Evaluation of Arthrocentesis Site Bacterial Flora before and after 4 Methods of Preparation in Horses with and without Evidence of Skin Contamination
Abstract
Objective— To evaluate the effectiveness of four methods of povidone–iodine preparation on skin bacterial flora of arthrocentesis sites, in horses, with and without evidence of skin contamination.
Study Design— Prospective randomized study.
Animals— Twenty-four adult horses.
Methods— Horses were assigned to either the clean or contaminated group based on housing environment and visual evidence of contamination. Using a moist sterile swab, microbial culture samples were obtained from the skin over the distal interphalangeal joints immediately before and after preparation. Each site was aseptically prepared with 1 of 4 povidone–iodine techniques: 10-minutes scrub, 5-minutes scrub, three 30-second scrubs, or commercial one-step iodophor surgical solution. Colony forming units (CFUs) were determined for each sample, 24 hours after inoculation, on blood agar plates.
Results— Mean (±SD) pre-scrub CFUs/mL was significantly higher in the contaminated group (9588.33±1223.65) compared with the clean group (4489.00±3842.03) (P<.01). After preparation of the arthrocentesis sites, there were no significant differences in post-scrub CFUs/mL among the 10 minutes (mean clean, 46.00±64.36; mean contaminated, 28.67±18.04), 5 minutes (mean clean, 84.17±109.80; mean contaminated, 40.33±44.52), three 30 seconds povidone–iodine scrubs (mean clean, 95.50±172.29; mean contaminated, 46.67±56.94), or application of a commercial one-step iodophor surgical solution (mean clean, 102.17±161.78; mean contaminated 117.67±143.78); or between the clean (81.96±131.69) and contaminated groups (58.33±85.90) (P<.01).
Conclusions— Preparation of the distal interphalangeal joint arthrocentesis site with each of these techniques significantly reduces the bacterial flora to a similar level for arthrocentesis in horses with and without evidence of skin contamination.
Clinical Relevance— Aseptic preparation of the skin over the distal interphalangeal joint may be accomplished with any of these techniques.
Introduction
ARTHROCENTESIS AND INTRA-ARTICULAR administration of medications are common procedures performed by equine veterinarians for localization and treatment of lameness. Arthrocentesis can be associated with inoculation of bacteria and the development of septic arthritis, a potentially devastating complication. In one report, intra-articular injection was the cause of 22.4% (43 of 192) cases of septic arthritis or tenosynovitis.1 In 69% of these cases, Staphylococcus aureus was isolated from the joint or tendon sheath. In another report, the most common bacterial isolates for 233 horses with septic arthritis, tenosynovitis, or osteomyelitis that developed after fracture repair were Enterobacteriaceae (28.8%), non-β-hemolytic streptococci (13.0%), and coagulase-positive staphylococci (11.8%).2 Other potential pathogens, such as coliform and fecal Streptococcus spp. are also present in relatively large numbers at arthrocentesis sites in horses with and without evidence of contamination.2 Inoculation with as few as 33 colony forming units (CFUs) of S. aureus can produce sepsis in a normal joint.3
Appropriate skin preparation and asepsis during arthrocentesis is critical to minimize the risk of septic arthritis. The ideal technique would rapidly decrease the number of microorganisms to minimize the risk of inoculation during arthrocentesis. Preparation of arthrocentesis sites can be time and labor intensive. It is desirable to use the most effective and efficient technique when preparing arthrocentesis sites to maximally reduce bacterial flora and organic debris, without creating skin irritation.
Given these general considerations, and because techniques used to prepare arthrocentesis sites are highly variable among practitioners, our purpose was to compare the efficacy of 4 different povidone–iodine preparation techniques for reducing bacterial counts at arthrocentesis sites of horses with and without contamination. Based on previous reports of surgical site preparation techniques, we hypothesized that all 4 arthrocentesis site preparation techniques; 10, 5 minutes, and three 30 seconds povidone–iodine scrubs, and application of a commercial one-step iodophor surgical solution, would produce equal reductions in skin bacterial flora in horses with and without contamination of the arthrocentesis site.
Materials And Methods
Horses
Twenty-four adult horses, free of dermatitis or edema of the distal aspects of the limbs, were selected from our teaching herd. Horses were divided into two groups of 12 horses. Group 1 (clean) consisted of horses with minimal skin contamination, groomed daily for ≥5 days, and housed in a clean stall environment. Group 2 (contaminated) horses were housed in a paddock environment for ≥5 days, during a time of high precipitation. These horses had visual evidence of mud and fecal skin contamination of the distal aspects of their limbs. All horses were sedated with detomidine hydrochloride (0.01 mg/kg, intravenously) and placed in treatment stocks for sampling and preparation.
