Evaluation of Primary Critical Ischemia Time for the Deep Circumflex Iliac Cutaneous Flap in Cats
Supported by Companion Animal Fund, Michigan State University. Presented at the 38th Annual Scientific Meeting of the American College of Veterinary Surgeons, October 9–12, 2003, Washington, DC.
Abstract
Objective— To evaluate the primary critical ischemia time for the deep circumflex iliac (DCI) cutaneous flap in cats.
Study Design— In vivo model.
Animals— Thirteen young adult female cats.
Methods— An island skin flap was created on the right side of each cat based on the angiosome of the ventral branches of the DCI vessels. The cats were randomly assigned to a flap ischemia time ranging from 1 to 3 hours in 10-minute intervals. Microvascular clamps were used to occlude the artery and vein for the designated time. Flaps were sutured into position after the ischemic period. On day 3, fluorescein dye was administered and the flaps were evaluated under ultraviolet light to assess percent area of perfusion. On days 7 and 14, the percent area of survival was determined for each flap based on cutaneous morphometry.
Results— All flaps had 100 percent area of survival throughout the study. On day 3, all flaps fluoresced uniformly compared with the surrounding skin. On days 7 and 14, all flaps were uniformly viable as confirmed by skin color, consistency, bleeding, and hair re-growth.
Conclusion— The DCI cutaneous flap in cats can withstand up to 3 hours of ischemia with predictable survival.
Clinical Relevance— In a clinical setting, high success rates can be expected with microvascular transfer of the DCI cutaneous flap in cats when the ischemia time is <3 hours and precise surgical technique is used.
Introduction
MICROVASCULAR FREE tissue transfer (MFTT) is a commonly performed wound reconstruction technique in humans and has been associated with a high success rate (92–96.4%).1,2 MFTT results in early and definitive wound reconstruction, restoration of function, potential salvage of impending amputation situations, and improved esthetics. It is also associated with decreased hospitalization, patient morbidity, and expense.1,2 Indications for use of MFTT for reconstruction include large defects created by trauma, thermal injury, ablative oncologic surgery and radiation therapy and congenital defects unable to be closed by conventional reconstructive techniques. MFTT allows for an early, 1-stage reconstruction of these defects with minimal patient morbidity.
MFTT of cutaneous, muscular, osseous, omental, myoperitoneal, and myocutaneous flaps for the reconstruction of wounds have been described in veterinary patients.3–18 The success of MFTT in dogs compares favorably with that reported for human reconstructive surgery.6–8 MFTT has only recently been investigated for clinical use in cats.3 Potential situations in cats where MFTT could be used include reconstruction after ablative oncologic surgery (vaccine associated sarcoma) and the repair of chronic non-healing wounds of the axillary and inguinal regions.3,19–22
The most devastating complication of MFTT is ischemia and necrosis of the transferred tissue. Transferred skin flaps are entirely dependent on the vascular pedicle for perfusion for the first 3–14 days until the in-growth of vessels from the wound bed is complete.23–25 Maintenance of a patent vascular pedicle is dependent upon careful tissue handling and accurate anastomosis of the vessels with prevention of kinking, torsion, or tension on the pedicle. Another important factor for successful MFTT is the critical ischemia time of the tissue being transferred. Critical ischemia time is described as the maximum length of time that tissue can tolerate ischemia and yet remain viable once perfusion is restored. Beyond this critical time threshold, the tissue will uniformly undergo necrosis despite restoration of perfusion. This is also described as the no-reflow phenomenon.26
The no-reflow phenomenon is caused by a complex network of interacting factors occurring secondary to ischemia and reperfusion culminating in narrowed vessels and sluggish blood flow with ultimate occlusion of the microvasculature. These factors include vascular sludging, perivascular edema, and increased endothelial permeability with cellular disruption and swelling. Furthermore, adenosine triphosphate consumption, decreased deformability of erythrocytes, aggregation and activation of platelets, chemotraction and accumulation of neutrophils contribute to progressive vascular occlusion and ischemia. In addition, free radical production results in perpetuation of cellular damage and no reflow.27,28
A free flap has a primary and secondary critical ischemia time that may contribute to the no-reflow phenomenon. Primary critical ischemia time is defined as the intraoperative ischemia time that occurs between the transection of the donor vascular pedicle to anastomosis with the recipient vascular pedicle. Secondary critical ischemia time is defined as the ischemia time secondary to postoperative obstruction/occlusion of the artery, vein, or both until perfusion is reestablished. Primary critical ischemia times for the pig have been described with 50% skin flap survival occurring after 9–13 hours of ischemia, 90% survival occurring after 7–7.5 hours of ischemia, and 99% survival occurring after 3 hours of ischemia.29–32 In rats, primary critical ischemia times have been reported with 14–53% skin flap survival occurring after 10–11 hours of ischemia and 100% survival after 8 hours of ischemia. Therapeutic alterations, in several studies using rats, have extended skin flap survival to 40–100% after 10–12 hours of ischemia.33–37 One study in rats demonstrated 50% skin flap survival occurring after 23.4 hours of ischemia.27 For rabbits, a primary critical ischemia time has been reported with 80% skin flap survival after 8 hours of venous occlusion and 100% flap failure after 10 hours of venous occlusion.38
