Association between alloantibody specificity and autoantibodies to red blood cells
Abstract
BACKGROUND: Alloantibodies (ALLOs) to red blood cells (RBCs) are frequently associated with autoantibodies (AABs). An association between ALLO specificity and AABs has not yet been described.
STUDY DESIGN AND METHODS: All patients and healthy blood donors screened for RBC antibodies between 2000 and 2006 were included. The odds ratio (OR) for ALLOs in patients with AABs compared to those without AABs was correlated with the OR of general ALLO prevalence in patients with AABs (normalized OR).
RESULTS: ALLOs were found in 4,626 of 204,330 patients and healthy blood donors (2.3%). The ALLOs were associated with AABs in 413 cases (8.9%). Among the specificities, anti-S with a normalized OR of 2.9 was overrepresented. This was most evident in pregnant women who showed a normalized OR of 15.1 for anti-S and AABs. The normalized OR revealed an additional association between Rh antibodies and AABs. No association was found between ALLOs to the Kell glycoprotein, Duffy protein, Lewis, or glycophorin A (M/N) and AABs.
CONCLUSION: The majority of associated ALLOs and AABs are directed against neighboring antigens of the Rh complex and glycophorin B.
ABBREVIATIONS:
-
- AAB(s)
-
- autoantibody (-ies)
-
- ALLO(s)
-
- alloantibody (-ies)
-
- ES
-
- epitope spreading.
It is estimated that approximately 3 percent of patients develop alloantibodies (ALLOs) to red blood cell (RBC) antigens.1 Antibody prevalence can be used to determine the immunogenicity of an antigen based on two criteria: if the prevalence of the antigen is known2 and if the antibodies are being developed independently from one another. This does not seem to be the case, however, because some patients may simultaneously develop more than one antibody after a single stimulation, that is, a blood transfusion.3 In addition, our group4 and others2,5-8 have shown that alloimmunization to RBCs may simultaneously stimulate the production of RBC autoantibodies (AABs). To date, there is no explanation for these findings.
In this study, we analyzed the specificities of ALLOs to RBCs and observed a predominant association between anti-S and AABs and, to a lesser extent, between Rh antibodies and AABs.
PATIENTS AND METHODS
All inpatients, outpatients, and healthy blood donors screened for RBC antibodies at the Charité from January 2000 through December 2006 were included. The majority of referred outpatient samples were from pregnant women.
Standard serologic and statistical methods were used as described.4 All RBC ALLOs and warm-reactive AABs were included. Cold-reactive AABs were not included.
Data were condensed to include each patient only once. The frequencies of the antibodies in patients with RBC AABs and in patients without RBC AABs were used to calculate the odds ratio (OR). Overrepresentation of ALLO specificities was expressed by relating the OR to the general ALLO OR (normalized odds ratio [NOR]; Equation 1).
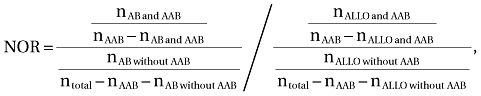 (1)
(1)where AB is the specific antibody (e.g., anti-S), ALLO is all patients with ALLOs, and n is number of patients. Hierarchical cluster analysis was performed with the analysis of variance–like Ward method.
RESULTS
A total of 124,408 patients and 79,922 blood donors were investigated (Fig. 1). ALLOs were detected in 3,864 patients (3.1%) and 757 blood donors (0.9%). AABs were present in 1,130 patients and 169 blood donors (Fig. 2). Among these antibodies, anti-S with a normalized OR of 2.9 was overrepresented (Table 1). This association was much more pronounced in pregnant women (normalized OR, 15.1; Table 2). The relationship between AAB and antibodies to S and s antigens that are located on glycophorin B could be confirmed by cluster analysis (Fig. 3). To a lesser degree, associations with AAB were also found for antibodies to the RHCE protein (Table 1; normalized OR, 1.6 for Cw, C, c, E, e, or f). This includes all allelic forms of the protein (i.e., Ce, cE, ce, and CE). No association with AAB was found for antibodies to glycophorin A (anti-M, anti-N), the Kell glycoprotein, Duffy protein, Lewis, P1, and various others. As expected, high associations were found for naturally occurring antibodies against antigens of lower prevalence such as anti-Wra, anti-Dia, anti-Vw, and anti-Mit.
Number of patients and donors investigated in relation to age.
