What Is Resource Consumption and How Can It Be Measured?
Theoretical Considerations
Summary
When analyzing the metabolism of our economy, the usual choice for a measure of resource consumption is the throughput of matter and energy. This, however, cannot be sufficient, since consumption by definition is always relating to the destruction or transformation, and hence a change in quality, not only in quantity, of material or energy flows. Here, an approach is presented that takes the entropy production associated with any process as a measure for the resource consumption of that process. Entropy production is thereby used to approximate the intuitive notion of consumption, which can best be described by the term loss of potential utility. This article delivers theoretical evidence for the validity of this choice, and a second article in a future issue will present an application taken from the metallurgical sector. The related concept of exergy analysis is discussed and compared against the entropy approach.
What Is Missing in Life Cycle Assessment, Materials Flow Analysis, and Other Tools
One of the main fields of research within industrial ecology is the measurement of the metabolism of regions, industrial systems, production processes, or product systems. Many tools have been developed to assess this metabolism, and most of them are applied very successfully. Still, what most of these tools do not (or only inadequately) address is the question of resource consumption. Resource consumption is undoubtedly an important aspect of the human society's metabolism, and a low resource consumption is also undoubtedly a key factor necessary for a sustainable way of living. Thus, the industrial ecologist's toolbox should contain an appropriate measure for resource consumption. The most promising approach to adequately describe resource consumption so far has been exergy analysis, an analysis of the available energy that is lost in the course of material and energy transformations. In a later section of this article, Related Concepts: Entropy Production and Exergy Loss, I will explain the exergy concept in more detail and show how it has been applied to measuring resource consumption. An approach that has gotten far less attention, which is also based on the second law of thermodynamics and is presented here as an alternative, is entropy production analysis (cf. Gößling 2001). I will show how it is related to exergy loss and how it avoids some of the conceptual shortcomings of the exergy approach. First, however, let us turn towards the importance of analyzing resource consumption.
Material and energy flows are at the heart of any analysis of the interactions between the anthroposphere and its environment. Different methodologies were developed to assess these flows and the ecological impacts associated with regions, processes, and products. The methodologies are different in their objective and their scope, but they share a common feature: they are built around the material and energy exchange with the environment in terms of a quantitative material flow model of the respective system or region. Some of the methods are product-oriented, such as life cycle assessment (LCA) and material input per unit of service (MIPS), while others focus on spatial or social units, including materials flow analysis (MFA), substance flow analysis (SFA), and ecological footprint (EF). The assessment in any of these cases is usually based on a detailed inventory, comprising all relevant flows of matter and energy exchanged with the environment. Which flows are considered relevant, however, and which ones are not, depends very much on the methodology used and the analyst applying it. LCA has generally the most complete list of flows.
If impacts on the environment are considered (as is the case with LCA), these impacts are calculated from the quantitative material flow model by applying impact categorization and impact factors to the flows. The results are then interpreted and can be compared to the results for other (equivalent) products and processes.1
The idea behind these approaches based on material flow models is that the total effect on the environment associated with a process can be approximated by measuring and analyzing the material and energy flows between the environment and the process or region. In other words, the industrial metabolism is measured and analyzed at its interface with the environment. At first glance, this approach seems to be valid. There are limitations, however, and most of the current methodologies have their blind spots. One of the limitations stems from the fact that the effect of resource exploitation and emissions on the environment is not always known in all cases or has not even been discovered yet, which is mainly due to the complexity of nature. Thus, some impacts might not be reflected in the pattern of categories and aggregation methods currently used. As research in this direction continues, more impacts will be discovered and integrated into the assessments. This is covered in the debate around life cycle impact assessment. Another shortcoming is the rather large number of categories and scores, which sometimes makes the process of decision making based on these results cumbersome. This circumstance can force decision makers to focus on a few impact categories while neglecting others. Solutions to this problem are discussed within the research community under the heading of aggregated sustainability indicators, and more specifically in terms of impact weighting (see e.g. Van der Voet et al. 2004 and 2008).
