An Animal Model for Interface Tissue Formation in Cemented Hip Replacements
Funded by a grant from CNPq Brazil and Stiftung ESM, Switzerland.
Abstract
Objective— To create a model in sheep for investigation of early changes related to the formation of an interface membrane in hip prosthesis.
Study Design— Experimental study.
Animals— Twenty-four female adult Swiss Alpine sheep.
Methods— Sheep were divided into 2 groups of 12 for unilateral cemented total hip arthroplasty. In Group I, the prosthesis was fixed with retrograde cement gun injection to achieve a complete cement mantle, whereas in Group II a primary cement mantle defect was produced. Groups I and II were further divided into 2 sub-groups with study end points of 2 and 8.5 months after surgery. Radiographs were evaluated postoperatively and at euthanasia for migration of the femoral component and bone resorption. Histologic sections were evaluated semiquantitatively for changes in cell types and numbers, and bone reactions; and quantitatively for size of interface membrane and new bone formation.
Results— Radiographically, there tended to be an increase in bone resorption and periosteal bone formation throughout the femoral shaft in Group II compared with Group I, but this was only statistically significant at the region of the femoral neck (R5) at both time periods (P<.05). Semiquantitative histologic evaluation revealed significant increases (P<.05) in cellularity, numbers of fibroblasts, giant cells, macrophages, and mononuclear cells, in Group II primarily at 2 months after surgery. This was also true for interface membrane formation and bone remodeling. Quantitative data showed an increased in the size of the interface membrane and area of bone formation at 8.5 months in Group II.
Conclusions— The cement defect model offered controlled and repeatable production of an interface membrane. The results suggest that a primary cement mantle defect could be a possible trigger for implant instability, eliciting a cascade of biomechanical and molecular events in bone tissue leading to aseptic loosening.
Clinical Relevance— The results show the effect of defects in the cement mantle in promoting interface membrane formation. Long-term and biochemical studies are required to evaluate the relevance of this interface membrane formation.
Introduction
ASEPTIC LOOSENING is the major cause of failure with cemented total hip replacement in humans1 and has also been reported in dogs.2 It is characterized by the formation of an interface membrane between the bone and the cement, resulting in implant loosening without infection.1 Both micromotion and wear particles have been implicated in eliciting host-specific inflammation responsible for the pathologic osteolysis typical of aseptically loosened prostheses.3–8 Early micromotion may be caused by the normal adjustment of the implant within the bone or by technical failures in surgical technique.9,10 The cementing technique plays an important role in the stability of cemented hip systems.10–14 Some investigators suggest that instability induced by implant migration increases fluid pressure locally at the bone–implant interface, which in turn increases local bone resorption around prosthesis implants.15 Alternatively, localized stress reduction, identified by measurements of bone mineral density with dual-energy X-ray absorptionmetry (DEXA) in human patients treated with cemented total hip replacements, may cause bone remodeling.16
Wear particles originate from the normal wear of the polyethylene components of the prosthesis, the femoral stem, or the bone cement.1 Recent studies suggest that wear particles are primarily responsible for aseptic loosening.7,9,17–21 However, most of the information available is based on retrieved failed materials from clinical patients, making it difficult to interpret the pathophysiology of early clinical aseptic loosening of cemented hip prostheses.
A consistent animal model of aseptic loosening would enable investigators to study the formation of interface membranes under controlled conditions. Although various animal models of aseptic loosening have been created either by addition of wear particles7,22–26 or initial placement of a loose implant27 they fail to adequately simulate the clinical situation or allow study of early changes encountered in an initially well-fixed total hip prosthesis. A standardized animal model of early loosening resembling the clinical situation would be appropriate for investigating factors initiating aseptic loosening, observing early events in the course of the disease, and potentially solving the clinical problem.7,22–29
Our goal was to create a consistent animal model for investigating early changes in the formation of an interface membrane and the resulting aseptic loosening in cemented total hip replacements. Our hypothesis was that micromotion caused by an incomplete cement mantle could be the initial trigger for interface membrane formation.
Materials and Methods
Experimental Animals
Twenty-four female Swiss alpine adult sheep, weighing from 45.2 to 90.1 kg (mean, 60.8 kg), were divided into 2 groups of 12. All sheep had a unilateral cemented modular total hip prosthesis with right or left hind limbs randomly assigned for treatment. The prosthesis was fixed with retrograde cement gun injection to achieve a complete cement mantle in Group I whereas a standardized primary cement mantle defect was produced in Group II. Each group was further divided in 2 sub-groups of 6 sheep with study end points of 2 or 8.5 months. After surgery, all sheep were confined to small stalls in groups of 2 for 2 months. Sheep surviving for 8.5 months were allowed to move freely in larger stalls in groups of 4–6 after 2 months.
