Evaluation of Laser Doppler Flowmetry for Measurement of Capillary Blood Flow in the Stomach Wall of Dogs During Gastric Dilatation-Volvulus
This work has been supported by a grant (#99CA-16) from Morris Animal Foundation.
Dr. Pelsue's current address is 855 E Peckham Ln, Reno, NV 89502.
Abstract
Objectives— To validate laser doppler flowmetry (LDF) for measurement of blood flow in the stomach wall of dogs with gastric dilatation-volvulus (GDV).
Animals— Six purpose-bred dogs and 24 dogs with naturally occurring GDV.
Study Design— Experimental and clinical.
Methods— Capillary blood flow in the body of the stomach and pyloric antrum was measured with LDF (tissue perfusion unit (TPU) before and after induction of portal hypertension (PH) and after PH plus gastric ischemia (GI; PH+GI) and compared with flow measured by colored microsphere technique. Capillary flow was measured by LDF in the stomach wall of dogs with GDV.
Results— PH and PH+GI induced a significant reduction in blood flow in the body of the stomach (P=.019). A significant positive correlation was present between percent changes in capillary blood flow measured by LDF and colored microspheres after induction of PH+GI in the body of the stomach (r=0.94, P=.014) and in the pyloric antrum (r=0.95, P=.049). Capillary blood flow measured in the body of the stomach of 6 dogs that required partial gastrectomy (5.00±3.30 TPU) was significantly lower than in dogs that did not (28.00±14.40 TPU, P=.013).
Conclusions— LDF can detect variations in blood flow in the stomach wall of dogs.
Clinical Relevance— LDF may have application for evaluation of stomach wall viability during surgery in dogs with GDV.
INTRODUCTION
GASTRIC DILATATION-VOLVULUS (GDV) is a life-threatening condition affecting mostly large breed dogs. Surgical treatment includes repositioning the stomach, gastrectomy if the stomach wall is necrotic, and then pexy to prevent recurrence.1,2 Necrosis of the stomach wall along the greater curvature occurs in 10–25% of GDV dogs that have surgery.3–7 Necrosis results from occlusion of the caudal vena cava and portal vein, and ischemia from reduced splanchnic blood flow because of decreased cardiac output. Ischemia is also induced by compression of capillaries in the stomach wall. Gastric necrosis is associated with a high morbidity.5,8
Surgical evaluation of stomach wall viability is subjective. Palpation of stomach wall thickness, serosal surface color, presence of peristalsis, and evidence of serosal capillary perfusion are the subjective criteria most commonly used to evaluate stomach viability during GDV.5 Subjective evaluation of stomach viability requires experience but was 85% accurate in an experimental model.5 Misinterpretation of criteria can occur in 40% of dogs with naturally occurring GDV and will result in stomach rupture or dehiscence if gastrectomy for removal of compromised or non-viable wall is incomplete.5 Fluorescein dye and scintigraphy have been investigated as objective measurements of stomach wall viability.9,10 Fluorescein was accurate in only 58% of dogs with GDV.9 Scintigraphy for detection of stomach wall ischemia was accurate in 91% of dogs in a canine model of GDV and in 79% of dogs with clinical GDV.10,11 However, scintigraphy is not widely available, does not provide specific anatomic landmarks for gastrectomy, and poses health risks for surgeons. An objective method of assessing gastric wall perfusion with high sensitivity and specificity that could be rapidly and conveniently applied intraoperatively would facilitate intraoperative decisions related to the need for gastrectomy after correction of GDV in dogs.
Laser doppler flowmetry (LDF) has been used as a quantitative, reproducible method for assessment of bowel perfusion intraoperatively under sterile conditions.12,13 LDF has been used to evaluate capillary blood flow in the gastrointestinal tract, kidney, myocardium, skeletal muscle, bone, teeth, retina, skin and brain in experimental and clinical situations.14–24 However, LDF flowmetry has not been validated for evaluation of the variation of blood flow in the stomach wall of dogs under conditions similar to GDV. Because GDV is associated with portal hypertension (PH) and gastric ischemia (GI), we decided to first validate LDF under these conditions. Our hypothesis was that LDF was a valid technique for evaluation of capillary blood flow in the stomach wall in dogs after induction of PH and PH+GI.