A shortcoming that might be even more problematic, however, is the fact that the above mentioned methodologies in their current form are mainly dealing with flows into and out of the technosphere, but pay little attention to the ways these flows are dealt with within the technosphere (von Gleich 2001). While it is true that many of the effects on nature can best be described by measuring the inputs and outputs, the actual solution to environmental problems is most often found within the technosphere (cf. von Gleich 2006). In the same way as the effects on the environment can only be quantified when there exist impact models, linking environmental impacts to matter (and energy) flows, we also need models describing the internal transformations of matter and energy to assess the efficiency (and sustainability) of the technosphere. Thus, if we want to avoid environmental problems, we should ask ourselves, “How can we better manage our internal flows of material and energy, and how can we make better use of them?” One of the immediate effects will then be the decrease of inputs from and outputs to the environment. The question remains, “How can we assess the changes in the matter and energy flows along their path through the technosphere?” This sets the stage for the development of a measure for the transformations of matter and energy taking place inside the technosphere (or inside a given process, respectively). Looking at the transformations of matter and energy will focus the attention of ecological assessment on the quality of flow transformations in addition to the exchanged quantities.
Throughput Versus Consumption
Coming back to the need for quantifying the transformations of matter and energy inside the technosphere, the term resource consumption already refers to such transformations, as can be seen, for example, from its dictionary definition:
“Consumption (noun): the utilization of economic goods in the satisfaction of wants or in the process of production resulting chiefly in their destruction, deterioration, or transformation.” (Merriam-Webster 2004)
For our purposes, I will try to further define resource consumption more precisely. Still, it is instructive to highlight the conceptual differences between resource use and resource consumption, two commonly used terms, which are sometimes confused.
Whenever a material or energy flow enters a production system, and is either transformed or not, and then either leaves the system or is stored within it, one can speak of the material or energy being used. The material or energy (i.e., the resource) was used to produce a product (or service). If this flow is transformed inside the system, either by changing its quantity or quality, then and only then, has it been (partly or wholly) consumed. Thus, use and consumption have related but different meanings. The term use is broader in its meaning, which implies a certain vagueness when one tries to measure resource use.2 As an example, consider the use of one ton of water in three different scenarios: (1) a hydro power station, (2) in the cooling tower of a fuel fired power plant, and (3) in a cleaning process. In each case, one could imagine the same amount of water entering the process and finally leaving it again. If one then asks for the respective resource use in each case, the answer would be the same in all three cases: one ton. Unfortunately, this answer, though formally correct, is totally blind to the actual quality of the process; specifically, the way in which the water is transformed. In the hydro power example, the potential energy of the water is decreased (and transformed into another energy form); in the cooling tower example, the heat contents of the water have changed; and in the cleaning process, the water is now really a complex mixture of many different materials. The water flowing from the hydro power station can still be used in many different processes, as it is chemically and physically indistinguishable from fresh water (except for its potential energy). This is not the case for the water output from the cooling plant and the cleaning process. Their potential utility has decreased (quite markedly in the cleaning example). If we asked for the consumption of water (instead of the use), the answer should really contain a reference to this decrease in potential utility. For the cleaning process, this would include the change in composition (taking the polluting substances into account), and for the hydro power example, this would include the generation of heat at ambient temperature (and thus requires the closing of the energy balance). A measure based on only mass, volume, or energy, however, will not be able to map this change correctly, as it will only measure the actual throughput of matter and energy. As argued in the next section, entropy production might serve as a proxy for measuring real consumption covering the physical aspect of transformations.
For completeness, there is another concept that needs to be mentioned in this context—resource depletion. This term usually refers to the decrease in quantity of a naturally occurring resource and is thus more limited in its scope than resource use or resource consumption. While use and consumption can apply to any form of material or energy, depletion applies only to the materials found in nature. This is not to say that the measurement of resource depletion is irrelevant! On the contrary, with diminishing reserves of fossil fuels and minerals, the depletion of these resources is an important factor for the sustainability of our economy. Measuring resource depletion, however, means focusing on the input side only, neglecting the transformations following the extraction. Thus, depletion needs to be accompanied by other measures that allow for a more detailed look into these transformations, yielding insight into the causes for depletion and starting points for optimization.