Limb Preparation
All 4 limbs of each horse were used. An area on dorsal midline of each limb, approximately 1 cm proximal to the coronary band, served as the sampling location. This site was selected to simulate the arthrocentesis site of the distal interphalangeal joint. Each limb on the horse was randomly assigned to 1 of 4 treatment groups using a random number table. All techniques were used on each horse, each arthrocentesis site served as its own control.
Before limb preparation, the body and upper limbs of each horse were wiped with a damp towel to remove dirt and dust in an attempt to minimize the amount of bacteria laden debris that could fall onto the arthrocentesis sites. The distal aspect of the limbs was dry before preparation. Before initial swab sampling, each arthrocentesis site was cleaned with 3 strokes from a currycomb, and 3 strokes with a soft brush to remove large pieces of organic debris. The hair at the arthrocentesis site was left intact. The 4 preparation techniques were performed simultaneously on each horse by 4 individuals, and each technique was performed by the same individual on each horse. Clean latex examination gloves were worn for all preparations.
Sampling Technique
Samples for bacterial culture were taken from the arthrocentesis site on each limb immediately before and after preparation. All cultures were taken by the same individual, wearing clean latex examination gloves. Sampling of the arthrocentesis site was performed as previously described.4 Samples were collected using a sterile swab that was dampened by placing the swab in a sterile blood collection tube (Vacutainer, BD Vacutainer, Preanalytical Solutions, Franklin Lakes, NJ) containing a 1 mL aliquot of sterile saline (0.9% Sodium Chloride Injection USP, Baxter Healthcare Corporation, Deerfield, IL) solution before sampling. Excess saline solution was removed from the swab by gently rolling the swab on the inner surface of the sterile tube as it was withdrawn.
The surface area sampled was standardized by placing the side of the swab beneath the hair, in contact with the skin at the arthrocentesis site and rotating the swab in the dorsal plane one complete revolution from distal to proximal.4 This allowed sampling of both the skin surface and the hair. A ballpoint pen was used to mark the handle of the swab so that one revolution could be determined. After sampling, the swab was returned to the sterile tube containing saline solution, agitated for 30 seconds, and the fluid expressed from the swab on the side of the tube. The swab was discarded, and the fluid was submitted to the laboratory for processing.
Preparation Techniques
Each limb was prepared using 1 of 4 techniques and preparation sites were evaluated at 24 hours for evidence of irritation or reaction. Each prepared area was approximately 2″ square.
Technique 1. A 10 minutes scrub in a circular motion using 4, 3″× 3″ gauze sponges soaked in povidone–iodine (Betadine Scrub and Solution, Purdue Frederick Co., Norwalk, CT) scrub solution (0.75% available iodine). Excess povidone–iodine was removed from the arthrocentesis site by a single 70% isopropyl alcohol wipe with a 3″× 3″ gauze sponge. The arthrocentesis site was blotted dry with a single sterile gauze sponge before sampling, by digitally pressing the sponge on the arthrocentesis site for 10 seconds. Samples were obtained immediately after blotting.
Technique 2. A 5 minutes scrub in a circular motion using 2, 3″× 3″ gauze sponges soaked in povidone–iodine scrub solution. Excess povidone–iodine was removed by a single 70% isopropyl alcohol wipe with a 3″× 3″ gauze sponge. The arthrocentesis site was blotted dry with a single sterile gauze sponge before sampling, by digitally pressing the sponge on the arthrocentesis site for 10 seconds. Samples were obtained immediately after blotting.
Technique 3. Three 30 seconds scrubs in a circular motion using 2, 3″× 3″ gauze sponges soaked in povidone–iodine scrub solution for each scrub. After each scrub, excess povidone–iodine was removed using a single 70% isopropyl alcohol wipe with a 3″× 3″ gauze sponge. The arthrocentesis site was blotted dry with a single sterile gauze sponge before sampling, by digitally pressing the sponge on the arthrocentesis site for 10 seconds. Samples were obtained immediately after blotting.
Technique 4. Blotting the arthrocentesis site with 6 mL of a commercial 1-step iodophor surgical solution (DuraPrep Surgical Solution, 3M Health Care, St Paul, MN), using a single use custom applicator. The solution was allowed to dry for 2 minutes and immediately sampled.