Surgery
Sheep were administered tetanus antiserum before surgery. Gentamicin (4 mg/kg intravenously [IV]) and penicillin (30,000 U/kg IV) were administered 30 minutes before and for 7 days after surgery. Buprenorphine (0.01 mg/kg IV) was administered immediately after surgery, then carprofen (4 mg/kg IV) once daily for 4 days. Sheep were sedated with medetomidine (5 μg/kg intramuscularly [IM]) and anesthesia induced with ketamine (2 mg/kg IV) and diazepam (0.01 mg/kg IV) and maintained with isoflurane in 100% oxygen.
A standard craniolateral approach was used to expose the hip joint; the femoral head was luxated and resected with an oscillating saw. The shaft cavity was prepared for cementing the prosthesis. The cement was mixed (Surgical Simplex P, Howmedica International Ltd, Limerick, Ireland) according to the manufacturer's recommendations, and the liquid phase carefully injected in a retrograde fashion. The prosthesis (size 9 femoral and 27 mm acetabular cup, BioMedtrix, Boonton, NJ) was positioned within the shaft. Care was taken to avoid air bubbles and attain an even distribution of the cement mantle. A standardized, primary cement mantle defect was achieved by introducing a small-sized osteotome at the lateral side of the femoral canal after the liquid phase cement injection (Fig 1). The osteotome was removed just before the cement polymerized completely, leaving a primary, standardized cement defect along the lateral aspect of the femoral shaft prosthesis. Implant stability was manually assessed by the surgeon before wound closure. All surgical procedures were performed by an experienced surgeon (M.O.) assisted by the same surgical assistants (B.v.R., A.E.-W.).
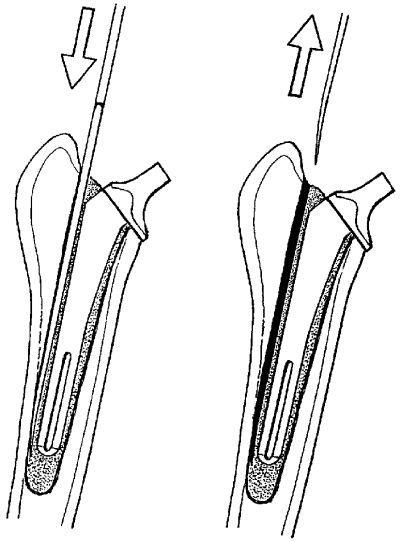
Schematic drawing illustrating the production of the cement mantle defect: An osteotome was placed on the lateral side of the femoral canal (left) and slowly removed before polymerization of the cement. The defect in the cement mantle is on the lateral side of the femur throughout the length of the femoral shaft (right).
Clinical Observation
After surgery, sheep were recovered in small stables under continuous observation until they were able to ambulate and started eating (approximately 40–60 minutes after surgery). Sheep were observed 3 times daily for 2 weeks and at least once daily thereafter for signs of pain (food intake, teeth grinding, alertness and responsiveness) and for gait abnormalities (limping or abnormalities in stance and sitting positions). If abnormal clinical signs were noted the animal was fully examined. If fractures occurred the sheep was excluded from the study.
Radiographic Evaluation
Ventrodorsal and lateral radiographs of the pelvis and treated femora of each sheep were made postoperatively and immediately after euthanasia. Care was taken to ensure the same distance between the radiographic film and the limb. Radiographic images were examined for evidence of periosteal reaction; the width of osteolysis between the bone and the cement, the quality of the cement mantle and postoperative femoral prosthesis movement.
Each femur was divided into 5 regions using a standard template based on the size of the femoral prosthesis stem. Femoral cortical width was measured in each of the 5 regions from both lateral and craniocaudal radiographs made immediately after surgery and at euthanasia (Fig 2). The difference between cortical widths measured at the 2 periods was calculated to quantify bone resorption. The analysis of cement mantle quality was based on evenness of cement distribution around the femoral implant, and the presence or absence of cement cracks or deficits visible on the lateral and craniocaudal postoperative and posteuthanasia radiographs. Femoral prosthesis movement was determined by measuring the angle formed between the longitudinal axis of the implant and the longitudinal axis of the femur on immediate postoperative radiographs and those at euthanasia, and calculating any difference.