Our primary objective was to correlate variation in capillary blood flow to the stomach measured by LDF compared with colored microspheres after induction of PH+GI. A secondary objective was to evaluate capillary blood flow in the stomach of dogs admitted with GDV.
MATERIALS AND METHODS
Validation Study
Normal Dogs. Eight purpose-bred dogs (28.00±3.10 kg) were studied; each dog was its own control. Blood flow in the gastric wall was measured at baseline, after induction of PH for 1 hour, and after induction of PH plus GI for 30 minutes. Blood flow was measured with colored microspheres (gold standard) and LDF.
Anesthesia. Food was withheld for 12 hours. Dogs were administered atropine (0.03 mg/kg), benzodiazepam (0.2 mg/kg), and morphine (0.8 mg/kg) subcutaneously then general anesthesia was induced with propofol intravenously (4–6 mg/kg) and maintained with isoflurane in oxygen. End-tidal CO2 was maintained between 35 and 40 mm Hg.
Instrumentation. With the dogs positioned in dorsal recumbency, the abdominal and thoracic cavities were opened by median incision and self-retaining retractors were used to maintain exposure. The portal vein was dissected at the level of the epiploic foramen. An ultrasonic flow probe (SB 10 mm Probe, Transonic Inc., Ithaca, NY) was placed around the portal vein cranial to its most cranial tributary and connected to an ultrasonic flow analyzer (T206 Ultrasound flowmeter, Transonic Inc.). A Rummel tourniquet was placed around the portal vein cranial to the flow probe. An 18 g over-the-needle catheter was inserted in a jejunal vein and secured with 3-0 polypropylene. A pressure transducer (CDXpress™, Argon, Maxxim Medical, Athens, TX), zeroed to the level of the right atrium, was connected to the jejunal catheter with a fluid-filled extension set.
The abdominal aorta was exposed distal to the aortic hiatus. An 18 g over-the-needle catheter was inserted in the aorta and secured with a 3-0 polypropylene mattress suture with Teflon pledgets placed in the wall of the aorta. A pressure transducer zeroed to the level of the right atrium, was connected to the arterial catheter with a fluid-filled extension set. An 18 g over-the-needle catheter was inserted in the cranial vena cava and secured with a 3-0 polypropylene mattress suture with Teflon pledgets placed in the vena cava wall. A pressure transducer (CDXpress™, Maxxim Medical), zeroed to the level of the right atrium, was connected to the central venous catheter with a fluid-filled extension set.
The pericardium was incised and sutured to the edge of the sternotomy with 3-0 nylon. An ultrasonic flow probe (SS 20 mm Probes, Transonic Inc.) was placed around the ascending aorta and connected to an ultrasonic flow analyzer (T206 Ultasound flowmeter, Transonic Inc.). A 20 g over-the-needle catheter was introduced in the left atrium and secured with a 3-0 polypropylene mattress suture with Teflon pledgets. A microtip pressure transducer catheter (Mikro-tips, Millar, Houston, TX) was inserted in the apex of the left ventricle and secured with a 3-0 polypropylene mattress suture.
A laser doppler flow probe (Type R, Transonic Inc.) was sutured on the pyloric antrum, at mid-distance between the smaller and the greater curvature of the stomach, with four 4-0 polypropylene simple interrupted sutures. Another laser doppler flow probe (Type P, Transonic Inc.) was sutured on the body of the stomach close to the short gastric arteries in a location where the stomach is typically at risk for necrosis with GDV (Fig 1). Both probes had the same characteristics (2 m long cable, transmitting and receiving optical fiber diameter was 0.0625 mm, and the transmitting and receiving optical fibers were 0.5 mm apart at the probe tip). The surface area of the window at the tip of the flow probes was 1 mm2. The flow probes were connected to a laser doppler flow analyzer (laser doppler flow meter BLF 21, Transonic Inc.). We used an infrared laser (780 nm wavelength, optical power of 2 mW). The laser doppler flowmeter (LDF) had an analog output time constant of 0.1 seconds and a Doppler signal band width of 24 Hz–24 KHz.