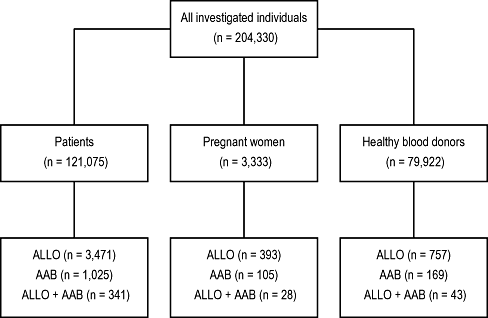
Auto- and alloimmunization in investigated subjects.
| Specificity† | Cases with ALLOs | Normalized OR | |
|---|---|---|---|
| And AABs | Without AABs | ||
| Anti-D | 64 | 1036 | 0.5 |
| Anti-C | 97 | 404 | 1.8 |
| Anti-c | 31 | 231 | 1.0 |
| Anti-Cw | 59 | 268 | 1.6 |
| Anti-E | 143 | 800 | 1.4 |
| Anti-e | 5 | 47 | 0.8 |
| Anti-f | 4 | 13 | 2.2 |
| Anti-K | 52 | 595 | 0.6 |
| Anti-k | 0 | 3 | |
| Anti-Kpa | 19 | 61 | 2.3 |
| Anti-Kpb | 0 | 3 | |
| Anti-Ku | 0 | 2 | |
| Anti-Fya | 21 | 233 | 0.7 |
| Anti-Fyb | 3 | 37 | 0.6 |
| Anti-Jka | 38 | 247 | 1.1 |
| Anti-Jkb | 9 | 63 | 1.0 |
| Anti-Lua | 23 | 217 | 0.8 |
| Anti-Lub | 0 | 17 | |
| Anti-Lu4 | 0 | 4 | |
| Anti-Lu6 | 0 | 1 | |
| Anti-Lu8 | 0 | 5 | |
| Anti-M | 9 | 311 | 0.2 |
| Anti-N | 2 | 16 | 0.9 |
| Anti-S | 35 | 88 | 2.9 |
| Anti-s | 3 | 16 | 1.3 |
| Anti-U | 0 | 2 | |
| Anti-Vw | 2 | 3 | 4.8 |
| Anti-Mta | 0 | 3 | |
| Anti-Mit | 1 | 2 | 3.6 |
| Anti-M1 | 0 | 1 | |
| Anti-He | 0 | 1 | |
| Anti-Lea | 9 | 237 | 0.3 |
| Anti-Leb | 2 | 85 | 0.2 |
| Anti-Xga | 0 | 1 | |
| Anti-P1 | 4 | 125 | 0.2 |
| Anti-P | 0 | 3 | |
| Anti-Wra | 61 | 59 | 7.7 |
| Anti-Dia | 3 | 6 | 3.6 |
| Anti-Coa | 0 | 5 | |
| Anti-Cob | 3 | 11 | 1.9 |
| Anti-Dob | 0 | 1 | |
| Anti-Ge | 0 | 6 | |
| Anti-Yta | 0 | 16 | |
| Anti-Ytb | 2 | 7 | 2.0 |
| Anti-Sc2 | 1 | 0 | |
| Anti-Inb | 0 | 1 | |
| Anti-AnWj | 0 | 1 | |
| Anti-LWa | 0 | 8 | |
| Anti-Ch | 1 | 61 | 0.1 |
| Anti-Rg | 1 | 8 | 0.9 |
| Anti-Kna | 0 | 21 | |
| Anti-KnMcC | 0 | 19 | |
| Anti-McCa | 0 | 2 | |
| Anti-Sla | 0 | 1 | |
| Anti-Yka | 0 | 11 | |
| Anti-Csa | 2 | 6 | 2.4 |
| Anti-JMH | 0 | 5 | |
| Anti-Vel | 0 | 12 | |
| Anti-Lan | 0 | 2 | |
| Anti-Jra | 0 | 2 | |
| Anti-MAM | 0 | 1 | |
| Any ALLO | 412 | 4209 | 1.0 |
- * A total of 204,330 patients and healthy donors were investigated.
- † Antibodies were observed either alone or in combination with other antibodies.
| Specificity | Normalized OR | |||
|---|---|---|---|---|
| All patients and donors | Healthy blood donors | Local patients | Prenatal screening | |
| Anti-C + AAB | 1.8 | 2.1 | 2.0 | 1.4 |
| Anti-E + AAB | 1.4 | 1.1 | 1.4 | 1.8 |
| Anti-Fya + AAB | 0.7 | 1.5 | 0.6 | |
| Anti-Jka + AAB | 1.1 | 1.5 | 1.3 | |
| Anti-S + AAB | 2.9 | 2.9 | 2.9 | 15.1 |
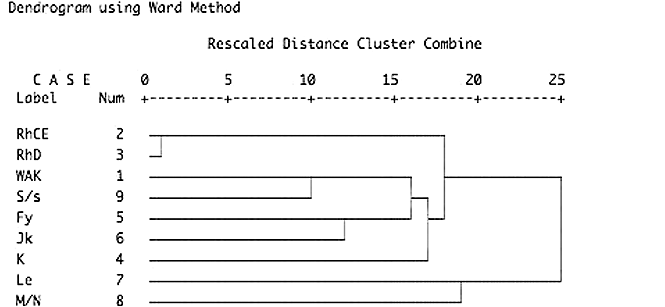
Blood group similarity dendrogram. Hierarchical cluster analysis of patients and donors (n = 204,330) of blood group antibody similarity. Close associations are marked by linking bars on the left-hand side of the graph. The looser an association is, the more it appears as a linkage to the right (i.e., left = similar; right = unequal). Owing to the hierarchical procedure, only the first linkage and closest association can be interpreted.
