Suboccipital Craniectomy, Dorsal Laminectomy of C1, Durotomy and Dural Graft Placement as a Treatment for Syringohydromyelia with Cerebellar Tonsil Herniation in Cavalier King Charles Spaniels
Abstract
Objective— To evaluate retrospectively the efficacy of the suboccipital craniectomy and dorsal laminectomy of C1 with durotomy and placement of a dural graft for treatment of syringohydromyelia (SHM) because of cerebellar tonsil herniation in Cavalier King Charles spaniels (CKCS). This technique is used with great success in human medicine.
Study Design— Four CKCS diagnosed by Magnetic resonance imaging (MRI) of SHM because of cerebellar tonsil herniation and not responsive to medical therapy underwent a suboccipital craniectomy and dorsal laminectomy of C1 (2 dogs) and of C1 and partial C2 (2 dogs) with durotomy and placement of a dural graft. Three dogs were evaluated neurologically 24 hours, 1 month, and 3 months postoperatively and evaluations were compared with preoperative neurological examination. Repeat MRI took place 3 months postoperatively.
Results—
Neurological examinations showed neither improvement nor progression of clinical signs 3 months postoperatively. MRI showed no regression of syrinx size 3 months postoperatively.
Conclusion— Improvement was not seen. Given the progressive nature of the disorder, evaluation over a longer period of time is necessary to detect if progression has stopped. Some modification to the surgical technique is needed to accomplish the same results as in human medicine. A study of a larger population is needed to attain more reliable information.
Clinical Relevance— Suboccipital craniectomy and dorsal laminectomy of C1 with durotomy and placement of a dural graft is a feasible technique in CKCS, but needs some modification to accomplish the same results as in human medicine.
Introduction
SYRINGOHYDROMYELIA (SHM) is a combined term used to describe abnormal fluid-filled cavities in the substance of the spinal cord (syringomyelia) or within the central canal (hydromyelia).1 SHM from cerebellar tonsil herniation is known to occur in Cavalier King Charles spaniels (CKCS).2,3 Clinical signs of SHM associated with cerebellar tonsil herniation in CKCS are usually noticed between 6 months and 2 years of age, sometimes up to 10 years.4 Spinal hyperaesthesia is the major neurologic sign with a typical persistent scratching of the shoulder and neck region,3,4 especially when excited on a leash.4 Scoliosis has also been described.5
Usually, SHM is not detected on plain radiographs.1 Myelography is not always diagnostic. Sometimes filling of the central canal and subsequently the fluid-filled cavity in the parenchyma of the spinal cord (syrinx) with contrast medium or an image of intramedullary spinal cord swelling may be observed.6,7 Magnetic resonance imaging (MRI) may show T2-weighted hyperintense lesions in the spinal cord or T1-weighted hypointense lesions. Cerebellar tonsil herniation can be detected on MRI, but is not easily seen because the caudal margin of the foramen magnum is hard to identify (cortical bone is similar to subarachnoid space).8 Dynamic studies of CSF can demonstrate areas of abnormal CSF flow in the region of the foramen magnum.8
As therapeutic options medical management with corticosteroids, acetazolamide, nonsteroidal anti-inflammatory drugs (NSAIDs), or oral opioids have been used with most often an improvement but not complete resolution of signs.2,4,9 Some authors believe that dogs with progressive signs should be managed surgically.9 The surgical procedure of first choice for SHM from cerebellar tonsil herniation in humans is suboccipital craniectomy, dorsal laminectomy of C1, and durotomy with placement of a dural graft. Specific surgical techniques for SHM from cerebellar tonsil herniation have not been described in veterinary medicine.
Our purpose was to retrospectively evaluate if suboccipital craniectomy and dorsal laminectomy of C1 (and partial C2) with durotomy and placement of a dural graft was as efficient for treatment of SHM from cerebellar tonsil herniation in CKCS as has been reported in persons.
Materials and Methods
Between 1999 and 2002, 9 CKCS (6 months–5 years in age; mean, 27.2 months) were suspected at admission to have SHM from cerebellar tonsil herniation after a complete physical and neurologic examination (severe scratching of neck region, scoliosis, fly-chasing, and air snapping). Six dogs had MRI; owners of 3 dogs refused MRI. After premedication with a combination of acepromazine (0.01 mg/kg, intravenously [IV]) and methadone (0.1 mg/kg IV), anesthesia was induced and maintained with sodium pentobarbital (20% IV to effect) during MRI. Sagittal and transverse T1- and T2-weighted images (Magnetom Symphony 1.5 T, Siemens, Germany; Signa 1 T, GE Medical Systems, Milwaukee, WI) were taken from the brain and cervical and thoracic spinal cord to confirm SHM from cerebellar tonsil herniation. All 6 dogs were diagnosed with cervical SHM and cerebellar tonsil herniation. Prednisolone (1 mg/kg orally, once a day) therapy was initiated. Surgery was scheduled for 4 dogs because no clinical improvement was detected after 3 months and owners had a hard time living with these dogs because of the constant scratching. The 2 remaining dogs improved slightly to the satisfaction of the owners and surgery was not pursued.