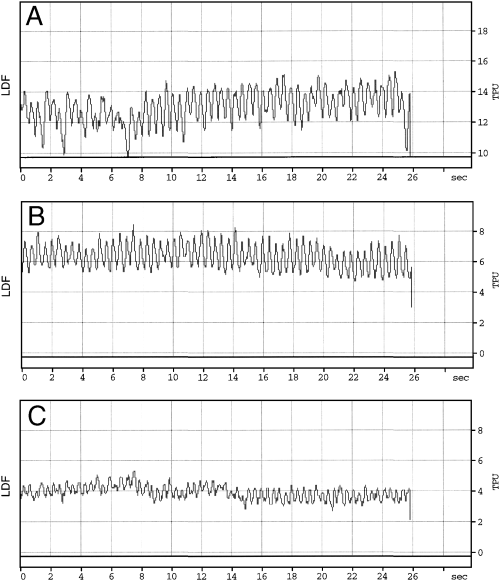
Capillary blood flow tissue perfusion unit (TPU) measured in the body of the stomach of a dog with the laser doppler flowmetry (LDF) at (A) baseline, (B) after induction of portal hypertension (PH), (C) after induction of portal hypertension plus gastric ischemia. At baseline the blood flow was not stable during the first 8 seconds because a peristaltic wave was going underneath the probe.
The flow probe analyzer and pressure transducers were connected to a data acquisition system (Superscope, GWI Instrument, Somerville, MA); data sampling rate, 195 Hz. Data acquisition software was calibrated for 0 and 10 tissue perfusion units (TPUs) with the calibration mode of the LDF. The zero value from the LDF corresponded to a biological flow of zero in the tissue. Data were stored in a computer for further analysis. For each measurement, data were collected for 3 minutes. LDF was determined from the tracing on the data acquisition system at 3 different points after blood flow stabilized. The median of the 3 measurements was then calculated.
Experimental Model Description. A water manometer (Allegiance Health Care Corp., McGaw Park, IL) was connected to the catheter in the portal vein. PH was induced by increasing portal pressure from baseline to 20 cm H2O by gradually closing the portal vein with the preplaced Rummel tourniquet. Gastric body wall ischemia was induced by ligating the short gastric arteries, the left gastroepiploic artery, the distal part of right gastroepiploic artery at the level of the body of the stomach, the left gastric artery, and the distal part of the right gastric artery.
Colored Microsphere Technique. A colored microsphere (Dye track Microspheres, Triton Technology, San Diego, CA) technique was used as a gold standard for measurement of gastric blood flow. Determination of tissue perfusion requires injection of microspheres in the left atrium and concurrent withdrawal of blood at a constant rate from the descending aorta. To prevent agglomeration of microspheres, dogs were pretreated with an intravenous injection of 1 mL of 0.05% Tween 80 (Fischer Scientific, Pittsburgh, PA). Yellow microspheres (1 × 106) were injected in the left atrium to measure baseline blood flow. Blue microspheres (2 × 106) were injected after induction of PH, and red microspheres (3 × 106) were injected to measure blood flow after induction of GI. Microspheres were injected as a bolus followed by a saline solution (0.9% NaCl) flush. Microspheres were 15 μm diameter. Blood collection during each injection was from the descending aorta at 6 mL/min, starting 10 seconds before microsphere injection and finishing 100 seconds after microsphere injection.