Established Measures for Resource Consumption
To motivate the introduction of an entropy-based measure for resource consumption, I would now like to discuss the way in which consumption is evaluated in the aforementioned methods (LCA, MIPS, MFA, SFA, and EF).
Evaluation in these methods occurs in different ways, but usually without reference to transformations of materials or energy. The LCA methodology speaks of the “depletion of abiotic (and biotic) resources” (Guinée 2002), which is to be distinguished from resource consumption, but is sometimes still used synonymously. While depletion can be interpreted as a decrease in the available amount, for the purpose of my argument, consumption is to be understood as decreasing the (overall) utility (see the section on resource consumption). In LCA studies, resource depletion and/or consumption is often analyzed only marginally. In the minimal case, only the amount of fossil fuels entering the life cycle of a product is considered. Indicators for the depletion of basically all natural resources are described in the literature (Guinée 2002); these are based on ultimate reserves and current deaccumulation rates. The numerical values of this indicator of course depend on our current knowledge of the reserves of the corresponding resource. In view of the approach taken in this article, the more prominent disadvantage of the reserves/deaccumulation approach is that it is only considering the extraction of resources from the environment (and correspondingly their input to the economic system), but neglecting the transformations these resources are subject to within the economic system.
Another approach to resource depletion is given by Müller-Wenk and operationalized in the Eco-Indicator 99 method (Müller-Wenk 1998). It is not based on the questions of how long resources last and how much they are decreased by current practices, but instead evaluates the effects on future generations by examining the future additional investment (in energy terms) due to the extraction of resources in the present time (Goedkoop and Spriensma 2001; Müller-Wenk 1998). This approach has been further refined by including the functionality of the resources, the ultimate quality limit to deliver this functionality, and the backup technology needed to deliver the functionality when the limit is crossed (Stewart and Weidema 2005). This approach does not take into account resource consumption in the sense of a transformation of resources. It is rather based on a resource's actual utility, in terms of the functions it performs (as opposed to the potential utility as explained below). A problem with this approach is that the actual utility of a resource is dependent on the current state of technology and knowledge, and thus the question of whether a resource can perform a certain function or not (in the latter case it would have reached its ultimate quality limit) can only be answered for the present time.
An assessment method within the LCA methodology, which is closer to the one presented here and is also based on the second law of thermodynamics, is exergy analysis. It can be seen as an extension to the conventional LCA methodology. This approach takes into account all resource consumption in the form of lost exergy (see e.g., Ayres et al. 2006; Cornelissen 1997; Dewulf et al. 2001; Dewulf and van Langenhove 2002a, 2002b; Finnveden and Östlund 1997). I will come back to this later in more detail.
In the MIPS approach, resource consumption (which is also referred to as resource use) is not distinguished from material movement (Ritthoff et al. 2002). Whenever a certain mass of material is moved in the course of a production or consumption process, it is considered to be a material input to this process and is counted as material consumption. This also applies to materials not entering the actual process (e.g., overburden from mining minerals), which are sometimes termed hidden flows. There is no reference made to the physical or chemical changes this material is subjected to in the process (apart from the fact that it is changing its positional parameters).
MFA treats resource consumption very much like the MIPS concept, but is usually applied on the national or regional level (as opposed to the product/service level). The parameter most closely related to resource consumption in this approach is direct material consumption (DMC), consisting of the domestic extraction of fossil, mineral, and biotic materials plus imported materials and minus the exports (Matthews et al. 2000). In essence, DMC describes the mass of the materials entering into and remaining in the economy/region (as stocks). Though the term consumption is used explicitly, no reference is made to the change in physical, chemical, economic, or other parameters describing the material flows. MFA also considers hidden flows, but they do not enter the definition of DMC.