Often in small animal MFTT, intraoperative ischemia times are shorter than the critical ischemia times resulting in high flap survival rates. In 2 case series of dogs, the primary ischemia time for microvascular free skin flap transfer was between 35 and 210 minutes with a reported flap survival rate of 93–100%.6,7 In another case series, the primary ischemia time for a microvascular-free musculocutaneous flap transfer in the dog was 75–130 minutes with a reported 100% flap survival rate.8 In an experimental study in cats, primary ischemia times for microvascular free heterotopic transfer of a muscle flap of 65–130 minutes resulted in a 75% flap survival rate and primary ischemia times of 40–100 minutes for orthotopic transfer resulted in 100% flap survival.4 Preliminary data from a recent study reported a possible association between flap failure in cats and ischemia times of >2 hours.3 This suggests that skin flaps in cats potentially have a significantly shorter primary critical ischemia time than that found clinically in the dog or reported experimentally in the rat, pig, and rabbit. By determining the primary critical ischemia time, future microvascular free transfer of skin flaps can be performed in cats with a predicted risk of flap failure because of the no-reflow phenomenon.
Therefore, the overall goal of our study was to evaluate the primary critical ischemia time for the deep circumflex iliac (DCI) cutaneous flap in cats. Our hypothesis was that the primary critical ischemia time for the DCI cutaneous flap in cats was <3 hours.
Materials and Methods
Animals
Thirteen young adult, female, specific pathogen free, domestic, shorthaired cats were studied. All cats were healthy based on history, physical examination, and the results of packed cell volume and total plasma protein concentration. Each cat was feline leukemia virus and feline immunodeficiency virus negative. All cats were admitted to an animal housing unit at least 1 week before surgery to become acclimated.
Anesthesia
Each cat was administered morphine (0.6 mg/kg intramuscularly [IM]) and acepromazine (0.1 mg/kg IM) 20 minutes before induction. Anesthesia was induced with ketamine (5.5 mg/kg intravenously [IV]) and midazolam (0.25 mg/kg IV). Placing 1–2 drops of 2% lidocaine on the arytenoid mucosa before intubation facilitated orotracheal intubation. Anesthesia was maintained by administering isoflurane in oxygen with a semi-closed anesthesia system. Physiologic variables (heart rate, respiratory rate, oxygen saturation, and indirect blood pressure) were monitored during anesthesia. After completion of anesthesia, cats were observed in recovery until they were able to maintain sternal recumbency on their own. Morphine (0.6 mg/kg IM) and acepromazine (0.1 mg/kg IM) were administered every 6–8 hours as needed for postoperative analgesia. All cats in this study were anesthetized for the same length of time (measured from induction to the time the anesthetic vaporizer was turned off).
Surgery
Cefazolin sodium (22 mg/kg IV) was administered at induction and every 2 hours during surgery. The right side of each cat extending from the caudal thorax to the caudal pelvis and from the dorsal to the ventral midline extending down the hind limb to the level of the hock was aseptically prepared and draped for surgery using a hanging limb technique. One surgeon (B.T.) performed all surgical procedures. To identify the angiosome of the DCI vessels, the iliac crest and the axis of the femur were drawn on the skin using a sterile felt-tipped surgical marker (Devon 150 Skin Marker and Ruler, Kendall/The Ludlow Co. LP, Chicopee, MA). The outline of the flap was drawn on the lateral thigh using the greater trochanter as the proximal landmark and 2 cm proximal to the patella as the distal landmark. The shaft of the femur was used to orient the cranial and caudal dimensions. The cranial margin was drawn 4 cm cranial and parallel to the femoral shaft and the caudal margin was drawn 1 cm caudal and parallel to the femoral shaft consistently creating a flap 5 cm in width and 7 cm in length within the described angiosome (Fig 1).3 The outline of the flap was incised, and the plane of dissection was developed between the underlying muscle and the subcutaneous tissue. The flap was elevated in a distal to proximal direction allowing for direct observation of the vessels within the skin and subcutaneous tissues using transillumination. The DCI artery and vein were identified and isolated as they exited the flank fold fat pad creating an island skin flap. Blood vessels encountered as the flap was elevated were sealed using point cautery.
