Retrospective Review of 707 Cases of Spinal Cord Stimulation: Indications and Complications
Abstract
Appropriate patient selection and minimizing complications are critical for successful spinal cord stimulation (SCS) therapy in managing intractable pain. We thus reviewed electronic medical records of 707 consecutive cases of patients who received SCS therapy in the Cleveland Clinic from 2000 to 2005 with an emphasis on indications and complications. SCS was used to treat complex regional pain syndrome (CRPS) (345 cases), failed back surgery syndrome (235 cases), peripheral vascular disease (20 cases), visceral pain in the chest, abdomen, and pelvis (37 cases), and peripheral neuropathy (70 cases). CRPS and failed back surgery syndrome accounted for 82% of the cases. The implant-to-trial ratio was 75% on average, with the highest for CRPS type 2 (83%) and the lowest for peripheral vascular diseases (65%). The only documented complication associated with SCS trials was lead migration in 5 of 707 patients (0.7%). There were no permanent neurological deficits or deaths as a result of SCS implant or its complications. Hardware-related complications were common (38%) and included lead migration (22.6%), lead connection failure (9.5%), and lead breakage (6%). Revisions or replacements were required in these cases. Biologically related complications included pain at the generator site (12%) and clinical infection (4.5%; 2.5% with positive culture). The rates of infection varied among the different diagnoses with the highest in failed back surgery syndrome (6.3%). Patients with diabetes had an infection rate of 9%, over the 4% in non-diabetics. Infections were managed successfully with explantation and antibiotic therapy without permanent sequela.
INTRODUCTION
Spinal cord stimulation (SCS) has been used for the treatment of various conditions including complex regional pain syndrome (CRPS),1,2 failed back surgery syndrome (FBSS)2–4 peripheral vascular disease (PVD), neuropathic pain,5 refractory angina,6 and more recently, visceral pain7 including pelvic pain.8 Outcome research has shown that SCS for FBSS is effective to improve quality of life, increase activities of daily living,9 and reduce health care costs for a long run.4,10–14 It is important to prevent, recognize, and minimize complications,15,16 given the increasing popularity of this treatment, the initial high costs associated with it, and the expanding range of indications for its use.
The complications of SCS can be many and the incidences have been reported at 30% to 40% in multiple studies. Hardware-related problems such as lead failure and migration are more common than biological complications that include infection, pain, and wound breakdown.2,16,17 Infection is one of the major complications of SCS with incidences of 4% to 10%,9,17 and is a common cause for explantation of the device. This incidence is higher than the 2% to 5% rate associated with any surgery in the U.S.A.,5 though the presence of a foreign device may be the reason for the higher incidence. The sites of the infection include the subcutaneous pocket at 54%, the lead track (17%), the lead entry site over the lumbar spine (8%), and multiple sites (14%).18 Complications add to the overall cost of the procedure and recent studies have recommended 18% budgetary allocation average per patient per annum for the maintenance of the therapy including complication management.15
The purpose of this study was to look into the common indications for SCS and, to identify factors associated with the commonly identified complications, and to recognize patterns that may help the practitioner prevent, predict, and manage these complications. We attempted to address this by reviewing a large number of cases and look into the comorbidities and diagnoses and stratifying the biological and mechanical complications. In addition, we attempted to determine the correlation between failed trials to diagnosis and other comorbidities.
METHODS
The study protocol was approved by the Cleveland Clinic Foundation Institutional Review Board. A retrospective analysis of 707 consecutive patients that underwent SCS in a 5-year period from 2000 to 2005 was carried out. Data related to the demographics, diagnosis, comorbidities and complications were gathered from the hospitals Electronic Medical Record system.
All the patients underwent the procedure at the Pain Management Department at the Cleveland Clinic Foundation. Each patient underwent the SCS trial and/or implantation during this period for management of chronic nonmalignant pain. The patients underwent investigations as clinically indicated prior to the procedure. The length of follow-up was from between 3 months to 7 years at the time of data collection with a mean of 3 years and 5 months.