Environmental Samples
Environmental samples were collected by removing the lid of a new 100 mm diameter blood agar plate for 10 minutes each hour during the study. The plate was placed approximately 3 ft away from the horse at the level of the arthrocentesis sites, to evaluate potential fallout contamination from both the horse and the environment.
Sample Storage and Preparation
Samples were stored in the sterile collection tube overnight in a 4°C refrigerator and processed the next day. The samples were vortexed for 5 seconds to disperse the bacteria throughout the saline solution. CFUs/mL of supernatant were determined by plating decreasing volumes of supernatant on blood agar plates. The plates were inoculated with 0.25, 0.1, 0.05 mL of supernatant, respectively. Plates were streaked in 3 overlapping accordion patterns. CFUs were manually counted after 24 hours aerobic incubation at 37°C. The maximum growth recorded for any sample was limited at 10,000 CFUs/mL by the highest dilution. The predominant bacteria cultured from the pre- and post-scrub samples were identified using standard techniques.5
Statistical Analysis
A 3-way ANOVA (clean versus contaminated, pre- versus post-preparation, and the 4 preparation techniques), with repeated measures on pre versus post, and over the 4 techniques, was used to assess the significance of differences in the number of CFUs/mL present before (pre-scrub) and after (post-scrub) skin preparation using each of the 4 techniques in both clean and contaminated groups. P-values were adjusted for the Huynh–Feldt correction when applicable. Post hoc tests, when appropriate, were done using Bonferroni corrected t-tests. Significance for all tests was set at P<.05. All analyses were performed with the Number Cruncher Statistical System (NCSS Statistical System for Windows, Kaysville, UT, 2001).
Results
There was no significant difference between limbs, or group of limbs selected for each scrub technique, with respect to pre-scrub CFUs/mL. The mean number of pre-scrub CFUs/mL was significantly higher in the contaminated group (9588.33±1222.65) compared with the clean group (4489.00±3842.03; P<.01). After preparation of the arthrocentesis sites, there were no significant differences in post-scrub CFUs/mL among the 10 minutes (mean clean, 46.00±64.36; mean contaminated, 28.67±18.04), 5 minutes (mean clean, 84.17±109.80; mean contaminated, 40.33±44.52), three 30 seconds povidone–iodine scrubs (mean clean, 95.50±172.29; mean contaminated, 46.67±56.94), or application of a commercial one-step iodophor surgical solution (mean clean, 102.17±161.78; mean contaminated 117.67±143.78), or between the clean (81.96±131.69) and grossly contaminated groups (58.33±85.90; P<.01; Fig 1). All 4 techniques were equally effective, regardless of the presence of pre-scrub contamination, in significantly reducing the total number of CFUs/mL between the pre- and post-scrub samples (P<.01). There was substantial environmental fall out (227.80±139.70 CFUs) when sampled for 10 minutes each hour.
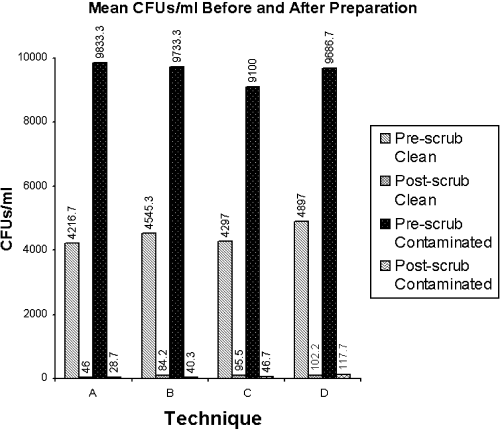
Mean number of CFUs/mL sampled from horses in the clean and grossly contaminated groups before and after preparation of the arthrocentesis site with povidone–iodine preparations consisting of a 10 minutes scrub (A), a 5 minutes scrub (B), three 30 seconds scrubs (C), or a commercial 1-step iodophor and isopropyl alcohol surgical solution (D).
The organisms isolated before preparation were predominantly environmental organisms (Bacillus spp., Acinetobacter spp.) and normal flora of the skin and mucous membranes (Moraxella spp., Streptococcus spp., Corynebacterium spp., coagulase-negative Staphylococcus spp., and Micrococcus spp.). After preparation, the only organisms cultured were environmental organisms (Bacillus spp.).6,7 Bacteria on the environmental control plates consisted of predominantly Bacillus spp. and Streptomyces spp. None of the horses had an adverse reaction to any preparation technique when evaluated 24 hours after preparation.