Anesthesia and Preoperative Preparation
Dogs were premedicated with methadone (0.1 mg/kg IV) and anesthesia was induced with thiopental (12 mg/kg IV) and maintained with sevoflurane in oxygen. Ventilation was controlled to maintain end tidal CO2 between 3% and 3.5% to minimize intracranial pressure. Additional analgesia during surgery was provided with a constant rate infusion of fentanyl (5 μg/kg/h). Amoxicillin (10 mg/kg IV), amoxicillin-clavulanic acid (8.75 mg/kg subcutaneously; Synulox RTU®, Pfizer, Louvain-la-Neuve, Belgium) to prevent infection and prednisolone sodium succinate (30 mg/kg IV) to minimize brain edema and inflammation were administered preoperatively. Lactated Ringers solution (5 mL/kg/h) was administered during surgery. Mannitol (20%, bolus of 1 g/kg) was administered before surgery to further lower intracranial pressure. Postoperatively analgesia was provided with buprenorphine (10 μg/kg) at 6-hour intervals for 48 hours.
Surgical Technique
Dogs were positioned in sternal recumbency with the head flexed at 90° and care taken to maintain a patent airway. The head was shaved from the eyebrows to C2–C3, scrubbed with chlorhexidine, and disinfected with alcohol and povidone-iodine.
Surgical magnification was used during most of the surgery. A median incision was made from 1–2 cm rostral to the external occipital protuberance to the caudal border of the crista axis. Musculature was dissected and retracted from the midline of the occipital bone and the lamina of the first cervical vertebrae (C1) and in 2 dogs from part of the second cervical vertebrae (C2) using unipolar electrocautery. The atlanto-occipital membrane was detached from the suboccipital bone and the lamina of C1 by use of a nerve root retractor and a scalpel blade. The dorsal part of the lamina of C1 (and cranial lamina C2) was removed using Kerrison and Duckbill double-action rongeurs. Occipital bone as far as the external occipital protuberance, laterally not past the nuchal crests, was removed using the same instruments. The atlanto-occipital membrane was further detached from the underlying dura again using a nerve root retractor and scalpel blade. All hemorrhagic tissue was electrocoagulated and blood clots were removed before opening the dura.
A median incision of dura and arachnoidea was made at the level of C1 (Fig 1), extended into a Y-shape over the cerebellum, providing visualization of the vermis, the medial part of the cerebellar lobes, and the dorsal aspect of the spinal cord. Any adhesions present in the cerebello-pontine angle were dissected. All blood clots were removed before closing. The dura was closed with a loose-fitting diamond-shaped dural graft (3 cm × 2 cm) taken from the fascia of the occipital muscles to ensure that the area did not get constricted again, using 5-0 polyester. Absorbable gelatine sponge (Gelfoam®, Pharmacia & Upjohn, Puurs, Belgium) was placed on top of the dura so that the overlying muscles would not adhere to the graft. This was preferred to fat deposition because of the moisture absorbing capacity of gel foam. The musculature was closed in 3 layers with 2-0 monofilament polyglyconate, the subcutis continuously with 3-0 polyglecaprone, and the skin intradermally with polyglactin 910.
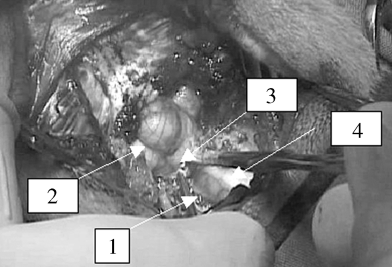
Dura mater opened and retracted to the side (1) above cerebellum (2), craniocervical junction (3), and C2 (4).
The dogs had a complete physical and neurologic examination 24 hours, 1 month, and 3 months after surgery. Repeat MRI examination was performed at 3 months.
Results
History
Four CKCS (mean age, 27 months; range, 21–42 months; 1 neutered bitch and 3 intact males) had surgical intervention for SHM. Each had a history of >6 months of progressive, severe scratching of the neck region that was exacerbated with excitement. The bitch also had urinary incontinence at the time of surgery, 18 months after initial consultation. No abnormalities were detected by dermatologic and otoscopic examination.
Physical and Neurologic Examination
All 4 dogs had severe scratching of the ear and neck area, 3 dogs on the right side and 1 on the left. Dog 4 had bilateral mechanical strabismus that was worse on the right side. Because of the continuous scratching without evident reason, the dogs were suspected of SHM from cerebellar tonsil herniation and were scheduled for MRI examination.