The patients underwent a trial period of stimulation ranging from 5 days to 2 weeks, prior to a decision to implant was made. Following the implantation, the patients were treated with pharmacological agents as deemed necessary by the treating physician. The patients continued to receive medications as prescribed by other specialists.
The data were collected and entered into Microsoft Excel (Seattle, WA, U.S.A.) and analyzed with descriptive statistical methods and chi-square tests for comparisons between incidences wherever appropriate using Sigmastat 3.1. (Systat Software, San Jose, CA, U.S.A.)
RESULTS
Of the total of 707 patients, 299 (42.3%) were male and 408 (57.7%) were female. The mean age was 46 with a standard deviation of 15 and a range of 14 years to 87 years. The most significant comorbidity we considered was that of diabetes. Of the 707 cases, 56 patients (8%) carried a diagnosis of diabetes.
Indications of SCS
The primary pain problems treated by SCS included CRPS, FBSS, PVD, chronic pain in chest/abdomen/pelvis, peripheral neuropathy, and other conditions. The distribution of the cases is tabulated in Table 1 and shown schematically in Figure 1. Of the various diagnoses, CRPS cases were most common with 317 (45%) patients of CRPS type 1 (CRPS I) and 28 (4%) patients of CRPS type 2 (CRPS II). Unilateral upper extremity was most commonly affected with 170 (24%) cases, followed by unilateral lower extremity with 112 cases (16%) (Table 2). Of note is that 4 patients had all 4 extremity CRPS I. A total of 235 patients were treated for FBSS with SCS. As expected, the lower extremities were most commonly affected with 224 cases (Table 2). Twenty patients were treated for PVDs with ischemic disease being the most common diagnosis with 12 of the 20 cases. Seventy cases were treated for peripheral neuropathies.
| Diagnosis | Trial | Implant (%) | Infection (%) |
|---|---|---|---|
| CRPS | |||
| Type 1 | 317 | 251 (79) | 11 (3.4) |
| Type 2 | 28 | 24 (83) | 1 (3.5) |
| FBSS | 235 | 176 (75) | 15 (6.3) |
| PVD* | 20 | 13 (65) | 0 (0) |
| Visceral pain† | 37 | 29 (78) | 1 (2.3) |
| Neuropathy | 70 | 57 (81) | 4 (5.7) |
| Total | 707 | 527 (75) | 32 (4.5) |
- * PVD: peripheral vascular disease, including Raynaud's.
- † Visceral pain: chest, abdominal, and pelvic pain.
- CRPS, complex regional pain syndrome; FBSS, failed back surgery syndrome.
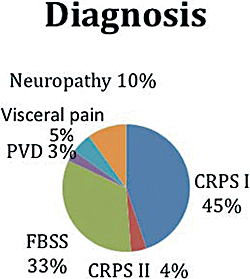
Composites of spinal cord stimulation indications. CRPS I, complex regional pain syndrome type I; CRPS II, complex regional pain syndrome type 2; FBSS, failed back surgery syndrome; PVD, peripheral vascular disease.
| CRPS I | CRPS II | FBSS | Total | |
|---|---|---|---|---|
| Unilateral UE | 154 | 16 | 5 | 175 |
| Bilateral UE | 22 | 6 | 28 | |
| Unilateral LE | 100 | 12 | 122 | 234 |
| Bilateral LE | 29 | 101 | 130 | |
| Bilateral LE + unilateral UE | 3 | 1 | 4 | |
| Unilateral UE + unilateral LE | 5 | 5 | ||
| All 4 extremities | 4 | 4 | ||
| Total | 317 | 28 | 235 | 580 |
- LE, lower extremity; UE, upper extremity.
The Implant-to-Trial Ratio
Of the 707 cases who had initial SCS trial lead placement, 527 patients were eventually implanted with implantable pulse generator (IPG). The implant-to-trial ratio varied by diagnosis (Table 1 and Figure 2), yet the differences were not statistically significant (chi-square tests). The average implant-to-trial ratio was 75%.
