Impact Assessment of Biodiversity and Carbon Pools from Land Use and Land Use Changes in Life Cycle Assessment, Exemplified with Forestry Operations in Norway
Summary
There is a strong need for methods within life cycle assessment (LCA) that enable the inclusion of all complex aspects related to land use and land use change (LULUC). This article presents a case study of the use of one hectare (ha) of forest managed for the production of wood for bioenergy production. Both permanent and temporary changes in above-ground biomass are assessed together with the impact on biodiversity caused by LULUC as a result of forestry activities. The impact is measured as a product of time and area requirements, as well as by changes in carbon pools and impacts on biodiversity as a consequence of different management options. To elaborate the usefulness of the method as well as its dependency on assumptions, a range of scenarios are introduced in the study. The results show that the impact on climate change from LULUC dominates the results, compared to the impact from forestry operations. This clearly demonstrates the need to include LULUC in an LCA of forestry products. For impacts both on climate change and biodiversity, the results show large variability based on what assumptions are made; and impacts can be either positive or negative. Consequently, a mere measure of land used does not provide any meaning in LCA, as it is not possible to know whether this contributes a positive or negative impact.
Introduction
Our strong dependence on and intensive use of fossil energy causes environmental and political concerns. There is clear scientific evidence that emissions of greenhouse gases arising from fossil fuel combustion and land use changes as a result of human activities are causing an increased rate of global warming. Alternative solutions based on renewable energy that are able to reduce the consumption of fossil fuels should therefore be promoted.
Bioenergy comes from multiple sources, but forest wood is one of the most widespread raw materials and can be used in many applications, including combined heat and power plants (CHPs), more traditional stationary combustion, and the production of second-generation biofuels, to mention a few. The overall impact from such utilization must be assessed to ensure that major impacts are not overlooked and that substitution for fossil fuels with renewable alternatives actually results in environmental improvements (Searchinger et al. 2008; Levasseur et al. 2010).
A thorough life cycle assessment (LCA) of forestry activities faces a number of challenges. Forestry activities differ substantially from region to region (Berg and Lindholm 2005; Johnson et al. 2005; González-García et al. 2009), and even within regions (Michelsen et al. 2008). The density of wood can also differ significantly (Stemsrud 1988), and thus the amount of biomass actually harvested; similarly, the rotation period can differ, and thus time and area requirements. How spatial and temporal boundaries for the assessments are defined is therefore of great importance. Presumably there are also important environmental impacts that should be included in LCAs of forestry and wood-based products that presently lack mature and agreed upon assessment methods. Land use and land use change (LULUC)-related impacts are among the most important of these; LULUC is recognized as the greatest threat to biodiversity (Chapin et al. 2000; Sala et al. 2000; Henry et al. 2008; Haines-Young 2009) and the importance of LULUC for changes in carbon pools and greenhouse gas emissions is widely recognized (Karjalainen et al. 2003; Cowie et al. 2007).
Even though an increasing number of publications on LCA of bioenergy take land use into consideration, with a particular focus on changes in soil carbon (e.g., Styles and Jones 2007; Cherubini et al. 2009; Brandão et al. 2010; Spatari et al. 2010), few take both land use and land use changes into account (Cherubini and Strømman 2011). Also, few studies present results in which aspects of biodiversity and greenhouse gas balances are combined for identification of potential conflicts and trade-offs, even though several incentives for increased carbon uptake in forests can conflict with protection of biodiversity (Cowie et al. 2007). One exception is a case study on vegetable oil (Schmidt 2010). A few studies outside of LCAs have tried to combine more aspects of LULUC (e.g., Haughton et al. 2009). But there is an obvious need to further elaborate methodologies for LULUC in LCAs in general, and in particular when it comes to studies on bioenergy.
The purpose of this article is to contribute to a better understanding of the complex issues surrounding LULUC, both related to methodologies on how to include LULUC in LCAs and to the importance of LULUC for the results of LCAs. Impacts caused by LULUC are here limited to global warming potential (GWP) caused by temporary and permanent changes in carbon pools and effects on biodiversity of changing land use. This is exemplified with a case study on logging and forestry operations in Norway. A newly proposed method for assessment of climate impacts from temporary changes in carbon pools (Cherubini et al. 2011) is here tested on a specific case study for the first time. This is combined with a method proposed by Michelsen (2008) for assessing impacts on biodiversity.