After euthanasia, 1 tissue sample each from the gastric body and pyloric antrum were collected where the flow probes were sutured. The serosa and muscularis layers were separated from the stomach wall and each sample weighed. Tissue samples (full-thickness stomach wall; serosa and muscularis; mucosa and submucosa) were digested with a 4 M KOH solution. After digestion of the tissue sample, the solution was filtered with a 10 μm filter (Filter Membranes, Triton Technology) to retain the microspheres. Filters were placed in a microcentrifuge tube and 300 μL dimethylformamide (Fischer Scientific) was added to each filter to extract bound dye from the microspheres. Blood samples were treated similarly by digestion with 8 M KOH. After centrifugation at 3000 ×g for 5 minutes, the supernatant of each microcentrifuge tube was transferred to a microcuvet and then to a spectrophotometer (Spectrophotometer, Beckman 640, Beckman Coulter, Fullerton, CA). Wavelengths of 448 nm (yellow dye), 620 nm (blue dye), and 535 nm (red dye) were used to measure absorption for each sample. A sample of 1 × 106 microspheres of each color was used to establish a reference for the absorption for a 1000 microspheres. Blood flow was calculated in the stomach wall for each situation by comparing number of microspheres in each tissue sample to the blood sample.25,26 Total blood flow for each location was calculated by adding the blood flow in the each sample (serosa and muscularis+submucosa and mucosa).
Data Analysis. Repeated-measures ANOVA (Statview, SAS Institute Inc., Cary, NC) was used to evaluate the effect of PH and GI on blood flow in the pyloric antrum and gastric body measured with LDF and colored microspheres. Percent variation in blood flow from baseline was calculated for each location (pyloric antrum, gastric body) and status (normal, PH, PH+GI). Correlation between variation in blood flow measured in the pyloric antrum and gastric body with microspheres and LDF was evaluated with ANOVA and a simple regression model. A P-value <.05 was considered significant. Data are reported as mean±SD.
Clinical Study—Dogs with GDV
Twenty-four dogs admitted for treatment of GDV were studied. After stomach derotation, stomach wall viability was determined by subjective criteria: serosal color, palpation of stomach wall, and peristalsis to determine if partial gastrectomy should be performed. Dogs were grouped: 1—gastrectomy (G), 2—no gastrectomy (NG). Blood flow in the pyloric antrum was measured by placing the flow probe at mid-distance between the lesser and the greater curvature. In the NG group, capillary blood flow in the body of the stomach was measured by placing the flow probe close along the greater curvature where the stomach typically undergoes necrosis during GDV. In the G group, capillary blood flow was measured with the flow probe in the center of the area to be resected, where the serosa was the darkest. Blood flow was measured in 3 locations for the pyloric antrum and body of the stomach. The LDF was applied to the serosa and maintained with gentle pressure. Four sponges were inserted between the probe and the fingers stabilizing the probe to provide cushioning to avoid placing too much pressure on the probes, which could have occluded the capillaries and artificially reduced capillary blood flow. Data was collected when the value of the blood flow on the digital display of the LDF was constant. Systolic, diastolic, and mean arterial pressures were simultaneously recorded from an 18 g catheter inserted in the dorsal pedal artery, and connected to a pressure transducer (CDXpress™, Maxxim Medical) and zeroed to the level of the right atrium.
Data Analysis. Repeated measures ANOVA was used to evaluate the effect of stomach viability (G, NG) on blood flow in the pyloric antrum and gastric body measured by LDF. A P-value <.05 was considered significant. Data were reported as mean±SD.