Discussion
The preparation techniques were chosen arbitrarily for evaluation. Veterinarians commonly use 3 of these techniques and the 4th (DuraPrep Surgical Solution, St. Paul, MN) is one used before arthrocentesis or intrathecal injection in human medicine. We chose to study the efficacy of these techniques for preparation of the arthrocentesis site for the distal interphalangeal joint because it is in close proximity to environmental contamination and thus likely subject to a higher bacterial load than other arthrocentesis sites.
The purpose of any arthrocentesis site preparation technique is to rapidly decrease the organic debris and local bacterial flora without damaging the skin. Bacterial populations at an arthrocentesis site can be categorized as resident and transient microflora.8 Resident bacterial populations inhabit the sebaceous glands and hair follicles and can be reduced by antimicrobial solutions but cannot be eliminated.7–9 The transient flora are those that are on the skin surface and are readily accessible to antiseptics, consisting mainly of environmental bacteria, and those bacteria that are resident flora of the oral cavity.7–9 In some locations, transient microflora can be easily reduced by simple washing and clipping of the hair.4,8 Resident and transient bacteria associated with the arthrocentesis site must be reduced or eliminated before arthrocentesis. Results of our study show that this population may vary considerably depending on the horse's environment. Aseptic preparation methods do not sterilize skin but substantially reduce CFUs, thus minimizing the risk of infection. An ideal preparation technique would eliminate the transient population of bacteria and organic debris, independent of the presence of contamination at the arthrocentesis site, and reduce the resident flora.
Rapid destruction of 99% of the accessible bacteria reportedly occurs within the first 30 seconds of application of povidone–iodine.10,11 This suggests that number of CFUs present at an arthrocentesis site should not be significantly affected by preparation time, and contact with povidone–iodine for periods of >30 seconds would not result in a temporal decrease in bacterial numbers. Others have suggested that iodophors require ≥2 minutes of contact time to allow sufficient release of free iodine to have a bactericidal effect.12 Five and 10 minutes povidone–iodine scrubs have been proven equally effective for preparation of the skin of operating personnel.13
The 1-step preparation technique of an iodophor surgical solution (DuraPrep Surgical Solution) requires only a fraction of the time (2 minutes to dry versus 10 minutes of scrubbing) normally required for preparation of an arthrocentesis site. The antimicrobial properties result from the combination of an iodophor–polymer complex and 70% isopropyl alcohol, which when applied to skin forms a water-insoluble film that serves as a chemical and physical barrier (Dura-Prep safety and efficacy studies, 3M health care). DuraPrep also maintains antibacterial activity at the preparation site >12 hours. For surgical preparation, DuraPrep was equal to, or better than a conventional 2-step skin preparation technique in reducing bacterial numbers for canine ovariohysterectomy.14 In humans, within 2 minutes of application, there was a 93% reduction in skin bacterial counts.15 When compared with a more traditional preparation technique (3 scrubs of povidone–iodine each followed by an isopropyl alcohol wipe), Duraprep had equivalent reduction of 99% of the CFUs for preparation of ventral celiotomy sites in horses.9
Total skin preparation time was >2 minutes for each technique studied. Like previous studies in humans and small animals, we found no significant difference in number of bacterial CFUs when povidone–iodine skin preparation was >2 minutes. This suggests that bacterial populations on equine skin, with or without contamination, are similarly susceptible to povidone–iodine antiseptics as those on human and small animal skin. Longer preparation time may physically mobilize more resident flora, making them accessible for destruction by povidone–iodine and may be beneficial in reducing the total bacterial flora at an arthrocentesis site.
A single wipe of the area was performed with an alcohol-soaked gauze sponge after 3 of the preparation techniques in this study, as previously reported.4 This removed most of the scrub from the preparation site that could inhibit bacterial growth on culture swabs. This was confirmed by bacterial proliferation on culture plates after inoculation with saline solution used to rinse the swabs. This method was chosen to standardize the exposure of each arthrocentesis site to alcohol. Multiple alcohol soaked gauze sponges are typically used in a clinical situation remove excess scrub from the arthrocentesis site, which should provide additional antiseptic activity, and removal of debris.