Case Study and Methodology
The main focus of this article is impacts from LULUC; the functional unit is the use of 1 hectare1 (ha) for 100 years of forestry operations. This time frame is a good approximation of an average rotation period in Norway. The potential product delivered is roundwood logs under bark, measured in cubic meters (m3).2
Methodological Framework
In this article forest management is regarded as land use, while establishment of a managed forest is regarded as land use change (cf. Eggleston et al. 2006). The impacts of LULUC on biodiversity and carbon pools are estimated based on the framework of Mila i Canals and colleagues (2007). Here, impacts caused by LULUC are regarded as a product of area, time, and land quality changes. In this case, changes are relevant to carbon pools and/or biodiversity in the area of interest (see figure 1). It is important to differentiate between the transformation impact, which here occurs when an unmanaged forest is transformed to a managed forest (occurring at t1 in figure 1), and occupation impact, which is the impact caused by the ongoing forest management. The occupation impact includes both the time when forestry operations are performed (from t1 to tfin in figure 1) and the time needed for the area to recover after the use of the area is concluded. This is referred to as the relaxation period and lasts from tfin to trel in figure 1. Even though the actual logging lasts for a limited time, the impacts must be considered over the whole of one rotation period (from t1 to trot1 in figure 1).
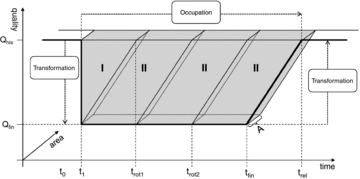
Schematic impact from land use (occupation) and land use change (transformation) based on quality changes, time of occupation, and area used (adapted from Mila i Canals et al. [2007] and Michelsen [2008]). The volume in “I” represents the transformation impact, while the volume in “II” represents the occupational impact over one rotation period. Here, three consecutive rotation periods are indicated before the area is left for relaxation. The solid line indicates assumed changes in the quality of the area over time, including transformation, multiple rotation periods, and a final relaxation of the area. Qhis= historic or original quality of the land; Qfin= final quality of the land; t0= time when land is in natural state, prior to forestry operations; t1= forestry operations begin; trot= land completes rotation period; tfin= forestry operations are finished and land enters relaxation period; trel= land completes relaxation period; A = area of land. See the text for more details.
Transformation and occupation impacts must be related to a functional unit, which is here the land use itself. Impacts from LULUC can also be related to the output from the system, measured in cubic meters of roundwood logs. The volume of “II” in figure 1 represents the impact on 1 ha over one rotation period; the time, area, and quality change are regarded as a postponement of natural relaxation (cf. Mila i Canals et al. 2007). Similarly, the volume of “I” represents the total transformation impact. In the figure, the relaxation time is equal to a rotation period (from tfin to trel equals from t1 to trot1). That is, the time needed for complete relaxation is equal to the rotation period for forestry. In this article, this assumption is applied to biodiversity. For carbon pools, it is assumed that the relaxation period is longer than one rotation if logging takes place in an unmanaged forest. This is described under the scenario assumptions below.
Figure 1 shows a schematic outline. It is also important to keep in mind that the quality can change during the occupation (between t1 and tfin), that Qfin can be higher than Qhis, and that the quality at t0 and trel can be different (see Mila i Canals et al. [2007] for more details).
Two measures of quality are used to assess impacts from LULUC in this study: the size of carbon pools (limited to above-ground biomass [AGB]) and biodiversity. These are included due to the great concern for biodiversity protection and greenhouse gas mitigation, as well as the potential conflict between these two (cf. Cowie et al. 2007). Limitation to AGB as opposed to complete coverage of changes in all carbon pools is due to uncertainty in data on below-ground biomass, soil organic content, and dead wood, which could all be included alongside AGB. It is also documented that forest management primarily influences the carbon pools in vegetation and not soil (Johnson and Curtis 2001; Gonzalez-Benecke et al. 2010), indicating that it is less important to include these pools. Because the focus is on changes, changes in AGB are limited to biomass of harvested stem volumes. It is assumed that branches, needles, and such, do not decompose at a higher rate than carbon is recaptured by new vegetation. The same assumption could be used for roots.
The climate impact of biogenic carbon dioxide (CO2) emissions (hereafter bio-CO2) from temporary changes in carbon pools is often omitted in unit-based environmental impact assessments, as the carbon is recaptured by new vegetation and the system is assumed to be carbon neutral. It is often taken for granted that carbon neutrality automatically means climate neutrality as well (Cherubini et al. 2011). This consideration ignores the time delay between emission and sequestration; because the CO2 is not instantly absorbed by vegetation, its presence in the atmosphere causes radiative forcing and contributes to climate change.
To assess the climate impact of temporary changes in carbon pools, a recent methodology is adopted based on the elaboration of atmospheric decay functions for bio-CO2 (Cherubini et al. 2011). These are used to estimate the contribution to global warming of bio-CO2 through a climate metric, the GWPbio index. For a 100-year rotation period the GWPbio is 0.427 and 0.077 CO2 equivalents (CO2e) for 100- and 500-year horizons, respectively.3 In other words, depending on the time horizon, bio-CO2 contributes 42.7% or 7.7% of the impact normally assumed for anthropogenic CO2. (These values will change if the rotation period is changed.)