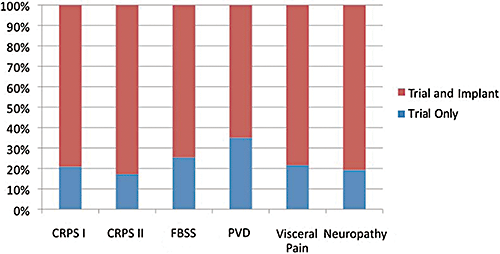
Implant-to-trial ratio. CRPS I, complex regional pain syndrome type I; CRPS II, complex regional pain syndrome type 2; FBSS, failed back surgery syndrome; PVD, peripheral vascular disease.
Complications
The documented complications included pain, seroma, lead migration and hardware failure, and infection. There were no permanent neurological deficits or deaths directly as a result of the SCS procedure or its complications recorded. There was 1 case of seroma noted without evidence of infection. Pain at the generator site as a complication was documented in 86 (12%) of the 707 patients.
Hardware-related complications were a prominent issue with SCS that required revisions. Among the 527 patients with implants, 119 cases (22.6%) had lead migration. Lead connection failure occurred in 50 cases (9.5%) and lead break in 33 cases (6%). The risks of these complications were very low in the trials. Among the 707 SCS trials, 5 patients (0.7%) had lead migration and there were no other hardware-related failures.
A total of 32 (4.5%) patients had documented infections (Table 1), of which 22 cases had deep infections (20 had IPG pocket infections and 2 had lead track infections). The rest 10 cases had superficial infections limited to the skin and subcutaneous tissues at the site of electrode entry over the spine (Table 3). None of the patients with superficial infections had abscess while 18 of the patients with deep tissue infections had documented abscesses. There was only 1 case of epidural infection without any evidence of abscess. This case was confirmed by a positive culture that was obtained during explantation surgery, from the epidural area. No infections were documented in any of the 707 SCS trials.
| Infections | Number of Cases | Pus Documented | Positive culture | Antibiotics |
|---|---|---|---|---|
| Superficial | 10 | 0 | 0 | Oral in all cases |
| Deep | 22 | 20 | 18 | HIVAT in 17 cases |
| Total | 32 | 20 | 18 | 32 |
- HIVAT, home intravenous antibiotic therapy.
The infection rates ranged from 0 to 6.3% among the various diagnoses (Table 1) and were not significantly different (chi-square tests). Of the total of 56 patients who had a diagnosis of diabetes mellitus, 5 patients developed infections. Hence, 9% of all diabetics developed infections. Among the 651 patients without diabetes, 27 developed infections with an incidence of 4%. However, this difference was not statistically significant from that of the diabetics (P = 0.188, chi-square test).
All the cases of infection were managed by explantation of the SCS system and antibiotics except 1 case that did not receive any antibiotics following explantation. methicillin-resistant Staphylococcus aureuswas isolated in 5 cases (15% of all infections), of which 2 were treated with home intravenous antibiotic therapy (HIVAT) only, 2 with a combination of HIVAT and oral antibiotics, and 1 with oral antibiotics only.
Of the 32 cases, 17 patients were treated with either HIVAT alone (12 cases) or in combination with oral medications (5 cases) (Table 3). Vancomycin was the most commonly used intravenous (IV) agent (in 11 of the 17 patients). Of the remaining 15 patients that received oral antibiotics, many of them received a short course of IV antibiotics while in the hospital before being transitioned to oral antibiotics. All the patients were completely cleared from the infection with 2 to 4 weeks treatments.
DISCUSSION
Treatment with SCS is an effective treatment for a variety of conditions in current pain practice. This study retrospectively evaluated our experience with this procedure over a 5-year period. CRPS I and II, FBSS, PVD visceral pain, and peripheral neuropathy remain some of the common indications for this treatment at our institution, with CRPS being the most common diagnosis in this period. The demographic differences were not significant and were consistent with the previously published data.
All of our patients underwent a trial of SCS prior to implantation of an IPG. This may have improved the selection of patients for long-term treatment with SCS as a fourth of the patients failed to respond adequately in trial, thereby identifying potential treatment failures with minimal risk. It is also clear that the trial stimulation was not associated with any more serious complications. There were in fact very few complications noted during the trial period. The absence of infections during trial stimulation was notable. The risk of hardware-associated complications was very low in the trials. Among the 707 SCS trials, there were 5 lead migrations (0.7%) and no other hardware-related failures. While the method of the procedure may vary between practitioners, it is generally agreed that a successful trial that demonstrates a significant reduction in pain and increased activity levels are desirable prior to the implantation of the IPG. Consistent with published data, this study suggests that the yield tends to be slightly lower in conditions like PVD.