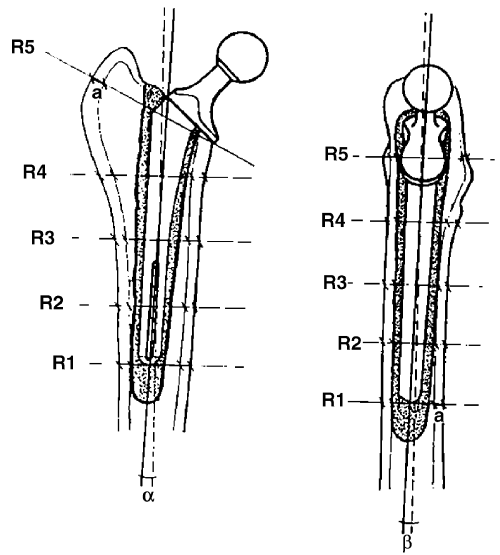
Schematic drawing illustrating the different regions (R1–R5) of the femur, the femoral cortex (a) and angles in grades formed between the longitudinal axis of the implant and the longitudinal axis of the femur (α and β).
Gross Evaluation
The treated femora were harvested for histologic evaluation. The shafts were sectioned (Exakt system, Nordstedt, Germany) transversely immediately below the level of the prosthesis. The femoral prosthesis was removed from the cement mantle by manual retrograde repulsion. Fixation of the femoral prosthesis was subjectively assessed during removal (loose=easy manual removal; tight=needed a hammer). Using the same radiographic template, the femoral shaft was sectioned into 5 regions (regions 1–5 (R1–R5), from distal to proximal femur, respectively) corresponding to the regions used for radiographic measurements. Two 3 mm sections were taken from each region. Each section was examined macroscopically for tissue characteristics such as color, size, form, and consistency of the interface membrane, and for the presence of defects, shrinkage, and cracks in the cement mantle.
Histologic Evaluation
One section from each region was processed to obtain undecalcified samples for histomorphometric evaluation. The 5 sections were stored in 4% paraformaldehyde solution at 4°C for 48 hours, then serially dehydrated using alcohol solutions (50%, 70%, 80%, 90%, 96%, and 100%) and defatted with xylene. The sections were infiltrated with acrylic resin based on methyl methacrylate (HistoDur, Leica, Zurich, Switzerland; 500 mL methyl methacrylate base, 30 mL plasticizer [phthalic acid dibutyl-ester], 5 × 1 g activator) as described.30 Sections were then infiltrated with HistoDur under vacuum at 4°C for 2–3 weeks depending on the size of the bone section. Sections were embedded with fresh infiltration solution in in-house manufactured Teflon molds in a water bath at 30°C for 12 hours. After polymerization, each section was ground to 200 μm using a sawing microtome (Sawing microtome Leica SP 1600, Leica), mounted on plexiglass slides (Wachendorf, Perspex GS Acrylicglas Opal 1013, Basel, Switzerland), and ground and polished to 30–40 μm using different grades of emery papers, diamond slurries, and lubricants (Struers, Copenhagen, Denmark and Merck, Darmstadt, Germany). The final sections were surface stained with toluidine blue.
The other section from each region was decalcified for evaluation of cellular morphology. Multiple serial slices were cut from each section using a microtome (Rotary microtome RM 2155; Leica). At least 10 slices from each region were transferred to chrome–gelatin coated slides (0.5% gelatin, 0.05% chromalaune), stretched in 96% ethanol at 42°C, and covered with polyethylene film. Excess ethanol was removed with pressure using a filter paper. Slides were stacked in pressure device and dried at 37°C for 1–2 days. Sections were deplastified (MME, Basel, Switzerland) and rehydrated in descending concentrations of ethanol and stained with toluidine blue or von Kossa/McNeal to evaluate new bone formation.
At least 10 slices from each region were fixed at 4°C in 10% paraformaldehyde, decalcified in 4% EDTA and further processed for routine paraffin sections stained with hematoxylin/eosin for evaluation of cellular morphology.
Semiquantitative Evaluation. All decalcified sample slides were evaluated by 3 investigators (M.H., B.v.R., A.E.-W.), without knowledge of the origin of the specimen, and simultaneously using a microscope with 3 oculars. Three consecutive slides from each region were evaluated and results compared after each assessment. If differences between observers occurred, the mean values were used for statistical analysis. Slides were evaluated for vascularity including cavity formation in the bone area adjacent to the interface membrane. The interface membrane was evaluated for cellularity, type of cells, thickness, and regularity. Bone reaction including bone resorption, new bone formation, or bone remodeling of existing Haversian canals was assessed close to the implant but also at the periosteal aspect of the bone. The uniformity of the slides was also evaluated; this feature was subjectively defined as the grade of similarity between regions of the same femur of the same sheep.
A semi-quantitative score was used, where vascularity, uniformity, cellularity, and bone reaction were graded as absent, low, medium, and high according to subjective evaluation of the investigators. The cell types (macrophages, fibroblasts, giant cells, mononuclear cells) were also semi-quantitatively assessed as absent (0), low number (1), medium (2), and high (3) number of cells or other variables (like areas of new bone etc) per power field.