SFA, which is sometimes seen as a submethod of MFA, focuses on one or a few substances and analyzes their mass flows into, through, and out of a regional or economic unit (for methodology and case studies, see Ayres and Simonis 1994). Transformations are covered to a certain extent when the flows of elements (e.g., N, P, Cu) are analyzed with regard to the chemical compounds they appear in. Consumption, however, is not the main aspect of SFA. It is mainly used for tracking environmentally or economically relevant substances in order to optimize their economic use or increase recycling rates. Consumption, as it is understood in this article, is not addressed; the focus is on throughput and stocks.
The EF approach determines the area necessary to sustainably support a given region (city, nation) by starting out with the national/regional consumption (production plus imports minus export of goods) (Wackernagel and Rees 1998). Here also, consumption is understood in the sense of materials entering the region, and no reference is made to their state or any respective transformations. Consumption is thus seen as the throughput of materials, and its relevance lies in the fact that these materials need to be created (and CO2 sequestered) by bioproductive land.
In conclusion, most of the standard tools in industrial ecology (IE) for measuring the metabolism of the technosphere refer to resource use, depletion, or consumption. Only LCA, however, has been equipped with a measure for assessing the actual consumption of resources (exergy loss); all others are only input- or throughput-oriented.
A More Detailed Look at Resource Consumption
Before introducing a measure for resource consumption, I would like to take a step back and give a working definition of the term resource. This will make the following discussion easier. We can distinguish at least three different definitions of resource: (1) the economist's view (any means that enter into the production of goods and services); (2) the physicist's view (energy, material, and information); and (3) the ecologist's view (naturally occurring components of the environment that can sustain or benefit organisms, populations, or communities within an ecosystem). While the ecologist's view includes only natural resources (those occurring in nature), the physicist's and the economist's view are more general. The physicist's view is the most general, but it lacks the common notion of usefulness and is therefore not precise enough. A set of materials or an amount of energy that could not be transformed into anything useful would not be considered a resource. In order to be as general as possible, without becoming too vague, I am choosing a definition that combines the generality of the physicist with the notion of purpose of the other two definitions:
“Resources are the flows and reservoirs of matter and energy that can sustain or benefit living systems.”
This definition includes natural and man-made objects, and it implies that a resource has utility. The utility can be defined more quantitatively by the notion and definition of potential utility, as described in the section on resource consumption and entropy production.
I said previously that resource consumption somehow refers to the transformation of material and energy flows. I would like to specify this reference in more detail. Since I am aiming at a thermodynamic description of resource consumption, much of the nomenclature is taken from this discipline. Since consumption is also a social construct, it is necessary to divert from a strictly thermodynamic description of consumption and to also use concepts from other disciplines to describe properties of the systems (like toxicity or structure). This also affects the meaning of such terms as transformation, property, process, and so on. Property in the following refers to all those characteristics of a material or energy flow that affect its potential uses, and transformations change these characteristics. Later I will narrow down the description of consumption to purely thermodynamic aspects, and the above terms will regain their usual meaning in thermodynamics.
The actual transformations associated with a process are of course manifold. Some materials are transformed into others, energy is freed up or dissipated, new substances are formed, matter is translocated, and so on. Many of these transformations can be described by physical, mostly thermodynamic, parameters that describe how the properties of the materials and energy flows change. Some transformations, however, create new properties in the affected material flows that have to be described by other disciplines, as for example toxicology. In the case of toxicity, it is not enough to know the physical and chemical state of the flows, one must also know the target system of these flows in order to assess their toxicity; different species and different ecosystems react very differently to the same substance! In any case, the existence of toxic properties in a material flow changes its usability in the sense that this flow has less potential applications. This has some bearing on the concept of consumption as explained below. Thus, for a complete assessment of consumption (beyond thermodynamics), toxicity has to be taken into account, even though it is not easily quantifiable. Apart from toxicity, there are other properties of material and energy flows that are difficult to quantify in simple physical terms, but which play a role in the assessment of consumption. A few examples are structure (e.g., nano-structured surface, fibrous material), odor, (dis)coloring, and so on. These properties can be changed, created, or destroyed in transformational processes, and this has an effect on the usability of the materials. All transformations thus somehow change the state of material and energy flows, such that their usefulness (for human purposes) changes also. I will come back to this shortly. For a list of some possible transformations of matter and energy flows and their associated property changes see table 1.