For permanent changes in carbon pools a simple average stock change approach is used (Cowie et al. 2007). An average increase or decrease of CO2 emissions to the atmosphere over the relevant time (100 years) is calculated and the impact is regarded as equal to the impact from anthropogenic CO2.
The impact from changes in carbon pools is related to a reference situation in which the pools develop as if there were no interference. This is in line with the framework proposed by Mila i Canals and colleagues (2007) in which the reference is a dynamic situation. The focus is on changes in radiative forcing during the period of concern (100 years); whatever happens to the carbon after this period is not considered, but is the subject for assessment of subsequent rotations.
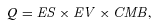 (1)
(1)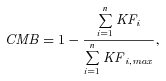 (2)
(2) (3)
(3)Case Study and Scenarios
The geographical location of the forestry activities is central Norway, ecoregion PA0608, the Scandinavian and Russian taiga (Olson and Dinerstein 1998; Michelsen 2008). The logged wood in the present situation is Norway spruce (Picea abies), a native species for the region. The final utilization of timber is assumed to be bioenergy of any kind and is assumed to be burned the same year that logging takes place.
The system boundaries for the forestry operations include planning of forestry operations, seedling production, soil scarification and planting, silviculture (mechanical cleaning of undesirable vegetation, fertilization, chemical cleaning and weed combating, and drainage), harvesting (felling, pruning, and cutting into logs), transport to pile at forest roads, construction and maintenance of forest roads, and transport from pile to a factory. Potential pretreatment before burning is not included.
Two scenarios for establishing a managed forest and two scenarios for forest management are included (figure 2). For comparison, a reference case is included where only the impact from logging operations is a factor, in which a best case (bc), average case (ac), and worst case (wc) are assessed based on log size and transport distances. The life cycle inventory of the forestry operations is performed as a hybrid LCA (Michelsen et al. 2008).
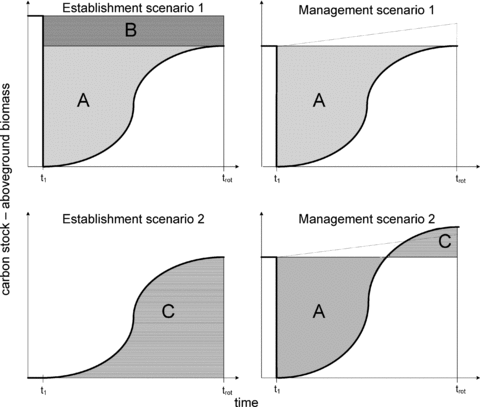
Changes in above-ground biomass (AGB) in the different scenarios. The bold line represents the changes in AGB. The gray areas (A) indicate a temporary loss of AGB due to logging, the shaded areas (B) represent a permanent loss of AGB due to transformation, and the horizontal lines (C) represent increased AGB due to transformation of the area in ES2 and introduction of a new species in MS2. The dotted line in MS1 and MS2 indicates the foregone sequestration, an increase in biomass that would have occurred if the areas were not logged at t1. See the text for details on the scenarios.
Establishment scenario 1 (ES1) represents establishment of a managed forest based on transformation from an unmanaged forest, while establishment scenario 2 (ES2) represents establishment of a forest based on transformation from a fallow field. Management scenario 1 (MS1) is continuous forest management with consecutive rotations of Norway spruce, while in management scenario 2 (MS2) Norway spruce is replaced with Sitka spruce (Picea sitchensis), an introduced species. Later rotations in this scenario (MS2*) are consecutive rotations of Sitka spruce after the replacement. Both MS1 and MS2 can represent continued forest management following ES1 and ES2, so these scenarios can be combined. All scenarios end with the area forested. It is important to keep in mind that since scenario ES2 represents the establishment of a forest on fallow field, there is no forest to be logged in this scenario, and consequently there is no impact from logging operations, only the impacts from planting and silviculture. ES2 can be regarded as an afforestation project, and potential changes in carbon pools after the period of concern are not included. ES2 can still be followed by forestry activities MS1 or MS2, and then also provide timber.