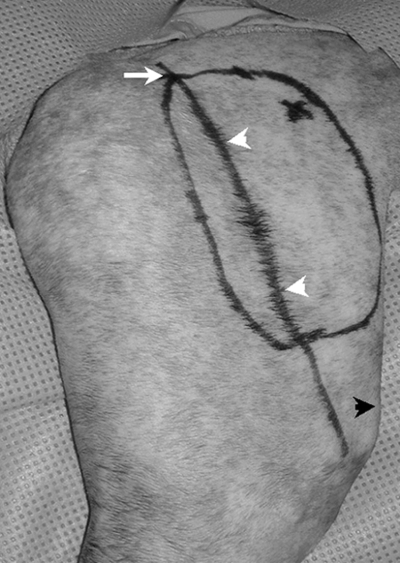
Photograph of the lateral thigh with the cranial direction orientated to the right side of the picture. This photograph demonstrates the margins of the island skin flap to be created relative to the labeled reference points—proximal aspect of the greater trochanter (white arrow), long axis of the femur (white arrow heads), and proximal aspect of patella (black arrow head). A skin flap 5 cm wide by 7 cm in length was consistently developed.
Ischemia of the skin flap was produced by occluding sequentially the artery and then the vein with microvascular clamps (Micro Aneurysm Clips, Miltex Instrument Company Inc., Lake Success, NY) for the allotted time (Fig 2). During ischemia, the caudal 1/3 of the flap was sutured back into place using 4-0 polydioxanone in a simple interrupted intradermal pattern allowing the microvascular clamps and vascular pedicle to remain visible. The 13 cats were randomly assigned an ischemia time ranging from 1 to 3 hours in 10-minute intervals with a random order of surgery created using a random number generator.
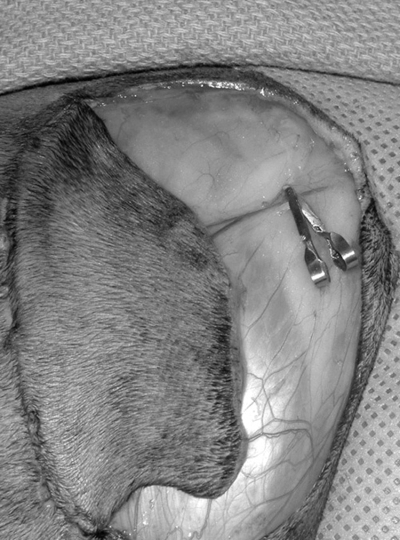
An enlarged photograph of the lateral thigh with the cranial direction orientated to the right side of the picture. This picture demonstrates an island skin flap (partially sutured back into place) based on the ventral branches of the deep circumflex iliac vessels. Microvascular clamps were used to occlude the artery and vein individually for the designated ischemia time.
Flap ischemia after clamp application and reperfusion after clamp removal was confirmed using an empty/refill test as well as gross observation of flap vascular cyanosis during the ischemic period and rapid resolution of the cyanosis after reperfusion. The empty/refill test consists of blanching the artery gently with a microvascular forceps moving distally from the point of the obstruction and confirming no filling proximally in the case of ischemia or confirming filling proximally in the case of reperfusion after release of the proximal obstruction.39 A similar manipulation is performed to evaluate the vein. After the ischemic period, the remainder of the flap was sutured to the wound bed using 4-0 polydioxanone in a simple interrupted intradermal pattern.
Postoperative Care
After completion of the surgical procedure, a soft padded bandage was applied to protect the surgical sites and to minimize edema and seroma formation. After recovery from anesthesia, the cats were returned to their cages. Examination of the cats' general health, examination of the skin flaps, monitoring of incisional healing and complications, and bandage changes were performed once daily until study end. The cats were administered amoxicillin-clavulonic acid (62.5 mg per cat) orally twice daily for 14 days.
Postoperative Flap Evaluation
Flap viability and healing were assessed daily. Flaps were also evaluated for wound complications like edema, seroma formation, incisional dehiscence, flap necrosis, or infection. On day 3, cats were sedated using morphine (0.6 mg/kg IM) and acepromazine (0.1 mg/kg IM) and fluorescein dye was used to evaluate the perfusion of the skin flap.40,41 Fluorescein (10 mg/kg IV) was administered and the flap was illuminated with an ultraviolet lamp 10 minutes after the injection. The presence of bright fluorescence of the skin flap, similar to the surrounding skin, was used as an indicator of perfusion. The percent area perfused was calculated by dividing the measured area that was fluorescent by the total flap area. On day 7, flaps were grossly examined to assess flap viability based on skin morphology. Cats were sedated with morphine (0.6 mg/kg IM) and acepromazine (0.1 mg/kg IM), and the skin flaps were sharply incised (2 mm) with an 11 scalpel blade to document bleeding and to associate gross skin appearance with actual tissue perfusion/bleeding. On day 14, the flaps were grossly examined to assess flap viability based on skin morphology and hair regrowth. At each time point, flaps were photo documented for morphometric analysis and calculation of percent area perfusion and survival. For days 7 and 14, the percent area of survival was calculated dividing the measured area that was viable based on skin morphology by the total flap area.
