Magnetic Resonance Imaging (MRI) Anatomy of the Ovine Stifle
Abstract
Objective
To describe magnetic resonance imaging (MRI) anatomy of the ovine stifle and investigate meniscotibial and cruciate ligaments anatomy.
Study Design
Descriptive ex vivo study.
Animals
Pelvic limbs (n = 44) from 22 adult Texel ewes.
Methods
Forty limbs (n = 40) were scanned using 3 Tesla MRI before gross anatomic dissection. Two other limb pairs were frozen and transected to obtain sections that were compared with MRI images for identification of anatomic structures.
Results
In all stifles, the craniomedial bundle of the cranial cruciate ligament inserted caudally to the cranial attachment of the medial meniscus. No transverse intermeniscal ligament was identified in 80% of stifles, whereas a few small ligamentous fibers were seen crossing from 1 cranial horn to the other in 20% of stifles. There was good differentiation of menisci, ligaments, and synovial cavities on MRI images. Two bundles were identified in all cranial cruciate ligaments on MRI. Sensitivity and specificity of 3T MRI for detection of transverse intermeniscal ligament were 42% and 84%, respectively.
Conclusion
3T MRI provided well defined reference images for menisci, synovial cavities, and most ligaments.
The ovine stifle has often been the joint of choice to evaluate surgical and medical therapeutics of osteoarthritis (OA),1-5 meniscal pathology,6-8 injuries of cruciate ligaments9, 10 and collateral ligaments.11 Knowledge of the anatomy of the joint is useful to plan surgical access and to assess the progress of the disease in research studies, either by gross anatomy or imaging techniques.
General descriptions have been provided in standard veterinary anatomy texts usually considering the sheep as a scaled-downed version of the cow, with little emphasis on the stifle.12-15 Allen et al proposed a detailed and useful description of the anatomy of the stifle based on cadaveric dissections and stifle radiographs of skeletally mature ewes of mixed breeds.16 The osseous anatomy,17 cutaneous innervation,18 menisci, and meniscofemoral ligaments7, 19 have been described and compared between species. The structure of ovine cranial and caudal cruciate ligaments are reportedly very similar to that in people.20 Surgical arthroscopy of the femoropatellar joint has been described.16, 21 The internal anatomy and injections of synovial cavities have been investigated using contrast radiography, computed tomography (CT), and gross anatomic dissection.22 Some discrepancies exist between descriptions of the insertion sites of the cranial attachment of the medial meniscus (meniscotibial ligament). Allen describes a transverse intermeniscal ligament (TIL) that joins the cranial horn of the medial meniscus to the cranial horn of the lateral meniscus; the craniomedial bundle of the cranial cruciate ligament inserts cranially to the TIL.16 According to others,22 the TIL was only present in a limited number of Texel sheep and it was suggested that variations exist between breeds or individuals similar to the anterior intermeniscal ligament in people.23 In that population of sheep, the insertion of the craniomedial bundle of the cranial cruciate ligament was caudal to the cranial attachment of the medial meniscus, and in consequence, was caudal to the TIL when this was present.22
In research studies using the ovine stifle model as a model of OA or meniscal pathology, non-invasive imaging techniques like magnetic resonance imaging (MRI) are useful. The few reports of the use of MRI in small ruminants mostly involved assessment of cartilage repair.24-27 Anatomic descriptions of the ovine stifle described from images acquired with MRI are lacking.
Our purpose was to describe the MRI anatomy of the ovine stifle and to investigate the anatomy of meniscotibial and cruciate ligaments.
MATERIALS AND METHODS
Specimens
Pelvic limbs (n = 44) from 22 Texel sheep, euthanatized for reasons other than pelvic limb lameness (mastitis), were disarticulated at the coxofemoral joint and collected within 12 hours of death. Sheep were 4−6 years old, weighed 50−70 kg, and came from the Sheep Centre of the University of Namur. The experimental protocol (KI 10/148) was approved by the local ethical committee for animal welfare. Limb specimens were moistened, wrapped in gauze, sealed in plastic bags, and stored at −20°C. Each limb was identified by a number. For all investigations, limbs were thawed to room temperature, clipped, and cleaned.