Table 1 and figure 2 show the assumed changes in timber volume in the different scenarios. The average timber volume in development class V (i.e., mature forest) in Norway is 218 m3/ha (Larsson and Hylen 2007). It is thus assumed that this is the volume at the end of scenarios ES1, ES2, and MS1, as well as the initial values in scenarios MS1 and MS2. ES1 is a transformation from unmanaged to managed forest. This transformation is assumed to cause a reduction in above-ground biomass of 20% (Eggleston et al. 2006), giving an initial timber volume value in ES1 of 272 m3/ha. MS2 represents a transformation from managed forest with Norway spruce to Sitka spruce. Sitka spruce has a higher potential for carbon capture and storage (Green et al. 2007; Øyen 2008), and above-ground biomass is assumed to increase by 35% (cf. Øyen 2008). Timber volume at the end of MS2 is thus 294 m3/ha.
| Scenario | Initial volume (= logged volume) | Processes | Final volume |
|---|---|---|---|
| Establishment scenario 1 (ES1) | 272 m3/ha | Logging: transformation from unmanaged to managed forest with Norway spruce | 218 m3/ha |
| Establishment scenario 2 (ES2) | 0 m3/ha | Establishment of a managed forest with Norway spruce on fallow land | 218 m3/ha |
| Management scenario 1 (MS1) | 218 m3/ha | Logging, consecutive rotations with Norway spruce | 218 m3/ha |
| Management scenario 2 (MS2) | 218 m3/ha | Logging, consecutive rotations where Norway spruce is replaced with Sitka spruce | 294 m3/ha |
| Management scenario 2* (MS2*) | 294 m3/ha | Logging, consecutive rotations with Sitka spruce | 294 m3/ha |
- Note: m3/ha = cubic meter/hectare.
Figure 2 shows the assumed changes in carbon pools in above-ground biomass in the different scenarios. Contrary to the schematic outline in figure 1, the quality (here above-ground biomass [AGB]) is not constant from t1 to trot. In order to assess the total impact over one rotation period, four different changes must be taken into consideration. First, there will be a temporary loss in above-ground biomass due to logging in scenarios ES1, MS1, and MS2. This is marked “A” in figure 2. In ES1, there will also be a permanent loss as a consequence of the land use change. This is marked “B.” ES2 and MS2 also include an increase of carbon pools: in ES2 as a consequence of transformation from fallow field to forestry, and in MS2 as a consequence of introducing a species with higher accumulation capacity (marked “C” in figure 2). Because these pools are present at the end of each scenario, the changes are considered permanent. Any potential reductions of these pools at a later time are allocated exclusively to the activities causing the reductions.
To assess the impact of the temporary loss (A in figure 2), the approach and GWPbio described in work by Cherubini and colleagues (2011) are used, while the permanent changes (B and C in figure 2) are assessed as average stock changes without any further valuation.
A fourth change that could come into consideration is loss of potential continued carbon accumulation in old growth forests, sometimes referred as “foregone forest sequestration,” as one rotation period is not enough to reach the level of biomass found in unmanaged forests (Cooper 1983; Luyssaert et al. 2008; Searchinger et al. 2008). Using the terminology from Mila i Canals and colleagues (2007), this foregone sequestration represents a postponement of relaxation. It is relevant for the management scenarios because the forests would have continued to accumulate carbon if they were not logged at t1. The GWP for bio-CO2 is also applied to these changes.
An average annual regrowth rate is used to allocate the impact from LULUC to the production of 1 m3 of roundwood. For a rotation period of 100 years, this rate is 2.18 m3/ha/year (yr), giving a time and area requirement of 0.459 ha/yr to grow 1 m3 of roundwood.
Based on density values from Lehtonen and colleagues (2004) and an assumed carbon content of 50% of dry mass, the carbon content of 1 m3 of roundwood is estimated at 192 kilograms (kg).4
Results
Greenhouse Gas Emissions
Table 2 gives an overview of greenhouse gas emissions from different stages in the forestry supply chain. Other impact categories can be found in the work by Michelsen and colleagues (2008).
| Planning | Seedling production | Planting | Silviculture | Logging | Transport by forwarder | Forest road construction | Road transport | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Best case (bc) | 55 | 271 | 65 | 48 | 618 | 435 | 443 | 535 | 2,470 |
| Average (ac) | 55 | 271 | 65 | 48 | 1,020 | 1,023 | 443 | 2,535 | 5,461 |
| Worst case (wc) | 55 | 271 | 65 | 48 | 1,830 | 2,962 | 443 | 5,712 | 11,387 |
- Source: Michelsen et al. 2008.
- Note: ha = hectare; kg CO2e = kilogram carbon dioxide equivalents.
The total impact on climate change from the different scenarios given different time horizons is shown in figure 3. An average impact from forestry operations is assumed for all scenarios. In scenarios ES1, MS1, and MS2 there is a temporary loss equivalent to 154 tonnes5 bio-CO2 due to logging and combustion of 218 m3 of timber; in MS2* 294 m3 of timber is combusted, giving a temporary loss equivalent to 207 tonnes bio-CO2. Using the GWPbio index, this is equal to, respectively, 66 and 88 tonnes CO2e with a 100-year time horizon, and 12 and 16 tonnes CO2e with a 500-year time horizon.