Preoperative MRI
All 4 dogs had clear herniation of the cerebellar tonsils on sagittal images at the craniocervical junction and had syrinx formation (Fig 2); dog 1 from mid-C2 to C4 and at T2–T3, dog 2 from C2 to T6, dog 3 from mid-C2 to C6 and from T1 to T9, and dog 4 from C2 to T1. On transverse images of the cervical spinal cord of dogs 1, 3, and 4 there was symmetrical placing of the syrinx, whereas the syrinx of dog 2 was deviated to the right dorsal side. In all dogs, the syrinx occupied >50% of the spinal cord diameter. The cervical and thoracic dilations were hyperintense on T2-weighted images and hypointense on T1-weighted images that match with accumulation of cerebrospinal fluid. Dogs 2 and 4 had asymmetrical lateral ventricles, both left being larger than the right. The lateral ventricles of dog 4 seemed mildly enlarged.
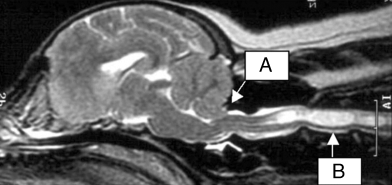
T2-weighted preoperative MR image of dog. Cerebellar tonsil herniation at craniocervical junction (A) and syringohydromyelia (B).
Surgery
Cervical laminectomy was performed as far as C1 in dogs 1 and 3 and more caudally to mid-C2 in dogs 2 and 4, in an attempt to achieve further decompression of the syrinx. Thickening of the arachnoidea and adhesion of the arachnoidea to the dura mater were noticeable as a thick, white membrane at the cranio-cervical junction in all dogs and was responsible for compression of the spinal cord at that location, so it was excised. Herniation of the cerebellar tonsils was visible in all dogs.
Postoperative Period
Three dogs recovered uneventfully from anesthesia. The other dog recovered very slowly and developed dyspnea and decreased oxygen saturation 6 hours postoperatively. Neurologic examination revealed nystagmus but otherwise normal cranial nerve reflexes. Mannitol was administered; intermittent positive pressure ventilation was started. Furosemide and doxapram were administered without effect. On thoracic radiographs there was a bronchointerstitial lung pattern and the insufficient oxygen saturation was attributed to noncardiogenic lung edema. Eight hours later seizures occurred, which were initially controlled with diazepam but later pentobarbital anesthesia was needed for control. The dog was subsequently euthanatized.
Neurologic examination at 24 hours revealed neck weakness and pain in the other 3 dogs. Dog 2 also had a head tilt to the right and a tendency to fall to the right. Dog 3 had a slight head tilt to the right. Dogs 1 and 3 were discharged at 3 days and dog 2 at 4 days.
Outcome
One month Dog 1 had returned to its preoperative neurologic state and was no longer incontinent. Scratching behavior for dog 2 was worse than before surgery and during play he still had some pain and did not want to jump up. Dog 3 also scratched more frequently, had a head tilt, a tendency to fall to the left, had difficulty lifting his neck, and was still quieter than before surgery.
Three months All dogs had similar neurologic status as before surgery. On MR images there was continued SHM in all 3 dogs (3, 4).
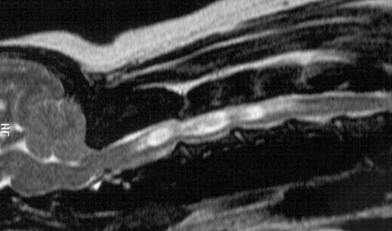
T2-weighted postoperative MR image of dog 1. Syringohydromyelia is still present.
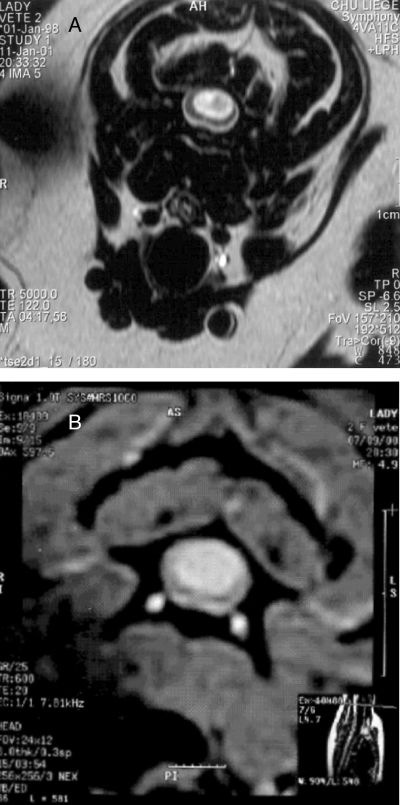
MR images of Dog 1. (A) Diameter of syringohydromyelia at C2 preoperatively, and (B) postoperatively.