| Transformation | Properties subject to change |
|---|---|
| Translocation | Coordinates of objects, potential energy, exergy |
| Energy transformation | Exergy, entropy, heat content, enthalpy |
| Diffusion or concentration of matter | Concentration, density, exergy, entropy, chemical potential |
| Chemical reactions | Chemical potential, exergy, entropy, enthalpy, concentrations, toxicity, density, structure |
| Phase transitions | Enthalpy, chemical potential, entropy, exergy, heat transferred, structure of condensed matter |
- Note. There are many more conceivable transformations; this table is only for illustrative purposes.
When analyzing processes with respect to their energy and material flows, we have seen that one can focus on different properties. Analysis of the properties that are subject to conservation laws is usually highlighting the throughput of materials and energy and is thus quantitative. Examples are the above mentioned DMC (measured in mass units) (see, e.g., Matthews et al. 2000), the MIPS measure (Ritthoff et al. 2002), or conventional energy analysis. Some properties, however, can be created or destroyed in the process, such as entropy, exergy, structure, or toxicity. In the analysis of these properties, the focus is rather on the quality of the material/energy transformations and on how the process changes these properties. Entropy and exergy analysis are examples of analyzing the quality of the energy content of material and energy flows. Currently, most approaches to the assessment of the ecological impacts of processes use a throughput-oriented analysis. The qualitative aspect of material and energy transformations is very often neglected. Since consumption, as I will explain in the next section, is very much related to the qualitative transformation of flows, we need to find a valid measure for these transformations. Here, entropy production is proposed for this purpose.
Resource Consumption and Entropy Production
One of the qualitative aspects of matter and energy transformations is what I call the potential utility. The potential utility, P, can be thought of as the size of the set of all possible uses of a given amount of resources, see figure 1.
When resources are consumed in a process, the actual set of possible uses or applications decreases. The size of this set can be viewed as the potential utility of these resources.
When a physical resource is called to be consumed, its potential utility is decreased. As an example, let's take the production of paper from wood, water, and energy. At first sight, a stack of paper might seem much more useful than the materials and energy it was made from, but in fact, the potential utility has decreased. Starting with wood, water, and energy (plus the right tools), one could produce all sorts of products: a table plus lots of extra water, a wooden swimming basin (filled with water), cardboard boxes, a boat and some extra drinking water rations, or, of course, a stack of paper. Each of these products could in turn be used in many different fashions, while the stack of paper can only be used as such—a stack of paper. The set of possible uses has decreased by transforming the materials and energy into paper. The size of this set would then correspond to the potential utility of the resources. It is to be noted, that the actual usefulness of the raw materials, or products respectively, is very much dependent on the preferences, abilities, and circumstances of the respective user. A sheet of paper is much less useful to someone who cannot write. Nevertheless, the set of potential uses is much more clearly defined. It will probably not be possible to precisely determine the size of this set, since it is also dependent on human ingenuity and technical possibilities. Still, it should be obvious that by choosing one production path, a certain subset of potential uses will no longer be available. It would certainly be desirable to measure the actual loss of potential utility and use this as a measure for resource consumption. Unfortunately, this seems to be impossible, for the same reasons that limit our knowledge of the actual size of the set of potential uses. Nevertheless, there is something in the transformations that reflects the loss of potential utility on a physical level—the irreversibility of the process.
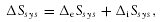 (1)
(1)Coming back to the discussion of consumption, potential utility, and irreversibility, I argue that the more irreversible a process, the more potential utility is lost. The basis for this argument is the fact that irreversibility describes for thermodynamic systems what the loss of potential utility describes for resources; namely the loss of potential pathways for development. Since entropy change measures irreversibility, I propose that the change in potential utility, ΔP (i.e., the consumption), that occurs inside a system, caused by an internal process or processes, is a negative and monotonically decreasing function of the entropy change, ΔS, caused by the process(es). Since the entropy change caused by these processes is equal to the internal entropy production, ΔiSsys, we found a physical approximation to the loss of potential utility—entropy production.