Quantitative Evaluation. Undecalcified ground sections that were surface stained with toluidine blue were digitized with the aid of a microscope camera (DC 200, Leica) coupled to a computer system. The images were captured in TIFF format and further prepared using Adobe Photoshop (Adobe, San Jose, CA). The interface membrane, new and old bone areas, and the empty spaces representing mesenchymal or fibrous tissue within the bone were colored to allow precise quantification with specialized software (Quips, Qwin; Leica). Each section was measured twice using an automated macro for measuring these areas and data were automatically exported to an Excel (Microsoft, Redmond, WA) data sheet, which later served as basis for statistical evaluation. The same sections were evaluated with polarized light to detect the presence or absence of wear particles.
Statistical Evaluation
One-way factorial ANOVA (StatView®, Abacus Concepts, Version 4.5 for MacIntosh, Berkeley, CA) was used to evaluate the degree of bone remodeling (based on the differences between femoral cortical sizes among groups over time), the amount of new bone formation (based on increased periosteal, endosteal bone formation), and the increase in interface membrane width between groups and regions over time. Unpaired t-tests were used to compare the presence of femoral prosthesis movement between groups over time. One-way factorial ANOVA was used to assess the semiquantitative scores for cellularity, vascularity, bone reaction, uniformity, and cell types between groups and regions over time based on differences of each investigator reading. Significance was set at P<.05.
Results
Surgery
No surgical complications occurred. All prostheses were considered as manually stable directly after implantation, despite the cement mantle defect in Group II. All sheep recovered uneventfully from anesthesia.
Clinical Observation
There was no difference in lameness or behavior observed between groups at 2 or 8.5 months. Two Group I sheep fractured the operated femur; 1 fractured the femur distal to the prosthesis at 2 days and the other sheep fractured the acetabulum at 1 week. Both sheep were euthanatized. Clinically undetected osteomyelitis was confirmed by histology in 1 Group I sheep at 8.5 months. These 3 animals were excluded from the radiographic study and histologic analysis.
Radiographic Evaluation
There was significantly more bone resorption in R 5 of group II sheep at 2 months (0.25 mm±0.1 for Group I, 0.392±0.1 mm for Group II; P<.05) and 8 months (0.5±0.0 mm for Group I, 0.7±0.12 mm for Group II; P<.05) after surgery (Table 1). Generally, there was more bone resorption occurring at the lateral cortices of the femora compared with the medial cortices.
| Regions | Control (mm) | Primary Cement Mantle Defect (mm) |
|---|---|---|
| After 2 Months of Observation | ||
| R1 | 0.2±0.0 | 0.21±0.3 |
| R2 | 0.237±0.2 | 0.2±0.3 |
| R3 | 0.16±0.1 | 0.15±0.0 |
| R4 | 0.28±0.3 | 0.31±0.24 |
| R5* | 0.25±0.1 | 0.392±0.1 |
| After 8.5 Months of Observation | ||
| R1 | 0.2±0.21 | 0.213±0.2 |
| R2 | 0.312±0.1 | 0.28±0.12 |
| R3 | 0.23±0.0 | 0.3±0.23 |
| R4 | 0.4±0.12 | 0.4±0.3 |
| R5* | 0.5±0.0 | 0.7±0.12 |
- * Statistically significant values.
- Mean values with respective standard deviations are given in mm.
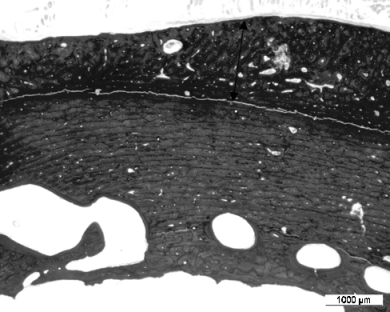
Cement cracks occurred in most Group II sheep and were predominantly located in R 1 which included the distal tip of the femoral implant. Radiolucent lines between the cement and the bone, and between the cement and the implant were present in Group II sheep especially at 8.5 months (a–e in Fig 3B). In contrast, these changes were not observed in Group I sheep (f in Fig 3B).
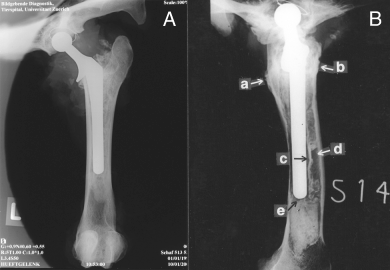
(A) a femur with a complete cement mantle at 8.5 months. (B) a femur with a primary cement mantle defect at 8.5 months, showing the proximal bone reaction (a, b), radiolucent lines between the implant and the cement and between the bone and the cement (c, d) and the presence of a cement crack at the tip of the femoral component (e).