DISCUSSION
There is increasing evidence that blood transfusion occasionally leads to the production of ALLOs and AABs.4,8,9 Although the pathogenesis of alloimmunization is largely known, there remains no explanation for the occurrence of autoimmunization in these cases. In this study, we found significant associations between the specificity of some ALLOs and AABs. The most predominant association appeared to be related to anti-S and, to a lesser degree, to Rh antibodies. In addition, the association between anti-S and AAB was most pronounced in pregnant women (normalized OR, 15.1).
We have previously demonstrated that pregnant women infrequently develop RBC AABs that did not seem to cause any significant hemolysis either in the mothers or in the newborns.10 The mechanism of this phenomenon might be similar to that observed in autoimmunization against RBCs after blood transfusion. In both cases, the host is confronted with foreign antigens that may stimulate an immune response. The reason as to why the immune response could also be directed against self-antigens is obscure. A possible explanation for this phenomenon might be via epitope spreading (ES). Various studies have demonstrated that ES contributes to the chronic pathogenesis of T-cell–mediated and antibody-mediated autoimmune diseases.11-14 The term ES refers to the development of immune responses to epitopes distinct from and non–cross-reactive with the dominant epitope.15,16 The response may spread to epitopes on the causative antigen (intramolecular ES) or to epitopes on other antigens (intermolecular ES). These epitopes are often cryptic epitopes on the same molecule or dominant epitopes on neighboring molecules. In addition, regardless of the initial antigenic stimulus, the specificity of the immune response spreads to include self-epitopes other than those that initiate the immune response.17-19
It has been previously demonstrated that the Rh proteins are directly associated with Band 3, and glycophorin B is associated with the Rh proteins.20,21 In this context, immunization against an alloantigen would spread to neighboring, common structures that would normally not be exposed to the immune system. This is supported by the correlation of alloimmunization and autoimmunization after blood transfusion and, less commonly, after pregnancy. In fact, the majority of associated ALLOs and AABs appear to be directed against neighboring antigens, which are the Rh antigens and glycophorin B (for, e.g., S antigen) that are jointly located within a supramolecular complex.21,22 Most importantly, Rh antigens that are lacking on Rhnull cells represent the most immunogenic and clinically relevant blood group antigens. The majority of all immune ALLOs are directed against Rh antigens, and the majority of AABs do not react with Rhnull RBCs.20 It is of importance to note that the target structures for RBC AABs are abundant on the RBC membrane.23,24 Accordingly, the coincidence of AABs and ALLOs is, if the theory of ES is correct, not surprising. One of the alternate interpretations might be that the S phenotype is linked to immune-regulatory genes that predispose to autoimmunization. This is supported by the fact that anti-s is less associated to AABs than anti-S.
In the case of antibodies associated with RBC transfusion, the primary immune response is directed against foreign antigens and may spread to self-antigens. Similarly, in circumstances of antibodies related to pregnancy, the primary reaction could be against the S antigen and may spread to self-antigens. The question as to how pregnancy can also lead to the production of AABs without ALLOs cannot be definitively answered. Roughly 25 percent of all pregnancies are ABO-mismatched, however, and mismatched fetal cells are repeatedly destroyed by the immune system of the affected pregnant women. This long-lasting inflammatory response may result in a secondary immune response against antigens presented on fetal RBCs as well as on the mother's RBCs, that is, autoantigens.
ES has been suggested to occur, if the immune system fails to immediately clear the target. In such cases, a more diversified inflammatory response might be observed.16 The lasting antigenic exposure in pregnancy, in contrast to that after blood transfusion, could explain why anti-S and AAB are predominantly identified in prenatal investigations.
Other causes for this association such as biasing by referring laboratories are unlikely, because methodologically similar complicated antibody combinations such as anti-C and/or anti-E together with AABs were comparably overrepresented in prenatal cases (Table 2). Furthermore, anti-Fya or anti-Jka in combination with AABs did not occur in a single prenatal case, despite being equally difficult to investigate when compared with anti-S and AAB.
The association between anti-S and AABs can be explained neither by cross-reactivity nor by mimicking allospecificity as described by Issitt and coworkers.25 Anti-S and AAB bind to different structures: anti-S to glycophorin B and AABs to Rh proteins. The S antigen on glycophorin B is destroyed by treating RBCs with suitable enzymes, whereas the Rh proteins become easily accessible via this modification. As a result, anti-S does not react with these cells, whereas AABs react considerably stronger. This illustrates that anti-S cannot be explained by AAB pseudoalloreactivity (mimicking specificity).
Surprisingly, anti-D was found to be inversely associated with AABs. The difference in the association between antibodies to the RHCE protein compared to those of the RHD protein might be explained by the different immunologic structure. Antibodies to antigens on the RHCE protein recognize a single-amino-acid difference, whereas anti-D is directed against the entire RHD protein with all its epitopes. The production of specific antibodies against antigens similar to self-antigens is more difficult than the production of antibodies to antigens that less resemble autoantigens. Thus, further cases resulting in accidental cross-reactive AAB production in the preceding case are more likely to occur.
ACKNOWLEDGMENTS
The authors thank S. Kamhieh for grammatical corrections. The authors declare no competing financial interests.