It is important to note that the entropy production always has to be analyzed for the whole system under investigation, including all flows into and out of the system. Entropy production does not measure the consumption of only one resource. Instead, all resources are considered together, and the overall consumption of resources is assessed. If, for example, we analyze a water reservoir with respect to its potential energy, we have to make sure we close the energy balance when the system undergoes transformations to account for all resource losses. If we lower the elevation of this reservoir by some means, we would of course lose potential energy stored in the water (or more precisely, stored in the gravitational field). Naively, one could argue that the entropy of the system would not change under this transformation, and thus entropy analysis would not detect any resource consumption. Nevertheless, if we close the energy balance, we must account for the lost potential energy somehow. It is either transferred to some other part of the system (e.g., by lifting a corresponding weight to a higher elevation), transformed into heat and transferred to the surroundings, or transformed into another form of energy (e.g., electricity). Whichever is the case, the analysis is only complete if all parts of the system are taken into account: the reservoir, the weight, and/or the interface between the system and the surroundings. Then the entropy production of the transformation tells us exactly how much of the resource, which is the potential energy in this case, has been lost. In the case of a lifted weight, there is no loss (apart from possible losses due to friction, which become visible in the balance as heat transfers to the surroundings). In the case of electricity production, the losses depend on the efficiency of the conversion. Again, these losses will appear at the interface of the system and the environment as heat flows (at probably ambient temperature). We can also see this from equation (1); the overall entropy change (of system and environment) caused by processes inside the system is ΔiSsys. The question that has to be answered correctly is: What is inside the system, or where do we draw the boundaries? If we define them in such a way that all losses occur outside the system, such as by defining the lifted weight to be part of the environment, we would of course have no irreversibilities inside the system, and all losses (due to friction when lifting the weight) would occur in the environment. Nevertheless, setting the system boundaries in such a way would be nonsensical when the goal of such an analysis is to measure resource consumption. When the system boundaries are set adequately, such that they include all relevant transformations of matter and energy, the term ΔiSsys in equation (1) correctly describes the irreversibility of the resource transformation.
We have seen how entropy change is related to resource consumption, but what about the absolute value of the entropy of a system? The absolute values of the entropy of a system has no direct meaning with regard to resource evaluation. Two identical water reservoirs at different elevations have the same entropy, even though the stored energy within, and thus their potential utility, is different. Thus entropy content cannot be used for evaluating the absolute utility of a resource; entropy production only accounts for changes in utility. Exergy is usually used for the purpose of estimating a resource's physical potential for doing useful work. The quantification of this potential, however, is based on the assumption of reversible processes. That is, it measures the physical work that could ideally, and reversibly, be extracted from this resource. Since real-life processes are always irreversible, and the minimum irreversibility is determined by many factors (including economic, social, technological, and environmental factors), the absolute exergy value is only of limited significance.
Related Concepts: Entropy Production and Exergy Loss
The relevance of entropy (production) in the discussion of resource consumption has a history that really dates back to the beginning of thermodynamics. After all, Sadi Carnot's main motivation was to find an expression for the efficiency of steam engines, specifically how much work could be produced from a given amount of steam (the resource in this case) (cf. Kondepudi and Prigogine 1998). Clausius later developed his ideas on the basis of the Carnot cycle and introduced the term entropy to describe the lost work potential (cf. Kondepudi and Prigogine 1998), which is a concept that is already close to resource consumption as understood in this article. Yet, to my knowledge, entropy analysis has not yet been used to assess the overall resource consumption of general industrial (or economic) processes. In the following paragraphs, I will focus on a few authors who have used second law analysis in an attempt to assess the economic consequences of resource consumption, the efficiency of industrial processes, or the environmental impacts of industrial processes.