Gross Evaluation
Generally, the femoral prostheses were more easily removed from Group II sheep at both 2 and 8.5 months after surgery whereas only 2 Group I sheep had easily removed femoral prostheses. The other Group I sheep had very stable femoral prostheses. However, an interface tissue with a scar-like, dark red coloration was detected macroscopically between the cement and the bone in both groups at 2 months; however, the interface membrane was more pronounced in Group II sheep (Fig 4A). At 8.5 months, the interface membrane was only observed in Group II sheep whereas Group I sheep had bone to cement contact without interposition of interface tissue (Fig 4B). Air bubbles were observed in the cement mantle of both groups despite the care taken during implantation of the prosthesis to avoid creating air defects. Furthermore, cement cracks and shrinkage with in-growth of the interface tissue into the cement mantle was observed in sheep of both groups.
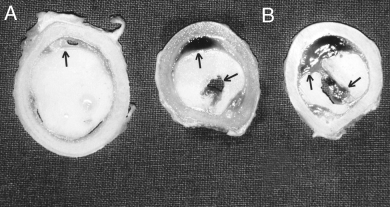
Photographs of transverse femoral bone sections after 2 months. (A) region 5 (R5) section from a sheep in the group with a complete cement mantle. (B) 2 region 1 (R1) sections from sheep with a primary cement mantle defect. An interface membrane was visible in both groups; however, it was more pronounced in the group with the primary cement mantle defect (arrows) in R1. Note that small air bubbles were present at the interface between cement and bone also in the group with the complete cement mantle (arrow head), where a local increase in the thickness of the interface membrane could be observed.
Histologic Evaluation
The transverse undecalcified bone slides from Group II sheep were more uniform histologically within regions when compared with Group I sheep (Fig 5).
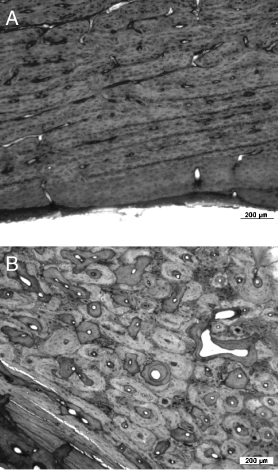
Photographs of transverse bone sections from sheep after 8.5 months. (A) section from a femur with a complete cement mantle; (B) section from a femur with a primary cement mantle defect: Note the more uniform structure of the lamellar bone in A compared with 5B indicating increased bone remodeling.
Bone remodeling was more intense and periosteal reaction more pronounced in Group II sheep at 2 and 8.5 months (Fig 6). This difference was most evident in sheep euthanatized at 8.5 months. Vascularity and cavity formation was more intense at 2 months in Group II sheep but had subsided somewhat by 8.5 months (Fig 7). New bone was primarily deposited close to the prosthesis in Group I sheep, whereas interface membrane was interposed and new bone was seldom deposited close to the cement mantle, especially in the most proximal (R5) and distal (R1) regions (Fig 8), of Group II sheep.
Periosteal reaction (arrow) is more pronounced in sheep with a primary cement mantle defect especially at 8.5 months; region R5. (ground section, toluidine blue).

Bone cavities were increased at 2 months in both groups, but were larger and more irregular in sheep with a primary cement mantle defect (A) compared with a complete cement mantle (B).
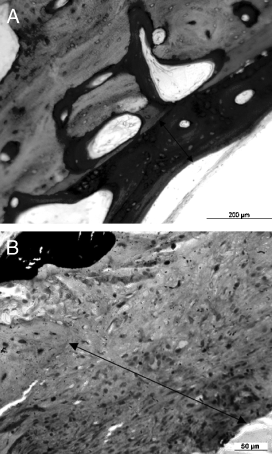
New bone deposition and osteoid production (arrow) was close to the cement mantle in sheep with a complete cement mantle (A) whereas in sheep with a primary cement mantle defect (B) a thick fibrous membrane was interposed.
Quantitative Evaluation. Histomorphometric measurements revealed a temporal decrease in new bone formation in most regions in both groups (Table 2). Temporally, there was significantly less new bone formation in R1 (P<.02) and R5 (P<.0069). There was a tendency for less new bone formation in all regions of Group I sheep in Group I compared with Group II at 2 and 8.5 months.