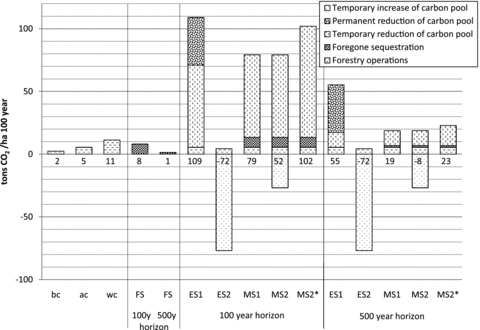
Impacts on climate change from the included scenarios. The first cases (best case [bc], average case [ac], and worst case [wc]) only include forestry operations. ``FS'' shows the foregone sequestration in the management scenarios with 100- and 500-year time horizons. For all establishment and management scenarios, changes in carbon pools are included, both with 100- and 500-year horizons. The values for each scenario show the total impact of 1 hectare (ha) over 100 years measured in tonnes of carbon dioxide equivalents (CO2 e). For descriptions of scenarios ES1, ES2, MS1, MS2, and MS2*, see table 1. CO2/ha = carbon dioxide per hectare; y = year.
In ES1 there is also a permanent loss of 54 m3 of timber causing emissions of 38 tonnes CO2, and in ES2 there is build-up of a new carbon pool in above-ground biomass equivalent to the final level in ES1.
In an unmanaged forest it can be assumed that the net accumulation of above-ground biomass is zero (at t0 in figure 1), but when a managed forest is logged, potential continued net accumulation (foregone forest sequestration) should be taken into account (Cooper 1983; Mila i Canals et al. 2007; Luyssaert et al. 2008; Searchinger et al. 2008). Here it is assumed that 50% of the carbon lost as a result of transformation to a managed forest could have been recaptured in AGB over 100 years if no more logging had taken place (cf. Harmon et al. 1990; Luyssaert et al. 2008). The foregone sequestration in MS1 is thus a result of impeding a potential continued regrowth of 27 m3. Because there are no old growth forests of Sitka spruce in the relevant region, the same number is used for MS2 and MS2*.
In MS2, Norway spruce is replaced with Sitka spruce. Following the temporary loss from logging and combustion of 218 m3, there is an increased carbon pool compared to the initial situation. The additional uptake is based on an additional 76 m3 of timber, which can remove 14.6 tonnes C/ha by the end of a rotation period of 100 years.
Impact on Biodiversity
Following Michelsen (2008), ES×EV= 0.535 in the relevant ecoregion, and ΔQ= 0.300 under the present forestry regime in Norway. The land occupation impact on biodiversity in MS1 is thus 30 (see “II” in figure 1).6
The land occupation impact of ES1 is the sum of the impact of MS1 and the transformation impact. The transformation impact for 1 ha is given by ([0.300 × 100]/2) × 1, which is equal to 15 (see “I” in figure 1). The total impact in ES1 is thus 45.
ES2 also represents a transformation. Here, the impact on biodiversity depends on the initial situation. The quality during active forestry is equal to the situation in MS1, but the CMB at time t0 must be assumed. If the fallow field had previously been intensively cultivated and relaxation into a forest postponed, then it can be assumed that all included KFis are zero, giving a CMB equal to zero, and a quality at t0 equal to zero. The quality at t1 is 0.235, as in ES1 and MS1, giving a ΔQ of −0.235. Assuming the improvement is a linear change during the 100-year period, the impact is then given by ([−0.235 × 100]/2) × 1, which equals −11.75 (or an improvement of 11.75) compared with the initial situation.
In MS2, Norway spruce is replaced with Sitka spruce. The impact from an introduced species is assumed to be major (Michelsen 2008). It is also assumed that the amount of decaying wood within such a plantation will be low; it is reasonable to assume that it will be less than 5 m3/ha. It is further assumed that the area set aside is kept constant, so the impact in this regard is moderate. Following the threshold values suggested by Michelsen (2008), this gives a CMB equal to 0.11 and a ΔQ of 0.476 for this scenario. The total impact is then 47.6. For biodiversity, the impact is equivalent in MS2 and MS2*.
Total Impact from Land Use and Land Use Changes
In table 3, the impact is allocated to 1 m3 of roundwood logs to adjust for different productivities in each scenario. Because ES2 is exclusively an establishment scenario, there is no logging and the impact cannot be allocated to timber production.
| Global warming potential (tonnes CO2e, 100-year horizon) | Impact on biodiversity (ΔQ× ha × yr) | |||
|---|---|---|---|---|
| ha 100 year | 1 m3 roundwood | ha 100 year | 1 m3 roundwood | |
| Establishment scenario 1 (ES1) | 109 | 0.40 | 45.00 | 0.17 |
| Establishment scenario 2 (ES2) | −72 | NA | −11.75 | NA |
| Management scenario 1 (MS1) | 79 | 0.36 | 30.00 | 0.14 |
| Management scenario 2 (MS2) | 52 | 0.24 | 47.60 | 0.22 |
| Management scenario 2* (MS2*) | 102 | 0.35 | 47.60 | 0.16 |
- Note: CO2= carbon dioxide; ha = hectare; yr = years; m3= cubic meter. NA = not applicable (since ES2 is exclusively an establishment scenario, there is no logging and the scenario's impact cannot be allocated to timber production).