RESULTS
Validation Study—Normal Dogs. Aortic blood flow, arterial pressures, left ventricular systolic pressure, and left ventricular end diastolic pressure remained constant during the study (Table 1). Portal blood flow was significantly reduced from 457.2±136.5 to 266.0±83.3 mL/min (P=.001) after induction of PH, but GI had no further significant effect.
| Variables | Baseline | PH | PH+GI | P-Value |
|---|---|---|---|---|
| SAP (mm Hg) | 99.00±17.40 | 90.50±12.50 | 96.70±19.10 | .36 |
| DAP (mm Hg) | 41.20±23.90 | 44.40±25.20 | 43.20±26.60 | .72 |
| MAP (mm Hg) | 60.40±11.60 | 59.70±17.80 | 61.00±19.00 | .93 |
| LVSP (mm Hg) | 98.70±38.90 | 96.90±14.20 | 100.30±20.40 | .96 |
| LVEDP (mm Hg) | 10.90±14.00 | 9.30±15.60 | 10.80±15.00 | .37 |
| AF (L/min) | 2.00±0.60 | 1.90±0.60 | 1.90±0.70 | .57 |
| PF (mL/min) | 457.20±136.50 | 266.00±83.30 | 250.50±111.00 | .001 |
| PP (mm Hg) | 7.50±0.70 | 13.80±0.70 | 14.50±1.40 | .001 |
- SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end diastolic pressure; AF, aortic flow; PF, portal vein flow; PP, portal vein pressure.
Blood flow measurement by microsphere technique was performed reliably in 6 dogs. In 1 dog, blow flow measured by microspheres was 0 mL/min/g at baseline whereas in the other dog blood flow in the gastric body increased 100-fold from baseline after induction of GI most likely because of microsphere aggregation in capillaries. PH and PH+GI induced a significant of reduction in total blood flow to the gastric body wall (P=.0189; Table 2). A similar effect occurred in flow to the serosa–muscularis (P=.039) and the mucosa–submucosa (P=.0183) of the gastric body. Capillary blood flow measured by LDF was significantly reduced after induction of PH+GI (P=.0397; Table 2, Fig 1). Pyloric antral blood flow measured by either technique was not significantly affected by PH and PH+GI (Table 2).
| Location Techniques | Baseline | PH | PH+GI |
|---|---|---|---|
| Body of the stomach | |||
| Colored microspheres | |||
| Full thickness | 0.63±0.50a,b | 0.09±0.11b | 0.23±0.11a |
| Serosa muscularis | 0.16±0.16c | 0.01±0.02c | 0.08±0.06 |
| Mucosa submucosa | 0.47±0.38d,e | 0.07±0.09e | 0.15±0.07d |
| Laser doppler | 25.30±19.32f | 20.10±23.40 | 7.00±6.6f |
| Pyloric antrum | |||
| Colored microspheres | |||
| Full thickness | 0.47±0.71 | 0.18±0.23 | 0.39±0.09 |
| Serosa muscularis | 0.16±0.22 | 0.01±0.02 | 0.09±0.03 |
| Mucosa submucosa | 0.31±0.51 | 0.17±0.21 | 0.31±0.11 |
| Laser doppler | 11.10±6.80 | 9.60±8.50 | 22.60±36.80 |
- Values with the same subscript letter are significantly different at P<.05.
Percent changes in blood flow measured with LDF were not significantly correlated with percent changes measured by microspheres in the pyloric antrum (r=.94, P=.056) and in the gastric body (r=.02, P=.96) during PH. However there was a significant positive correlation for percent change in blood flow measured by LDF and microsphere techniques after induction of PH+GI in the body of the stomach (r=.94, P=.014; Fig 2) and pyloric antrum (r=.95, P=.049; Fig 3).
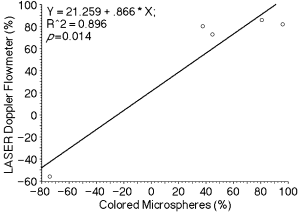
Relationship between percent changes in the blood flow measured with laser doppler flowmetry (%) and colored microspheres (%) in the body of the stomach after induction of portal hypertension plus gastric ischemia.
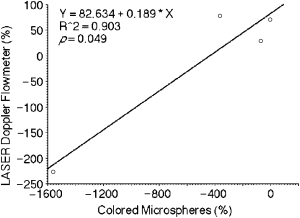
Relationship between percent changes in the blood flow measured with laser doppler flowmetry (%) and colored microspheres (%) in the pyloric antrum after induction of portal hypertension plus gastric ischemia.