| Group* | Time (months) | New Bone Formation (μm2) | Interface Membrane Size (μm2) |
|---|---|---|---|
| Complete-R1 | 2 | 2.5±0.707 | 2.0±1.414 |
| Defect-R1 | 2 | 2.8±0.447 | 2.8±0.447 |
| Complete-R2 | 2 | 2.5±0.707 | 1.0±0.0 |
| Defect-R2 | 2 | 2.25±0.957 | 2.0±1.155 |
| Complete-R3 | 2 | 1.0±0.0 | 1.0±0.0 |
| Defect-R3 | 2 | 2.2±1.095 | 2.6±0.894 |
| Complete-R4 | 2 | 1.0±0.0 | 1.0±0.0 |
| Defect-R4 | 2 | 2.5±1.0 | 2.75±0.5 |
| Complete-R5 | 2 | 2.667±0.577 | 2.0±1.0 |
| Defect-R5 | 2 | 3.0±0.0 | 3.0±0.0 |
| Complete-R1 | 8.5 | 1.25±0.5 | 2.25±0.957 |
| Defect-R1 | 8.5 | 2.0±1.155 | 3.0±0.0 |
| Complete-R2 | 8.5 | 1.6±0.894 | 1.4±0.894 |
| Defect-R2 | 8.5 | 1.8±0.837 | 1.6±0.894 |
| Complete-R3 | 8.5 | 1.5±0.577 | 1.0±0.816 |
| Defect-R3 | 8.5 | 1.4±0.894 | 2.0±1.0 |
| Complete-R4 | 8.5 | 1.5±0.577 | 1.25±0.5 |
| Defect-R4 | 8.5 | 1.5±1.225 | 1.833±0.983 |
| Complete-R5 | 8.5 | 1.0±0.0 | 1.333±0.577 |
| Complete-R5 | 8.5 | 2.2±1.095 | 1.8±0.837 |
- * Group I: complete; Group II: defect.
The size of the interface membrane varied over all of the regions but histomorphometrically the interface membrane decreased in size in both groups over time and significantly so in R3 (P<.05) and R4 (P<.036). There was a tendency for Group II sheep to have more interface membrane present compared with Group I.
Semiquantitative Evaluation. On decalcified sections, there were statistical differences for most variables (Table 3). Cellularity of interface membranes was significantly different overall (P<.0001), and if Groups I and II were compared (P=.0006), but not if time was considered as well. Cellularity was higher in Group II and was decreased at 8 months in both groups, but less pronounced in Group II with the cement mantle defect. Fibroblast scores were similar for both groups at 2 months, and although scores decreased in both groups at 8.5 months, there were significantly fewer fibroblasts in Group I sheep at 8.5 months (P=.01) compared with Group II sheep at 8.5 months. In Group I, giant cells were absent at 2 months and few in number at 8.5 months whereas they were higher in Group II (P<.0001) at 2 months and fewer at 8.5 months compared with Group I.
| Variables | Regions | Group I (Complete Cement Mantle) | Group II (Primary Cement Mantle Defect) | P-values | |||
|---|---|---|---|---|---|---|---|
| 2 Months | 8.5 Months | 2 Months | 8.5 Months | Time | Group | ||
| Fibroblasts | TR | 3.3±1.0 | 1.6±1.2 | 3.6±0.6 | 2.67±1.4 | 0.0001* | 0.01* |
| Giant cells | TR | 0 | 1.4±0.8 | 0.4±0.8 | 1.0±0.6 | 0.428 | <0.0001* |
| Macrophages | TR | 0.33±0.5 | 1.6±0.5 | 1.0±0.7 | 1.5±1.2 | 0.5 | 0.0005* |
| Mononuclear cells | TR | 1.0±0.9 | 1.8±0.4 | 1.4±1.4 | 1.67±0.5 | 0.85 | 0.046* |
| Bone remodeling | TR | 2.67±1.0 | 3.0±1.3 | 2.0±1.13 | 3.0±1.5 | 0.339 | 0.033* |
| Cellularity | TR | 2.67±1.0 | 3.2±1.01 | 1.2±0.4 | 2.34±1.3 | <0.0001* | 0.0006* |
| Uniformity | TR | 2.0±1.5 | 2.6±1.2 | 1.5±0.5 | 2.8±1.2 | 0.817 | 0.003* |
| Vascularity | TR | 3.0±1.5 | 3.0±1.3 | 2.8±1.0 | 3.16±1.2 | 0.97 | 0.522 |
| Interface | R1 | 2.5±1.6 | 3.6±0.8 | 2.75±1.4 | 4.0±0 | 0.772 | 0.001* |
| Interface | R2 | 1.0±0 | 2.5±1.6 | 1.6±1.2 | 1.8±1.2 | 0.426 | 0.068 |
| Interface | R3 | 1.±0 | 3.4±1.2 | 1.0±0.7 | 2.4±1.4 | 0.001* | <0.0001* |
| Interface | R4 | 1.0±0 | 3.5±0.9 | 1.25±0.5 | 2.17±1.4 | 0.0007* | <0.0001* |
| Interface | R5 | 2.34±1.3 | 4.0±0 | 1.34±0.5 | 2.0±1.1 | <0.0001* | 0.0005* |
| New bone formation | R1 | 3.0±1.1 | 3.6±0.8 | 1.25±0.5 | 2.5±1.6 | <0.0001* | 0.0002* |
| New bone formation | R2 | 3.0±1.1 | 2.75±1.4 | 1.8±1.2 | 2.0±1.1 | 0.013* | 0.591 |
| New bone formation | R3 | 1.0±0 | 2.8±1.5 | 1.5±0.5 | 1.6±1.2 | 0.012* | 0.038* |
| New bone formation | R4 | 1.0±0 | 3.25±1.4 | 1.5±0.5 | 1.8±1.6 | 0.01* | 0.018* |
| New bone formation | R5 | 3.3±1.0 | 4.0±0 | 1.0±0 | 2.8±1.5 | <0.0001* | 0.0004* |
- Mean values with respective standard deviations are given in scores. R, regions; TR, total of regions.