Discussion
In humans, suboccipital craniectomy in combination with cervical laminectomy of C1, durotomy and use of a dural graft is the procedure of choice for surgical treatment of SHM from cerebellar tonsil herniation associated with Chiari type I malformation. Opinions about technique differ.10–13 SHM is thought to occur because of hydrodynamic effects. Several theories have been postulated based on Gardners theory of outflow obstruction of CSF from the 4th ventricle, which forces fluid into the central canal instead of the subarachnoid space during arterial pulsation10–12 but none of these can explain all aspects of the SHM formation. A new hypothesis, advanced by Chang et al14 proposes that the cisterna magna functions as a shock absorber against the pulsatile CSF waves coming from the cranial side. Loss of shock absorption, because of the overcrowding of the caudal fossa, causes the CSF pressure wave propagated along the central canal to be markedly increased and will lead to leakage of CSF into the parenchyma, which precedes the formation of the syrinx.14 If this supposition is correct, then creating more space at the foramen magnum by decompressive surgery should improve the volume capacity and shock absorbing capability of the cisterna magna, which in turn should decrease the pressure wave in the central canal and diminish existing SHM.14
SHM from cerebellar tonsil herniation has been reported in dogs.1–4,6–9,15–17 Surgical intervention has been suggested1,2,7,9 but only reported once.17 Bagley described suboccipital craniectomy, dorsal Laminectomy of C1, durotomy, and covering the brain and spinal cord with foam sponge in a dog with occipital dysplasia and widening of the subarachnoid space at the level of C1.15 MRI was not performed, so a definitive diagnosis of SHM associated with cerebellar tonsil herniation was not established.
Treatment options proposed for SHM from cerebellar tonsil herniation are medical by administration of corticosteroids (decreased CSF formation, decreased intracranial pressure), acetazolamide (decreased intracranial pressure), NSAIDs, and opioids, or surgical intervention. Our 4 dogs did not improve sufficiently with corticosteroid therapy. Because of clinical progression and the relatively exhausting behavior of these dogs, surgical intervention was recommended.
The short follow-up was not ideal. One dog was euthanatized the day after surgery. Possible complications resulting in death are brain edema and/or brain stem tissue damage from surgical manipulation, continuous bleeding, infection, and herniation of the cerebellum from opening the occiput beyond the nuchal crests.9 Hemorrhage was controlled before closure and clots were removed. Necropsy of the euthanatized dog revealed no signs of bleeding or cerebellar herniation and infection seemed unlikely. Brain manipulation with consequent edema or tissue damage was most likely the cause of deterioration. Administration of mannitol during and after surgery is important and monitoring intracranial pressure (not possible at our clinic) would be useful to evaluate patient status. Minimal manipulation of brain tissue combined with good knowledge and experience of brain surgery is also crucial.
The condition of the other 3 dogs worsened after surgery, because of marked neck pain and weakness. Indeed recovery to the preoperative state required a minimum of 2–3 months. Atlanto-axial instability from removal of part of the crista axis may have contributed to neck weakness and neck pain in dog 2. Surgical stabilization was considered but the owners declined. Complete recovery or an improvement was only achieved in dog 1 who recovered from urinary incontinence. One possible reason for SHM continuing after surgery is the smaller size of the CKCS compared with humans. Graft placement may contribute to further compression of the cisterna magna. The dogs had a history of spinal cord compression for >6 months, so surgery at an earlier stage may have been more beneficial. In humans, improvement is usually observed within a couple of weeks13 but sometimes stabilization of symptoms rather than improvement is the outcome. In dogs, it is possible that SHM remains unchanged but the dogs no longer deteriorate; however, longer follow-up is needed to evaluate deterioration. Unfortunately, transverse MR images of the cervical and thoracic spinal cord were not made in all dogs postoperatively, so we cannot exclude a change in diameter size of the syrinx pre- and postoperatively. Only 4 dogs had decompressive surgery. Certainly, a larger population in a prospective study would provide more reliable information.
Surgical intervention is advised in patients who do not respond positively to medical therapy and have progressive signs of spinal cord compression. In our dogs, MRI after 3 months revealed that the craniocervical decompression did not eliminate or diminish SHM or improve clinical signs. Evaluation over a longer period is needed to determine if progression can be stopped. A report of 4 patients may be too small to make relevant conclusions but some alterations in surgical technique may be needed to achieve results similar to those reported in humans.
Acknowledgments
We would like to thank Dr. N. Mols (dogs 1, d 4), Dr. J. Devos (dog 2), and Dr. P. Lemmens (dog 3) for referring the patients and Prof. R. F. Dondelinger and Dr. J. H. Saunders for access to the MRI facilities at the University Hospital Center in Liege. Our thanks also to F. Clompen for the photographic material.