Figures 4 and 5 summarize these results both for impact on biodiversity and GWP for different LULUC scenarios. Figure 4 shows the absolute impacts from 1 ha over 100 years, while figure 5 allocates the impacts to the production of timber. In figure 5, the effects of allocating the transformation impacts to multiple rotation periods are also shown. The establishment scenarios can be followed by any management scenario. In the figure, ES1 and ES2 are both shown followed by a series of MS1, where the transformation impact is allocated evenly to all rotations. Consequently, the impact from the produced timber approaches the impact of MS1, indicated by the arrows in the figure. ES1 is also shown with a series of rotations of MS2, that is, one MS2 and a number of MS2*s. The result is a broken line that, in the second rotation, approaches MS2, but from the third rotation on approaches MS2*.
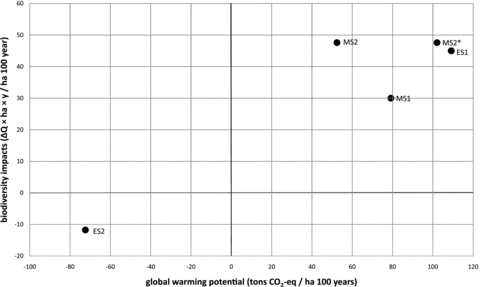
Absolute impact on biodiversity and global warming potential (GWP) from land use and land use change (LULUC) in the included scenarios (see table 1 for full descriptions), shown as impact per hectare over 100 years. Values for GWP are given in a 100-year time horizon.
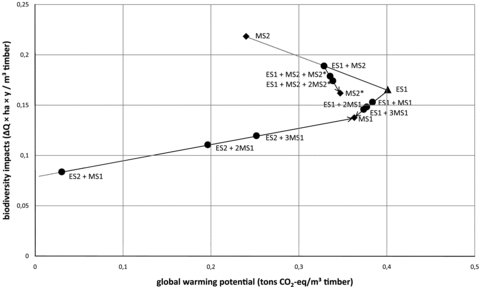
Impact on biodiversity and global warming potential (GWP) from land use and land use change (LULUC) allocated to timber production. (See table 1 for descriptions of scenarios.) The establishment scenarios are shown as triangles (ES2 is not shown because this cannot be allocated to timber production) and the management scenarios are shown as diamonds. The circles represent combinations of establishment scenarios and a number of management scenarios in which the transformation impact is allocated evenly to all included rotations. Values for GWP are given in a 100-year time horizon.
Discussion
This article demonstrates several of the uncertain aspects that exist in LCAs of forestry and wood-based products and the need to treat LULUC in a consistent way in LCAs.
The methodology for inclusion of LULUC in LCAs is immature. In this article the atmospheric decay functions proposed by Cherubini and colleagues (2011), giving GWP for bio-CO2 as a function of rotation periods and time horizons, are used for temporary changes in carbon pools. These are combined with the methodology proposed by Michelsen (2008) to assess impacts on biodiversity from LULUCs in different scenarios.
The results in this article show that LULUC is of major importance and should not be ignored in LCA studies. Two findings can be highlighted. First, following the methodology from Cherubini and colleagues (2011), it is shown that the impact from changes in carbon pools is more important than direct emissions from forestry processes when it comes to climate change. Second, the LULUC impact on carbon pools and biodiversity must be treated independently; a mere assessment of area used does not give any meaning in LCAs since it will be unknown whether this is a positive or a negative impact.
As long as biogenic CO2 stays in the atmosphere for a period of time, it will cause radiative forcing, which should be included. The GWPbio is sensitive to selection of time horizons (Cherubini et al. 2011). This is in line with all assessments of GWP, and there is a range of proposals on how to deal with this in LCAs. Here, a fixed time horizon following an intervention is used to assess the impacts, as all scenarios are assumed to have the same time span.
The temporal starting point of the assessments is also of major importance. In ES1 the starting point is an unmanaged old growth forest; thus, considerable amounts of carbon are stored in the vegetation. When the starting point is changed to a managed forest, as in MS1, or an area with almost no vegetation, as in ES2, the results change significantly. It can be argued that in a Norwegian context it would be right to assume that a managed forest is already present and that MS1 represents a “normal” situation. Also, if transformation impacts are evenly allocated to all rotations following the transformation, then the impact from each rotation will inevitably approach the impact in the management scenario (figure 5), strengthening the argument that the management scenarios could be used as standard values if it is not known from what type of transformation the timber originates.