Disposition of the Cats
Each cat's social behavior and appropriateness for adoption was evaluated at study end. Six cats were adopted.
Statistical Analysis
Percent area perfusion/survival (dependent variable) was correlated to the related ischemia time (independent variable) using correlation/regression analysis for estimation of the primary critical ischemia time.
Results
All cats, mean weight 2.84 kg (range, 2.5–3.18 kg), were 8 months at surgery. Each cat was anesthetized for 4 hours for the procedure. Mean immediate postoperative body temperatures were 96°F (93.8–98.5°F). At no time did any cat require the administration of postoperative analgesic medications based on clinical signs of discomfort (vocalization, elevated heart rate, increased respiratory rate, and overt signs of discomfort).
All flaps had 100% area survival. On day 3, all flaps had uniform fluorescence throughout 100% of the flap area as compared with surrounding unaffected skin. On day 7, all flaps had uniform viability of 100% of the flap area as confirmed by skin color and consistency and verified by bleeding from a skin prick. On day 14, all flaps had uniform viability of 100% of the flap area as confirmed by skin color, consistency, and hair regrowth. Based on these results, there was no relationship (R2=0.00) between ischemia time and percent area survival of the flap over the time period of 1–3 hours. In all cats, evaluations at days 3 and 7 were consistent with findings at day 14. Minimal flap or incisional complications were encountered throughout the study. Mild serosanguinous incisional drainage as well as mild flap swelling or edema was noted for the first 1–4 days in most cats. No complications were encountered with the use of padded bandages.
Discussion
When performing MFTT, flap ischemia and necrosis can occur secondary to either vascular pedicle occlusion or the no-reflow phenomenon. Thrombosis or occlusion of the vascular pedicle can be minimized with precise surgical techniques and careful tissue handling. Prevention of the no-reflow phenomenon depends upon completion of the microvascular anastomosis with reestablishment of perfusion before a known critical ischemia time.
In a controlled clinical setting with an experienced surgical team, the microvascular anastomoses associated with MFTT should be completed in <2 hours. Heterotopic microvascular transfer of the DCI cutaneous flap, latissimus dorsi muscular flap, and gracilis myocutaneous flap have been successfully demonstrated in the cat.3–5 Based on our results, predictable high success rates for cutaneous flaps can be expected if ischemia times are minimized to <3 hours, and careful and precise surgical technique is practiced.
Ischemia times of 1–3 hours were selected because of preliminary data, suggesting flap failure occurred at ischemia times >2 hours.3 Anesthesia times for each cat were kept constant to 4 hours to minimize variability in outcome related to anesthesia. Mean recovery body temperature was 96°F (94.0–98.5°F). It is well known that hypothermia has protective/preventative effects against ischemia and reperfusion injury in many tissues including muscle and skin.42,43 These effects may have contributed to the success of the skin flaps in this study. The authors feel that the anesthesia length, surgical procedure, and postoperative recovery temperature are representative of actual clinical cases and should not affect the clinical application of this information. The dimensions (5 cm × 7 cm) of the skin flaps created in this study were approximately 1 cm within the borders of the described angiosome of the DCI skin flap. This precaution was taken to prevent any partial or marginal flap necrosis related to extension of the flap beyond the margins of the angiosome of the DCI. Antibiotics were administered through the postoperative period to prevent overt clinical infection of expected devitalized and necrotic skin flaps. Soft-padded bandages were placed postoperatively and changed daily to help minimize flap complications including self-mutilation, incisional dehiscence, seroma formation, and flap edema. Postoperative complications of the skin flaps were minimal.
We expected a primary critical ischemia time of <3 hours. Therefore, a group size of 13 cats, with time points uniformly spaced between 1 and 3 hours at 10-minute intervals, was selected to allow detection of the hypothesized critical ischemia time (<3 hours). Based on previously reported values in other species35,37,44 and expected percent area of survival, this model allowed for prediction of percent area of survival based on ischemia time with calculation of the 95% confidence limits. Because of 100% area survival of each flap in all cats up to 3 hours, there was no relationship between ischemia time and percent area survival of the flap over 1–3 hours in the cat.
The DCI cutaneous flap in cats can withstand up to 3 hours of ischemia with predictable 100% survival. In a controlled clinical setting with an experienced surgical team, the microvascular anastomoses associated with MFTT should be accomplished in ≤2 hours. Therefore, high success rates can be expected with microvascular transfer of the DCI cutaneous flap in cats when the ischemia time is <3 hours and precise surgical technique is used.
Acknowledgment
The authors thank Sandy Wilkins L.V.T. and Marlee Richter L.V.T. for their technical support.