MRI Study and Gross Anatomic Dissection
Forty limbs (n = 40) were scanned using high-field 3 Tesla (3T) MR (Verio, Siemens, Erlangen, Germany) with IW FS FSE (intermediate weighting fat suppression fast spin echo) sequences detailed in Table 1. A 15 channel-knee coil (Siemens) was used. To highlight synovial anatomy, the common joint cavity was injected with liquid: to ensure adequate positioning of the needle and injection at the correct location, it was performed under fluoroscopic guidance with contrast agent. A paraligamentous technique was used22: a 21G, 1½″ needle was inserted along the medial aspect of the patellar ligament at mid-distance between its distal and proximal insertions. Radiopaque contrast solution (10 mL:7.5 mL melamine and sodium ioxaglate [Hexabrix 320, Guerbet, Villepinte, France] mixed with 2.5 mL saline) was injected with minimal pressure into the joint. Images were transferred on a medical digital imaging system (PACS, TELEMIS, Louvain-la-Neuve, Belgium) for analysis. Field of view extended from the base of the patella to 6 cm below the tibial plateau. Dorsal and transverse slices were respectively perpendicular and parallel to the tibial plateau.
| IW FS FSE sagittal | IW FS FSE dorsal | IW FS FSE transverse | |
|---|---|---|---|
| TE (echo time; milliseconds) | 37 | 37 | 35 |
| TR (repetition time; milliseconds) | 3200 | 3000 | 4400 |
| Acquisition time (minutes) | 3.26 | 2.11 | 3.06 |
| FOV (cm) | 16 | 16 | 16 |
| Volume pixel | 0.4 × 0.4 × 2 | 0.5 × 0.5 × 2 | 0.5 × 0.5 × 2 |
| Matrix | 384 × 384 | 320 × 320 | 320 × 320 |
| Slice thickness (mm) | 2 | 2 | 2 |
Gross anatomic dissection was performed in the 40 limbs to describe ligaments and menisci. Particular attention was paid to the cranial attachments of the menisci and to the hypothetical presence of a TIL. Digital photographs of the relevant stages of the dissection were acquired.
On two other frozen limb pairs (n = 4), stifle joints were sectioned into ∼3 mm slab sections in sagittal and parasagittal planes in two stifles, in dorsal planes in one stifle, and in transverse planes in one. Each gross section was photographed and compared to the corresponding MR images for identification of anatomic structures.
RESULTS
High quality MR images of the ovine stifle joint were obtained yielding good differentiation of synovial cavities, ligaments, and menisci (1-4). With intra-articular contrast, IW FS FSE images created an arthrogram like effect, with hyperintense (white) synovial fluid improving the delineation of the cartilage surfaces. Ligaments and tendons were seen as low-signal-intensity bands on all sequences except when they were orientated at 55° to the main field B0 and presented an intermediate signal because of the magic angle artifact on short TE (echo time) sequences.
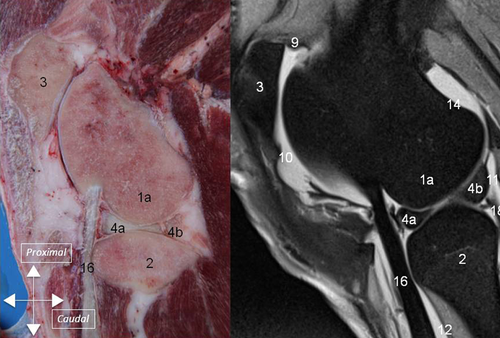
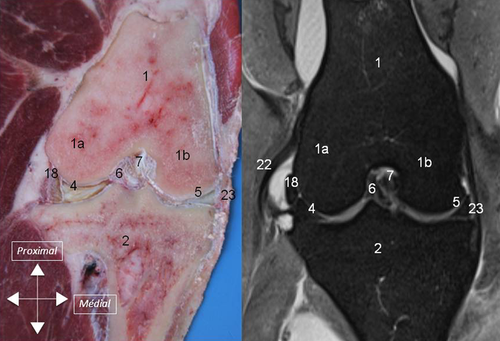
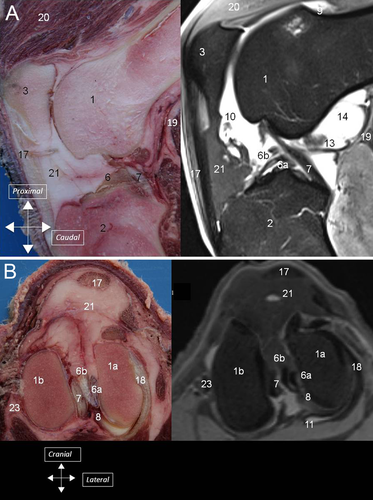
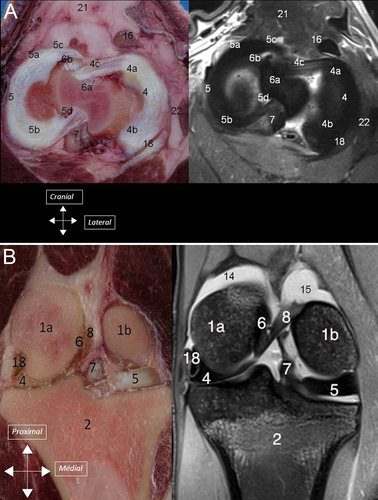
Synovial Cavity
The synovial cavity consisted of three major interconnected compartments: the femoropatellar, and medial and lateral femorotibial compartments (1). Major synovial recesses were identified: (1) a suprapatellar recess cranial to the distal aspect of the femur; (2) two recesses, medial, and lateral, caudoproximal to the femoral condyles (supracondylar recesses); (3) a recess under the tendon of the popliteus muscle laterally and caudally (sub-popliteus recess); and (4) a recess of the lateral femorotibial compartment which extends distally on the lateral aspect of the proximal tibia, in the extensor groove, creating a sheath for the common tendon of the peroneus tertius muscle, long digital extensor muscle, and medial extensor muscle (m extensor digiti III proprius) (sub-tendinous recess).