The targeted coverage included the upper and/or lower extremities and various combinations of these extremities in the cases of CRPS and FBSS (Table 2). These targets are also applicable to PVD and peripheral neuropathy in our practice. Additional targets were the chest, abdomen, and pelvis for visceral pain. Not reflected is the coverage of the back that has been employed in the last few years in our practice and in a few other centers for FBSS with predominantly axial back pain. With this recent advancement, the utilization of SCS in FBSS is likely to increase significantly as axial back pain is a common and predominant manifestation in many cases of FBSS.
The complications in this cohort included pain, seroma, lead migration and hardware failure, and infection. There were no permanent neurological deficits or deaths as a result of the SCS procedure or its complications. Consistent with published data, hardware-related complications were common and occurred in up to 38% of the cases of SCS implantation in this period. These complications included lead migration (22.6%), lead connection failure (9.5%), and lead breakage (6%). Revisions or replacements were required to correct the problems in these patients. The high rates of hardware-related complications may have since improved significantly with the recent advancement of SCS technology that provides better lead anchor, more reliable lead connections, and break resistant leads.
Infections were the most significant complications and the average incidence was 4.5% in this cohort, 2.5% of which with positive culture (Table 1). This is in the lower range of the 4% to 10% published in the literature. This may be due to our extensive experiences in these procedures, close postoperative follow-up, and the practice of routine perioperative prophylactic antibiotic therapy. The difference in infection incidence between diabetics (9%) and nondiabetics (4%) is potentially significant clinically, yet it did not reach statistical significance as the study was still underpowered given the low incidences of infection in this large cohort.
Diabetes has been identified as an important risk factor for complications in general and infection in particular for other surgical conditions. It appears to be applicable to SCS as well from this study. The risk of infection specific to SCS implantation is previously unknown. The clinician is faced with an interesting conundrum whereby treatment of diabetic neuropathy may present with an additional risk in this population. While previous studies have suggested a higher infection rate, concrete conclusions have not been drawn because of the limited number of cases recruited in the underpowered studies.5,19 The data of the present study suggest that special considerations need to be given to this population for the prevention of infections, such as strict sterile technique, reduction in surgical time, and perioperative prophylactic antibiotic therapy.
In addition, the data of this study show relatively low incidences of infection in patients with PVD and visceral pain and indicate that this procedure may be carried out safely in these patients. There are reports of delayed diagnosis and treatment of spinal infections in patients with FBSS20 without spinal cord stimulators. It is unknown if this risk is transferred in the case of implanted SCS. This study revealed an infection rate of 6.3% in FBSS patients, which is higher than the 3.4% in CRPS patents and the 4.5% average of this cohort. However, the differences are not statistically significant as the low incidences of infections in this cohort make this study underpowered to show statistical significances. The infections were managed with oral and/or IV antibiotics and favorable outcomes were achieved without permanent neurological or any other significant sequela.
Taken together, SCS has been established as an effective treatment for a variety of conditions. This therapy is relatively safe if it is done appropriately with careful patient selection. We would suggest that an adequate trial lasting from 5 to 14 days for appropriate patient selection is required as SCS implantation in poorly selected patients may not only be therapeutically unhelpful, but may also be potentially harmful and cause costly complications. Lower thresholds for suspicion of infection are suggested in patients with diabetes and FBSS in the perioperative period of SCS implantation. Close follow-ups and perioperative prophylactic antibiotics may reduce the infection rates, but definitive conclusions are dependent on further investigation. Continuing to improve surgical techniques, as avoiding subcuticular stitches with absorbable stitches, which we have adopted after the study, might help decrease the incidence of superficial wound infection. As simple as it sounds, proper aggressive irrigation and ongoing home instructions might improve the incidence of infections.




