biomarker for an increased risk of mild bleeding [100].
Two recent studies of VWD type 1 provide additional quantitative support for these conclusions. The Canadian Type 1 VWD Study included 155 informative families with an average of 1.9 affected persons per kindred. In this selected and well-characterized population, the proportion of VWD that was linked to the VWF gene was just 0.41 [14]. The European MCMDM-1 VWD included 143 families with an average of 2.9 affected persons per kindred. Linkage of the VWD type 1 phenotype to the VWF gene depended on the severity of VWF deficiency. If the plasma VWF:Ag was < 30 IU dL−1 in the index case, linkage to the VWF gene was always observed. But if the plasma VWF:Ag was > 30 IU dL−1, then the proportion of linkage was reduced to 0.51 [15]. The proportion of linkage decreased further if subjects with abnormal multimers or VWF:RCo/VWF:Ag < 0.7 were excluded. In addition, bleeding symptoms did not show significant linkage to the VWF gene, although there was a trend toward increased linkage for subjects with VWF:Ag < 30 IU dL−1 [15].
Correlation with response to therapy
Patients with VWD type 1 have a high probability of responding to desmopressin, whereas those with VWD type 2 or VWD type 3 usually do not respond [82,101,102]. The rare patients with VWD type 2 who do respond can be identified with a test dose. In addition, assays of plasma VWF during a desmopressin trial can be useful in order to resolve ambiguities among test results obtained at baseline and to facilitate the classification of some types of VWD.
Within the group of patients with VWD type 1, the likelihood of responding to desmopressin correlates with the initial VWF:Ag level, so that patients with VWF:Ag < 10 IU dL−1 often do not have a useful increment in VWF or FVIII level [82,103]. The subset of patients with VWD type 1 caused by accelerated clearance from the circulation (e.g. VWD Vicenza) may have an exaggerated but short-lived response to desmopressin, despite a very low VWF:Ag [31,33]. As discussed above under VWD type 2N, patients with relatively mild defects in VWF binding to FVIII often have a good response to desmopressin, whereas those with markedly abnormal FVIII binding usually do not [80–83].
These observations suggest that the less a patient's VWD phenotype deviates from normal or VWD type 1, the more likely it is that they will have a good response to desmopressin, and therefore the more useful a desmopressin test dose would be to assess their response.
Emerging techniques and opportunities in VWD classification
Standardized assessment tools are being developed to evaluate bleeding symptoms caused by defects in VWF. A questionnaire for this purpose was tested in a retrospective case–control study of VWD type 1 [104], and a revised version was used to compute a quantitative bleeding score for participants in the European MCMDM-1 VWD study [105]. Symptoms that discriminated between VWD type 1 and unaffected subjects included: bleeding after tooth extraction, nosebleeds, menorrhagia, skin bleeding, surgical bleeding, and bleeding after minor wounds. There was a strong inverse relationship between the bleeding score and the VWF level. However, the relationship between VWF level and bleeding score had limited prognostic value for individual subjects. For example, the severity of bleeding in the index case did not predict the severity of bleeding in other affected family members, and approximately one-third of subjects with significant bleeding (e.g. bleeding score ≥ 3) had VWF:Ag in the normal range (> 50 IU dL−1) [105]. Prospective studies using this approach will provide an opportunity to learn how the risk of medically significant bleeding depends on the level of plasma VWF.
The type of VWD generally correlates well with the probability of a useful response to desmopressin, but the correlation is weaker for intermediate VWD phenotypes that are hard to classify as either type 1 or type 2. Some of the difficulty is caused by lack of information about how best to use various VWF laboratory tests, but technical problems also contribute. For example, ristocetin-dependent assays of VWF function that are based on platelet agglutination or aggregation have low sensitivity and low reproducibility. Highly sensitive and reproducible assays of VWF platelet binding have been developed that use purified platelet GPIb instead of platelets [106–108]. Such assays could represent a substantial improvement for the diagnosis and classification of VWD.
As discussed in ‘Phenotypic classification of VWD’, mutations that cause VWD can be identified directly by sequencing the VWF gene [10] (http://www.shef.ac.uk/vwf/index.html). With a few exceptions, the location of VWF mutations correlates with the VWD type. Relationships between gene mutations and phenotype have been documented in detail for VWD types 2A, 2B, 2M and 2N, as well as for some forms of VWD type 1. In VWD type 2A, mutations in specific regions of the VWF subunit cause the selective loss of HMW multimers by several distinct mechanisms, and the location of these mutations can be predicted based on features of the plasma VWF multimer pattern [47]. Canadian and European studies are collecting similar extensive information for VWD type 1. As genetic testing strategies evolve, the results from other laboratory tests of VWF together with gene sequencing may increase our ability to predict responses to desmopressin or factor replacement therapy, which may lead to further improvements in the classification of VWD.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