External Ligaments
Several external ligaments connected the tibia, femur and patella (2). The medial collateral ligament inserted proximally on the tubercle of the medial condyle and distally on the tibia ∼3 cm below the articular surface. The lateral collateral ligament inserted proximally on the tubercle of the lateral condyle and distally on the proximal aspect of the fibula. The lateral meniscus was separated from the lateral collateral ligament by the tendon of the popliteus muscle. The medial collateral ligament was adherent to the medial meniscus. The tibiopatellar ligament joined the patella to the tibial crest (3).
Internal Ligaments
Caudal and cranial cruciate ligaments connected the tibia to the femur (3). The caudal cruciate ligament inserted on the caudal aspect of the tibia and coursed cranioproximally and medially towards the axial aspect of the medial condyle. The cranial cruciate ligament had 2 distinct and separated bundles, 1 caudolateral and 1 craniomedial. The distal insertion of the caudolateral bundle was located on the dorsal slope of the notch of the intercondylar eminence. It coursed proximally, joined the craniomedial bundle and inserted on the axial aspect of the lateral condyle. The craniomedial bundle inserted distally, caudal to the cranial attachment (meniscotibial ligament) of the medial meniscus. Distally, the two bundles were easily identified and separated by a small amount of fat. Proximally they appeared separated in 21 stifles whereas it was more difficult to differentiate them in 19 stifles. On dissection, the cranial cruciate ligament was composed of 2 bundles in all stifles.
Menisci
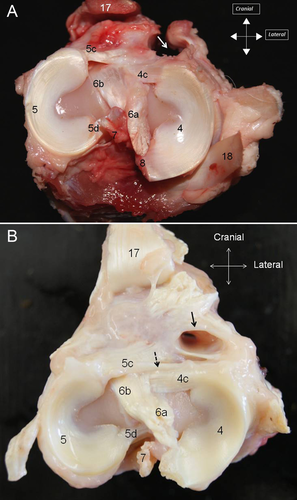
The medial meniscus and the lateral meniscus were attached to the tibia by meniscotibial ligaments (4A). Caudal meniscotibial ligaments arose from the caudal horn of each meniscus. The medial caudal meniscotibial ligament inserted cranially to the caudal cruciate ligament on the caudal slope of the intercondylar eminence. The lateral caudal meniscotibial ligament inserted laterally to the caudal cruciate ligament on the proximocaudal aspect of the tibia. Cranial meniscotibial ligaments arose from the cranial horn of each meniscus. The lateral cranial meniscotibial ligament inserted cranially to the caudolateral bundle of the cranial cruciate ligament on the cranial aspect of the lateral tibial condyle. The medial cranial meniscotibial ligament inserted cranially to the craniomedial bundle of the cranial cruciate ligament. The meniscofemoral ligament originated from the caudal horn of the lateral meniscus and inserted proximally on the medial intercondylar region of the femur (4B).
On dissection, the different parts of the menisci could be identified (cranial horn, middle segment, and caudal horn) together with the meniscotibial and meniscofemoral ligaments (5) In all stifles, the medial cranial meniscotibial ligament was cranial to the craniomedial bundle of the cranial cruciate ligament. No TIL ligament was identified in 32 stifles (80%), whereas a few small ligamentous fibers were identified crossing from the medial meniscus to the lateral meniscus on both stifles (5B) of four ewes (20%).