Clinical Study—Dogs with GDV. Based on subjective evaluation, 6 dogs required gastrectomy because of necrosis along the greater curvature. None of the NG dogs had gastric rupture. Systolic (P=.26), diastolic (P=.94) and mean arterial (P=.30) pressures were not significantly different between G and NG dogs (Table 3). Capillary blood flow in the pyloric antrum was 31.10±17.10 TPU in the NG group and 24.60± 5.90 TPU in the G group. Capillary blood flow in the gastric body was significantly reduced when gastrectomy (5.00±3.30 TPU; P=0.022; Fig 4) was required compared with the NG group (27.40±14.20 TPU).
| Treatment | Location | SAP | DAP | MAP |
|---|---|---|---|---|
| No gastrectomy | ||||
| Pyloric antrum | 100.80±18.40 | 66.90±12.90 | 77.60±13.80 | |
| Body of the stomach | 101.00±16.20 | 66.10±12.90 | 78.40±13.20 | |
| Gastrectomy | ||||
| Pyloric antrum | 89.70±16.20 | 54.00±12.10 | 64.70±13.10 | |
| Body of the stomach | 85.80±11.70 | 53.00±10.40 | 63.20±10.00 | |
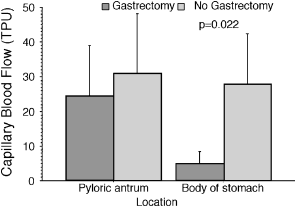
Capillary blood flow measured in the pyloric antrum and the body of the stomach in dogs presented for GDV.
DISCUSSION
Stomach blood flow changes were induced with PH and PH+GI. LDF accurately measured changes in capillary flow in the canine gastric wall when ischemia was present, suggesting that LDF could be used to evaluate stomach wall viability in dogs with GDV. Ultrasonic doppler has been unreliable for recognizing nonviable intestine because ultrasound has a relatively long wavelength.27,28 A monochromatic and coherent light with a short wavelength like a laser beam is required to measure capillary blood flow in tissue with doppler technology.28
Colored microsphere technology has been recognized as the gold standard technique for measurement of capillary blood flow in different organs25; in dogs, flow has been reported as 0.36–0.54 mL/min/g of tissue in the stomach wall during laparotomy.29 Delaney and Grim30 reported that the pyloric antrum received 0.32 mL/min/g whereas the body of the stomach had a flow of 0.61 mL/min/g. Our baseline results were similar. Low blood flow is associated with a low concentration of microspheres, and/or aggregation of microspheres. Therefore, we used a high number of microspheres with more dye bonded to them to improve the quality of our measurements.25,31 Dogs were pretreated with Tween 80 to prevent aggregation of microspheres in areas with low blood flow.25,31,32 Aggregation more likely occurred in the body of the stomach after induction of GI because total flow measured by microspheres increased whereas LDF flow decreased.
PH induced a reduction of blood flow in the stomach wall of rats,33–38 and resulted in portal hypertensive gastropathy in patients with chronic PH.39–42 The blood flow in stomach mucosa is the most severely affected, and regulation of blood flow in the mucosa is under metabolic and vasculogenic controls.41,43 The mucosa–submucosa of the body of the stomach had the most severe reduction in flow in our study.
Correlation between LDF and colored microspheres was only significant when GI was present. Variation in flow after complete arterial occlusion can be diagnosed more reliably than after venous occlusion with LDF.20,44–49 Ischemia is associated with a more abrupt reduction of the Doppler signal because there is less oscillation of red blood cells within the capillaries.20,44–49 Therefore, reduction of blood flow in the stomach wall might only be detected accurately with LDF if ischemia is present.