Clipping does not significantly alter the ability of a preparation technique to decrease bacterial flora on the skin surface,4 so we did not clip the arthrocentesis sites before disinfection. Further, arthrocentesis sites that have been clipped or shaved have higher bacterial counts before preparation than non-clipped sites.4 It is likely that clipping or shaving dislodges bacteria from hair follicles making more available for sampling. Intact hair also provided additional surface area for adherence of contaminants in this study.
Bacterial flora isolated from the skin of the clean and contaminated groups before preparation included large numbers of environmental organisms (Bacillus spp., Acinetobacter spp.)16 and normal flora of the skin and mucous membranes (Streptococcus spp., Corynebacterium spp., coagulase-negative Staphylococcus spp., Escherichia coli, Actinomyces spp., Micrococcus spp.).16,17 Preparation of the arthrocentesis sites removed most detectable normal flora, and most environmental organisms, usually leaving only low numbers of Bacillus spp. The bacterial species and numbers present after preparation were similar to those isolated on plates used to ascertain environmental contamination. Plates used to monitor environmental contamination were placed 3 ft from the horse to monitor air fallout that could contaminate the arthrocentesis site, throughout and after site preparation, from the horse as well as the environment. Results suggest that there may be rapid environmental contamination of the arthrocentesis site after preparation, or a lack of effectiveness for antiseptics to eliminate certain bacteria from the arthrocentesis site.
It has been reported that room air fallout bacterial flora should not exceed 20 CFUs/h in a well ventilated surgical suite.18 Number of CFUs per environmental culture plate collected over a 10 minute period were higher than this throughout the entire sampling period. The area where the preparation and sampling was performed was a semi-isolated area of the hospital; however, the movement of horses throughout the hospital can affect airflow through the area. Based on the level of fallout contamination, arthrocentesis should be performed in a closed environment with minimal contamination and away from the main hospital traffic flow, when possible.
The total numbers of bacteria that were cultured from each arthrocentesis site in this study was greater than the number shown experimentally to induce septic arthritis when combined with intra-articular medications.3 In our clinic, a 10 minutes povidone–iodine scrub has been effective in preventing iatrogenic septic arthritis. This difference is likely because of a larger area sampled with the swab in this study, and by the bacteria being administered in solution intra-articularly in the previous study. The actual amount of bacteria that are physically relocated into a joint from the skin surface during arthrocentesis in a horse has not been evaluated. The lack of difference found in this study between the 4 preparation techniques suggests that using the preparation techniques with less than 10 minutes of contact time does not increase the risk of iatrogenic septic arthritis.
Although there was not a significant difference in number of CFUs at the arthrocentesis sites after preparation with these 4 techniques in either clean or contaminated groups, the amount of organic debris that remained at the arthrocentesis site was not evaluated. Organic debris may be introduced into the joint during arthrocentesis, as may a small plug of skin.19 During arthrocentesis, the needle must pass through the hair, skin, and glands, and could inoculate the joint with any of the transient or resident bacterial population as well as organic material that was not removed. Organic material was visible on the swab after sampling the arthrocentesis sites prepared with DuraPrep solution and was most prevalent on contaminated sites. This organic material may be aseptic, however, any foreign material inoculated into the joint, may cause a local inflammatory reaction, as well as providing the opportunity for a subinfective dose of bacteria to cause septic arthritis.
The cost of preparation techniques was not evaluated. However, several factors should be considered when evaluating the economy of preparation protocols. Scrubbing time can be important in a busy practice, and increased time may result in increased labor cost as well as irritation to the skin surface. The technique selected for arthrocentesis site preparation should efficiently reduce bacterial numbers and organic debris, have some residual activity, be economical, have a low incidence of skin irritation; but most importantly, have a very low incidence of post-arthrocentesis infection.
Based on our results, there does not appear to be a difference in CFUs obtained from clean or contaminated equine arthrocentesis sites after povidone–iodine preparation using 10, 5 minutes, three 30 seconds povidone–iodine scrubs, or a commercial 1-step iodophor surgical solution. The techniques are equally effective for reducing the bacterial flora of the distal interphalangeal joint arthrocentesis site in clean and contaminated horses. Our study does not; however, address the organic debris, remaining at the arthrocentesis site that could be introduced into the joint. DuraPrep solution is an appealing alternative to conventional arthrocentesis site preparation techniques; however, this technique cannot be recommended until further evaluation of the importance of the residual organic debris has been evaluated, and the technique has proven safe and effective when arthrocentesis is performed experimentally.