Intermeniscal fibers, corresponding to the so-called TIL, were identified on MRI in four stifles but not on anatomic dissection. In five stifles, dissection revealed intermeniscal fibers not identified on MRI. In three joints, intermeniscal ligaments were identified by both techniques and in 28 joints, TIL was not detected by either MRI or dissection. Considering dissection as the gold standard for detection of TIL, positive and negative predictive values of 3T MRI for TIL identification were 37% and 84%, and sensitivity and specificity were 42% and 84%, respectively.
DISCUSSION
In ovine stifles, a characteristic anatomic landmark on MRI scans is the common tendon of the long digital extensor, peroneus tertius, and medial digital extensor muscles (1)16 which does not occur in people. It is clearly visible on the lateral aspect of the joint and has been described.22 It originates proximally and is inserted on the distolateral aspect of the lateral trochlear ridge of the femur. The proximal aspect of the m peroneus longus covers partly the proximal portions of the long digital extensor, peroneus tertius, and medial digital extensor muscles. The cranial tibial muscle surrounds also partly the proximal portion of those three muscles. The common tendon is included in a large synovial recess beneath the cranial tibial muscle and m peroneus longus; it is maintained in the extensorius sulcus by a thin band of fibrous tissue (Fig. 5A and B). This tendinous recess may have clinical useful application as it communicates with the articular synovial cavity. Synoviocentesis of the stifle joint could be performed by inserting a needle along the bone, under the common tendon, in that recess and might be the site of choice for injection because there would be a lower risk of iatrogenic articular damage.22
The 4 limbs injected with liquid had highlighted synovial cavities and on MRI, articular synovial cavities were delineated. The anatomy of the different compartments were consistent with previous observations obtained by contrast radiography, dissection (with injection of resin in the joint), and CT.22
Another characteristic of the ovine stifle, clearly visible on MRI, was the presence of two bundles within the cranial cruciate ligament, which were observed on all specimens. In people, the double-bundle anatomy of the anterior cruciate ligament was identified in 94% of patients using 3T MRI.28 The possibility of distinguishing the two bundles in the anterior cruciate ligament with 3T MRI has interesting clinical applications because it enables the study of different rupture patterns of the bundles.29 For example, it would enable monitoring the progress after surgical treatment of 1 torn bundle while the intact bundle is preserved.
We were unable to consistently identify a TIL. This structure was only present in 4 sheep as tiny fibers between cranial horns of menisci. In people, the TIL, called anterior intermeniscal ligament (AIL), also known as the transverse geniculate ligament, lies between the anterior horns of the menisci and is not a constant feature. AIL was found in 53% of patients and in three possible configurations.30 It has also been described in the dog and cat as a structure joining the menisci cranially.31 Barone describes this ligament in the dog and the pig, but not in the sheep.12 These findings confirm previous reports that variations between individuals or breeds may exist in sheep, and that the TIL or fibers joining both cranial horns are not a constant feature.22 In our study, MRI was not very sensitive nor specific for detection of the tiny fibers joining both cranial horns, likely because of the small size of the fibers. In people, the AIL, when present, is thicker and positive predictive values to identify AIL of 100% has been reported for 3T MRI.30
It was not our purpose to assess the efficacy of 3T MRI sequences to detect cartilage, our images illustrate the narrow thickness of ovine stifle cartilage, which may limit assessment of cartilage morphology and volume even with a 3T magnet. Frisbee et al reported that average articular cartilage thickness over several locations (lateral trochlear ridge, medial trochlear ridge, and medial femoral condyle) were 0.4–0.5 mm for sheep and 2.2–2.5 mm for people.32 To see early morphologic degenerative changes in cartilage, imaging with a resolution of 0.2–0.4 mm is required.33 This may have implications for research and necessitate the development of sequences with high resolution.
There is a growing demand for appropriate animal models for both OA and tissue engineering studies. Using large animals may provide a more reliable model of OA and offers several advantages including the opportunity to undertake topographic analysis of joint cartilage and serial aspiration of synovial fluid, to investigate animals of higher weight, and to more objectively measure pain and/or mobility through gait analysis. In addition, non-invasive advanced imaging techniques, like MRI are recommended for measuring outcomes in longitudinal studies.34 Thus, a fuller understanding of the anatomy of the ovine stifle, as well as similarities and differences to the human knee will be beneficial to both surgeons and radiologists involved in such research.