Variation in total capillary flow within the stomach wall measured with LDF correlated with variation in total flow of the stomach wall measured with colored microspheres. Therefore, LDF has been most likely measuring capillary blood flow of the entire thickness of the wall of the stomach and not just flow in serosa or mucosa. Johansson et al50 showed that the laser beam can penetrate in the stomach wall in dogs to a depth of 6 mm with a 780 nm laser wavelength. This is of major importance for evaluation of stomach perfusion after GDV because most likely the blood flow of the entire wall is affected and not just flow in 1 layer of the wall.9,51 Edema in the stomach wall during GDV might interfere with depth of penetration of the laser doppler beam. It is likely that edema was not present in our model because Lantz et al51 reported that edema was only obvious 4 hours after induction of GDV in dogs. Macroscopically, we could not appreciate any edema in the wall of the stomach at the end of our experiment. We did not perform histology to document presence of edema in the stomach wall.
LDF measures capillary blood flow in a small volume of tissue underneath the probe.44,52 Therefore, it has then been recommended that flow be measured at several different points to increase measurement accuracy.44,52 Because we sutured the probe, we only measured capillary blood flow at one point in the pyloric antrum and the body of the stomach for each step of the experiment. Because blood flow measurement with LDF is influenced by motion of the stomach wall, contact pressure, and contact angle of the probe tip with the serosa,53 the probes were sutured to ensure constant pressure of application, excellent contact with the serosa, and no motion of the probes relative to the stomach wall. In clinical GDV, we placed sponges over the probes to provide cushioning between the probe and the fingers of the operator maintaining the probes on the stomach wall and thus more constant pressure could be applied over the probe without compressing capillaries in the wall of the stomach.
R and P probes were used and have the same physical characteristics, so type of probe did not influence our data. The R probe has a right angle profile, which allows a better application on surfaces that are difficult to reach.
One potential application of LDF is evaluation of stomach wall viability during GDV in dogs. Our model based on Berardi et al,11 only reproduced the hemodynamic alterations that are present in the stomach wall during GDV. Because evaluation of stomach wall viability in clinical GDV is performed after derotation and decompression, we did not insufflate and/or rotate the stomach. In our model, capillary blood flow was reduced by 69% in the body of the stomach after induction of PH+GI which is similar to 80% reported by Lantz et al51 in a model of GDV with a nondistended stomach. GI was induced by ligation of multiple arteries, and, in particular, the left gastroepiploic artery. Blood flow in this artery could have been redirected toward the pyloric antrum and resulted in augmented flow resulting in a total reduction of flow in the pyloric antrum by 17% instead of 69% as occurred in the body of the stomach.
LDF has been used in surgery to determine intestine viability in humans with 100% sensitivity and 92.9% specificity.16,54 Because the magnitude of the LDF signal depends on several factors that differ between tissue, hematocrit, red blood cell velocity, vascular geometry, tissue properties, and point to point variation of blood flow within the tissue,20,44-46 LDF could be accurate in qualitatively assessing differences in blood flow within discrete areas of the same organ or vascular bed. Therefore, for dogs with GDV, criteria to determine viability of the stomach wall will have to be based on comparison of blood flow of another part of the stomach13,15,16; blood flow in the pyloric antrum could be used as a reference. After we determine criteria to establish acceptable capillary blood flow for a viable stomach wall, LDF could be used to delineate surgical landmarks for gastrectomy.
When used for GDV, capillary blood flow measured by LDF was significantly lower in the body of the stomach of dogs that required gastrectomy than in dogs that did not. The decision to perform gastrectomy was based on subjective criteria (color, peristaltic activity, and palpation).8 We did not confirm histologically that the removed stomach sections were non-viable. In the NG group, the stomach was viable because rupture did not occur postoperatively.
We concluded that objective information on stomach wall perfusion can be measured with LDF. Because LDF is more accurate when ischemia is present, it should be a valuable technique during GDV surgery to evaluate stomach wall viability; however, criteria to characterize viability need to be determined before clinical application.