Georgescu-Roegen argued, as far back as 1971, that the inevitable entropy increase described by the second law of thermodynamics will lead to the final stand-still of all economic activities3 (Georgescu-Roegen 1971). He claimed that the true resource is low entropy. Notwithstanding Georgescu-Roegen's enormous impact on economics, this interpretation is somewhat naive, because it is not the low entropy of a material flow that defines its resource character, as I have described here previously. His observation that economic activities produce entropy, that their inputs have a lower entropy than their outputs (if we include stocks and wastes in the outputs), and that the consumption of resources is thus linked to entropy production is without a doubt correct. However, he did not introduce entropy production as a measure for resource consumption. His argumentation was directed at qualitatively describing that economic activities lead to the inevitable degradation of matter and energy flows.
Bejan introduced entropy production (he uses the term entropy generation) as a means of thermodynamically assessing and optimizing the losses connected with heat transfer, fluid flow, and mass transfer irreversibilities (Bejan 1996). Bejan's entropy generation minimization (EGM) approach is basically a way to optimize the design of systems and to assess their efficiency. It requires knowledge of the geometrical setup of the machines and the thermodynamic properties of the internal flows of matter and energy inside the analyzed system (or model). Though it is based on the same principle, namely that entropy production means loss of physical usefulness, the EGM method is never explicitly applied to assess resource consumption, as I propose in this article, but it nevertheless is a method that optimizes resource consumption. It is rather obvious that increased thermodynamic efficiency (second law) of any device implies a reduction of resource consumption attributable to this device. Nevertheless, to fully assess the effects of optimizations at the process level, one has to carry out a life cycle-wide analysis. While Bejan's EGM method is aimed at improving the design of systems at a very detailed level, Georgescu-Roegen's argument is aimed at the consideration of entropy production at a very aggregate level; namely the whole economy. The approach presented here is situated in between these two extremes, at the level of production and consumption systems that span several plants and regions. It is not so much aiming at optimizing the design of specific systems, but at assessing the resource consumption accompanying the economic production and consumption of goods.
When talking about consumption and potential utility, another physical property of material and energy flows must be mentioned—exergy. The exergy concept was introduced in the 1960s by Rant (1956) and worked out in detail in the 1970s by Szargut and colleagues (1988) as a measure for the available energy incorporated in material and energy flows. Exergy analysis has been applied in a large number of case studies (see Göran Wall's web page for an extensive list, http://www.exergy.se) mainly as a tool for assessing the efficiency of industrial processes. Since exergy is defined as the useful energy stored in resources and intermediate process streams, an important application of exergy analysis is the (technical and monetary) optimization of energy conversion technologies; see for example the long standing series of conferences on Efficiency, Costs, Optimization, and Simulation of Energy Systems (ECOS) (see e.g., Ishida 2002). In this field, it has undoubtedly been most successful.
Exergy, or more precisely exergy loss, has also been proposed as a measure for resource consumption. Szargut, for example, has introduced exergy as a concept to account for ecological costs, defined as the cumulative depletion of nonrenewable exergy resources (for a recent article, see Szargut et al. 2002). He excludes renewable streams from the analysis, based on the assumption that the consumption of these does not come with a relevant cost to the environment. As long as the depletion rates stay below regeneration rates, this is of course justified. The exergy of natural resources, in this context, is understood as a common measure of their quality. For resources being consumed as energy carriers this bears significance, since their exergy content describes the potential useful energy that could be extracted from them. For non-energy resources, however, exergy as a quality measure is questionable, since their exergy content is probably never utilized. This is especially true for end-of-life materials that serve as secondary resources. For example, when comparing copper and iron scrap, their specific exergy differs by a factor of 3. But how does that correspond to their quality? One could argue that this difference in exergy value reflects the necessary expenditure when these metals are manufactured from the reference environment. But in the case that they are manufactured from scrap, what is the significance in the different exergy values? At least we can say this much: when the specific exergy of a material stream changes, it loses some of its quality in the sense that it is less available to further processing. If further processing is desired, new exergy has to be added to the process. For natural resources, a difference in specific exergy could thus mean a difference in energy requirements for further processing. Sulfidic copper ore (concentrate) is a good example; it has a fairly high specific exergy, such that the first pyrometallurgical step is almost energetically autonomous. After that, the remaining material flows (copper matte and slag) do not contain enough exergy to drive further refining steps on their own—exergy has to be added. Iron ore (containing Fe3O4 and Fe2O3), on the other hand, has a rather low specific exergy, and it needs large amounts of exergy for its metallurgy. In this respect, it makes sense to compare the two exergy values (of copper ore and iron ore) as they represent the dowry, provided by nature, of the two metal ores. But what is the relevance of this information? In the absence of alternative ores, this information is only purposeful in the sense that one should take good care of making use of the given exergy. This, again, is better done by analyzing the exergy losses within a process. In summary, the absolute values of exergy seem not to have an importance of their own. The interesting question is: How do we best manage the material and energy flows as to minimize exergy losses?