- * Statistical significance.
Macrophages (Fig 9) were significantly increased at 2 months in Group II (P=.0005) compared with Group I. The distribution of mononuclear cells was seemingly independent from the period of observation but cells were more numerous in Group II (P=.046) compared with Group I and were quite variable in Group I at 2 months. Plasma cells were detected in the interface membrane in only 2 Group II sheep. Differences in interface membrane formation were significant for groups in all regions (P<.05), but not always when comparing 2 and 8.5 months. Interface membrane formation was consistently higher in Group II.
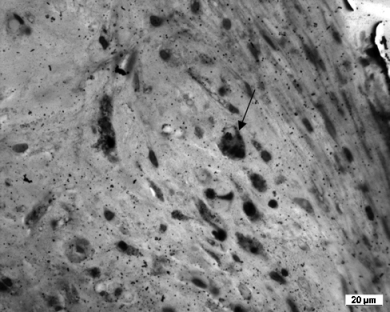
Macrophages in interface membranes were more prominent in sections from sheep with a primary cement mantle defect. Macrophages seemed to be filled with dense material in their cytoplasm (arrow).
There was significantly more bone reaction (remodeling activity) in Group II sheep compared with Group I at 2 and 8.5 months (P=.03). Whereas bone reaction decreased over time in Group I, it was maintained in Group II. Similarly, new bone formation at the periosteal or endosteal site was significantly higher in Group II in all regions except R2, where no difference was detected. New bone formation decreased over time in regions 1 (P=.0001), 2 (P=.013) and 5 (P<.0001), but not in R3 and R4. Bone quality was more uniformly lamellar in structure in Group I compared with Group II (P=.005).
No wear particles were detected with light microscopy using a polarized light source, in tissue, bone, or interface membrane from any of the sheep.
Discussion
Creating a primary cement mantle defect appeared to reliably induce formation of an interface membrane between the cement and the bone. Although an interface membrane was observed in both groups at 2 months, the membrane subsided almost completely at 8.5 months in sheep with a complete cement mantle, whereas it became more pronounced in sheep with primary cement mantle defects. This animal model apparently fulfills the requirements for studying the early radiologic and histologic changes after total hip replacement procedures, because it provides an early cause of aseptic loosening in the cemented prosthesis in a relatively well controlled repeatable manner.28,29
Our model creating a primary cement mantle defect using an osteotome at the lateral aspect of the femoral implant may possibly not withstand a critical biomechanical review. However, it may more closely mimic the clinical situation when compared with previous experimental animal models where the inflammation typical of aseptic loosening was induced by addition of wear particles7,22–26 or initial placement of a loose implant.27 These approaches seem unrealistic for studying aseptic loosening in cemented prosthesis, because wear particles are unlikely to be present during surgery or the immediate postoperative phase. Moreover, it may be safely assumed that the surgeon would not leave a loose prosthesis in place. However, it is possible that a primary cement mantle defect could be created unintentionally and missed at surgery. The quality of cement mantle has a crucial role in stability of the system,10–14 making the concept of a primary cement defect feasible as an experimental model even though it is not standardized sufficiently from an ex vivo biomechanical view. However, the animal model does not allow conclusions to be drawn about aseptic loosening in non-cemented hip prosthesis, where cause and pathogenesis may be very different.
It was of interest to note that none of the sheep (except those where fractures occurred) had clinical signs of lameness despite the fact, that some of the prosthesis were very loose in Group II sheep at 8.5 months. It is possible that the restricted movement of the sheep postoperatively obscured any lameness caused by loose prostheses. Additionally, the sheep were not observed on a treadmill or force plate, where ground reaction forces could have been measured. It is also possible that lameness in sheep with loose prostheses was too subtle to be detected by observation alone.