For the forest growth and carbon uptake, we used a simple stock change model compared with other models for forestry growth (e.g., Gonzalez-Benecke et al. 2010). This is done to keep the model simple and easy to relate to a functional unit. When using the proposal from Cherubini and colleagues (2011) on temporary changes, it is assumed that this will not have any significant impact on the results.
The growth rates of the forest are of major importance. These differ from species to species and from region to region (Larsson and Hylen 2007). The growth rates and rotation period will influence how fast the carbon is removed from the atmosphere, and thus the impact bio-CO2 has on climate change. The values used here, based on average timber volume and a 100-year rotation period, are close to the average values presented in the work of Stokland and colleagues (2003), indicating that the values are within the range of what could be expected. Still, the regrowth rate used for old growth forest is much lower than growth rates reported by Larsson and Hylen (2007) for spruce forests in development class V, indicating that the magnitude of foregone sequestration might be underestimated in this study.
The assumed density of the wood will also make a major contribution to the results. Here, values from the work of Lehtonen and colleagues (2004) are used, assuming a carbon content of 192 kg/m3. However, Stemsrud (1988) reports that the density of Norway spruce ranges from 300 to 640 kg/m3, with an average of 430 kg/m3. In most cases carbon is half of the mass, but this also varies (Lamlom and Savidge 2003). The ranges for Sitka spruce are even larger, and the carbon content of 1 m3 might be as low as 125 kg/m3. The direction of the results will not change in the cases presented here, but the exact numbers can change significantly. It can be questioned whether the traditional unit of timber volume is useful in LCAs in which timber is used for bioenergy generation.
In ES1 a reduction of 20% in biomass from an unmanaged to a managed forest is assumed. This number is from Eggleston and colleagues (2006), who report a reduction in boreal forests from 50 to 40 tonnes dry matter/ha. This number is extrapolated here to a 20% reduction independent of the amount of dry matter in the initial conditions. It is not known whether this assumption is valid at all ranges; as well, the initial value from Eggleston and colleagues (2006) is an approximation.
We have not included changes in below-ground biomass, dead wood, litter, and soil organic matter, although in boreal forests these might, in total, be larger than above-ground biomass (Harmon et al. 1990; Karjalainen 1996). However, forest management is shown to primarily influence carbon storage in vegetation (Gonzalez-Benecke et al. 2010). Still, below-ground and other carbon pools can fluctuate following the rotation periods, although not as abruptly as above-ground biomass (Johnson and Curtis 2001; Jandl et al. 2007). It is reasonable to assume that an inclusion of these carbon pools would make the storage of carbon an even more important part of the assessment (cf. Johnson and Curtis 2001). This would presumably strengthen the direction of the establishment scenarios; in ES2 there would most likely be an even larger build-up of carbon with the inclusion of soil carbon and below-ground biomass, while the loss in ES1 would be larger, particularly due to loss of wood debris (Harmon et al. 1990). Due to data uncertainty, this effect is not included here.
For the results presented in figures 3 and 4 and table 3, the framework proposed by Mila i Canals and colleagues (2007) is used; the transformation is allocated to the first rotation period, while the lost potential is attributed to the rotation period in focus, as this will constitute the dynamic reference situation (Mila i Canals et al. 2007). This means that consequential aspects are introduced in this part. The reference situation is a potential dynamic situation following an assumed relaxation potential, not, for example, a long-term average (cf. Cowie et al. 2007). The results so far indicate that the lost potential uptake is of minor importance compared to the other changes (figure 3), but this might be an underestimate due to the assumptions on carbon pools noted previously.
MS2 shows some of the conflicting issues related to LULUC. Replacing Norway spruce with Sitka spruce in MS2 would increase the carbon storage and reduce the climate impact compared to MS1, but the impact on biodiversity would increase. In table 3 and figure 5, in which the impacts are allocated to 1 m3 of roundwood, this is particularly visible. MS1 and MS2 are both on consecutive rotation periods, but MS1 has a lower impact on biodiversity, while MS2 has a lower impact on global warming. It is therefore important to focus not only on LULUC as such, but also on the specific impacts LULUC causes and the potential trade-offs between these.
Other aspects related to LULUC could also have been included. As shown in figure 4, ES2 has a positive impact both on climate change and biodiversity; the carbon pool increases and the conditions for biodiversity are improved. However, the assumption here is that formerly intensively cultivated agricultural land lying fallow is transformed (back) to a forest. This could influence food production and land use aspects in other parts of the world (Kløverpris et al. 2008) and cause other impacts not included here. In this scenario there is also no logging of timber.