Cement mantle cavities were observed in some Group I sheep. These may have been caused by use of regular viscosity cement, which produces a high porosity in the cement mantle, predisposing it to failure.13,14 Alternatively, a more consistent cement mantle might have been obtained by using methods such as the pressurization technique, a stem centralizer, high-viscosity cement, or by placing cement plugs.10,13,14 Although a recent study comparing contemporary cementing techniques showed that the clinical outcome was not significantly improved, it is nevertheless possible that new cementing techniques would guarantee a more predictable cement mantle in our sheep model.31
Radiographically and histologically, more pronounced changes were observed between the 2 groups at 8.5 months compared with 2 months. It is possible that micromotion created by the primary instability of the cement mantle defect was not fully effective yet in Group II sheep at 2 months. The decrease of variables within groups over time is most likely because of the resolution of surgical trauma. Surgical trauma alone may cause intense bone remodeling and the formation of a thin interface membrane in the first few months after surgery. If the implant is sufficiently stable and implanted in a sound and complete cement mantle, this reaction may subside and the fibrous tissue may be replaced by bone. In contrast, if the cement mantle is incomplete, this may favor micromotion between cement and implant and/or cement and bone leading to an increase of interface membrane formation and finally aseptic loosening of the prosthesis. This assumption is supported by our results, where histomorphometric measurements showed interface membranes decreasing in size in both groups over time.
Histomorphometric measurements confirmed that there was an accelerated rate of bone remodeling in most regions in both groups at 2 months after surgery. Progressive healing over 8.5 months resulted in slower bone remodeling and decreased bone reaction. The instability created by the cement mantle defect may have stimulated bone reaction. Well-fixed femoral stems generally produce less bone reaction31; however, there is also considerable variation in bone remodeling because of individual biological response.32 The size of the interface membranes was also highly variable between regions, an observation that agrees with results where cell profiles vary according to regional differences in the interface membrane6. Variations in bone remodeling and interface membrane formation could also correlate with biomechanical variation throughout the femoral stem and shaft.9
Sheep with the primary cement mantle defect had increased bone remodeling and increased periosteal reaction occurring primarily at the proximal region, a finding compatible with other data.9 There was also a significant difference in the presence of bone remodeling and periosteal reaction between the medial and lateral cortices in both groups. The increased mechanical stress applied to lateral cortex of the femoral shaft was probably exacerbated by the cement defect on the lateral side. In our opinion, the presence of cement fractures at the tip region could be explained by a possible “pivot” effect at this point also induced by the defect on the lateral side.
Vascularity and cellularity were not different between groups at 2 months; however, by 8.5 months vascularity and cellularity were increased in Group II compared with Group I. Macrophages (increased in Group II) are known to be involved in the inflammatory process of aseptic loosening6. Their release of cytokines and interaction with other cell types may underlie the bone loss characterizing pathologic bone resorption associated with aseptic loosening. A similar process would explain the presence of higher numbers of lymphocytes and giant cells in the interface membranes of Group II sheep. Furthermore, plasma cells were only detected in Group II sheep. A strong relationship between aseptic loosening and the immune response has been reported.33 Longer term study with this model would be required to observe the more complex cellular, biochemical and molecular responses. The overall profile of cell types, however, supports the hypothesis that an incomplete cement mantle defect may be responsible for the persistence of chronic inflammation. The persistence of high cellularity and even the bone remodeling in this group could be related to the chronic instability between implant and cement and cement and bone. The fibrous tissue reaction was more evident at 8.5 months in Group II sheep and it is likely this micromotion was preventing bone formation at the cement interface.32,34 Future biomechanical studies would be necessary to quantify micromotion as well as the effect of mechanical instability on tissue at these sites.
Wear particles have been incriminated in aggravating the inflammatory processes and subsequent osteolysis around prosthetic implants.7 The lack of wear particles observed in our model could be related to problems of particle detection because of microscopic technique or particle size. However, in another study in our laboratory (publication in preparation) titanium wear particles could be easily seen with polarized light in macrophages, even at earlier time points. It is possible that implants based on cobalt chrome are more resistant to tribocorrosion, (the interaction of both mechanical [wear] and chemical [corrosion] phenomena that leads to performance degradation of metal surfaces), compared with titanium-based implants. It is also possible the wear particles were small enough to escape detection within the macrophages, and even in Group II sheep, the macrophages were not present in large numbers. Alternatively, these results may support the possibility that wear particles are more likely to be a result of chronic instability and implant tribocorrosion instead of being the potential trigger for aseptic loosening15.
Although this technique for producing a cement mantle defect could benefit from further biomechanical standardization, it did create a repeatable model of aseptic loosening in a cemented femoral prosthesis. This model could be used for future studies evaluating early biochemical changes at the bone–cement interface, or therapeutic interventions including impregnating the cement with inflammation suppressive drugs or adding biointegrative materials to the surface of the prosthesis.