There is an obvious question about whether transformation impacts should be allocated to the first rotation or evenly across several rotation periods. The latter is problematic due to the length of the rotation periods, but the situation in ES2, in which first a forest must be established, clearly demonstrates the challenges of a complete allocation to the first rotation. Figure 5 shows the results of changing the allocation of transformation impacts. Allocation of transformation to multiple rotations will necessarily give a result closer to the management scenarios. The figure also shows that replacing Norway spruce with Sitka spruce has a limited effect over time. The transformation gives a climate benefit once, but when this is followed by consecutive rotations of Sitka spruce, the increased amount of carbon stored in the forest actually makes the MS2* scenario worse than MS1 due to higher carbon release from the area (table 3). Since the productivity is also higher, the impact per cubic meter of roundwood is still slightly lower (table 3).
It is important to keep in mind that there is a fundamental difference between assessing the impact from LULUC on biodiversity and on carbon pools. One of the challenges of including LULUC in LCAs is the lack of physical flows that can be accurately assessed (Udo de Haes 2006). While this problem is highly relevant for impacts on biodiversity, it is not equally valid for changes in carbon pools. In the case of carbon pools, the challenges are more about spatial and temporal boundaries of the assessment. The time horizon is of major importance, and the scenarios presented demonstrate the importance of consistent time horizons (figure 3). Longer time horizons strongly favor bio-CO2 since the vegetation is able to remove approximately all carbon initially released to the atmosphere. As a consequence, bioenergy will appear as a better climate change mitigation strategy with a longer time horizon.
Another critical point for the time horizon is the determination of when the different forestry activities and processes are assumed to occur. Here it is assumed that all processes are taking place in the same year. At first this might seem problematic: a tree cannot be planted and logged the same year, and consequently the release and capture of carbon will occur at different times. Still, this is a common assumption in LCAs of forestry products (Michelsen et al. 2008). The rationale is, first, that only the present management practice is known; since management practices hundreds of years from now cannot be foreseen, present management is used. Second, all processes are in fact going on simultaneously—not on the same spot, but across a larger area; all operations are in fact concurrent and the assumptions are thus justifiable.
Conclusions
In this article the impacts of LULUC on climate change and biodiversity are assessed. A new methodology for assessing the impacts of temporary changes in carbon pools is used and combined with a methodology for assessing impacts on biodiversity. This is the first case study in which the atmospheric decay functions proposed by Cherubini and colleagues (2011) are applied. Both methodologies show promising results for implementation in LCAs. As long as the rotation periods and timber volumes are known, the GWP for bio-CO2 from Cherubini and colleagues (2011) can be used to provide data for all energy crops.
A critical issue is the actual amount of biomass in the harvested volumes. The density range for wood is so large that the traditional reporting method of cubic meters is questionable.
The scenarios where this approach is used clearly demonstrate the importance of including LULUC in LCAs of forestry and wood-based products. The scenarios also demonstrate a high level of complexity, as many variables are involved—partly due to local conditions, partly to unknown conditions from long time spans. The scenarios also demonstrate the challenge of treating permanent and temporary changes in a consistent way using different time horizons.
It is here shown that the impacts on climate change from LULUC are more important than the impacts from forestry operations. It is thus crucial to continue the work on including LULUC in LCAs and further refining and developing proposed methodologies.
It is important to keep in mind that LULUC causes a wide spectrum of impacts, both positive and negative. In this article this is exemplified by biodiversity and carbon pools, which are presumably the two areas of protection most affected by LULUC. Assessments must take these into account and illuminate possible trade-offs between them. A mere measure of land used does not provide any meaning in LCAs since it is not possible to know whether the impacts are positive or negative.
The results are sensitive to both selection of time horizon and allocation of transformation impacts. This is no different than other LCA studies and studies on climate change. We have shown the results of different allocation choices and time horizons. From our point of view, it is justifiable to use values from management scenarios if it is not known whether the timber originated from a previously unmanaged forest. We give no recommendation on which time horizon should be taken, except that this must be harmonized with other choices.
Acknowledgement
This work is partly financed by the Bioenergy Innovation Centre (http://www.cenbio.no) in Norway. We also want to thank the three anonymous reviewers for their valuable comments.
Notes
References
About the Authors
Ottar Michelsen is research coordinator for the Industrial Ecology Programme at the Norwegian University of Science and Technology (NTNU) in Trondheim, Norway. Francesco Cherubini is a postdoctoral researcher at the Industrial Ecology Programme at NTNU. Anders Hammer Strømman is an associate professor at the Industrial Ecology Programme at NTNU.