In this line of thought, Ayres has long been an advocate of using exergy loss as a measure for resource consumption (Ayres et al. 1996, 2006). His work builds mainly on the groundbreaking work by Szargut (Szargut et al. 1988). In addition, Ayres has proposed exergy as a measure for resource quality (Ayres 1998) and as a measure of pollution (Ayres and Martinás 1994). The latter notion is rejected by other authors (especially Koshland and Connelly, and Szargut).
Connelly and Koshland (2001a, 2001b) consider exergy loss of natural resources as the ultimate measure for assessing the burden placed on the environment. Their reasoning is not based on the notion of exergy as a measure of the value of a resource (they explicitly reject this interpretation), but rather on the fact that resource extraction is always accompanied by environmental damage.4 Thus, to minimize this damage, the extraction of resources must be minimized. They propose three strategies for optimizing industrial systems towards this goal: (1) efficiency, (2) recycling, and (3) use of renewable resources. Indicators for all three of these strategies are derived from analyzing the exergy flows within and through the respective system. In this manner, they identify exergy removal (i.e., the irreversible loss of exergy) as a measure for resource consumption. They also take into account the possibility of upgrading a resource by adding exergy to it, enabling the distinction between consumption and depletion; a resource that is consumed and then refilled with exergy is not depleted. Depletion only takes place when a resource's lost exergy is not renewed.5
In the framework of LCA, exergy loss has also been used as a measure for resource consumption, as in the work by Cornelissen (1997), Cornelissen and Hirs (2002), and Ayres and colleagues (1996). In these analyses, exergy loss or exergy destruction (or both) is used to describe the consumption of renewable and nonrenewable resources during the life cycle of a product. It is basically an extension of Szargut's notion of cumulative exergy consumption, which includes all exergy consumption from resource extraction to final product (Szargut and Morris 1987). Dewulf and colleagues have used the cumulative exergy approach to analyze different waste treatment systems (Dewulf et al. 2001; Dewulf and Van Langenhove 2002b) and to define an exergy-based measure of the sustainability of technology (Dewulf and Van Langenhove 2002a). The life cycle-exergy analysis can also be used to measure depletion by distinguishing between renewable and nonrenewable resources. If only nonrenewable inputs to the life cycle are counted, the results (in terms of lost exergy) reflect the depletion of natural resources.
As mentioned above, exergy is mostly used in the optimization of processes. The relation with entropy then invites the interchange of the two concepts. Baumgärtner and de Swaan Arons (2003) use exergy and entropy to describe the efficiency of regular industrial processes, a term describing a basic class of industrial processes. Exergy and entropy in this context are used in two ways: (1) they allow the calculation of the amount of waste (referred to as unwanted byproducts) and (2) how much of it is due to thermodynamic inefficiency (as opposed to necessity). Specific entropy and specific exergy in their approach are also used as defining factors for discerning between raw materials (low exergy, high entropy) and fuels (high exergy, low entropy), or, respectively, products (high exergy, low entropy) and wastes (low exergy, high entropy). By demanding that the specific entropy of raw materials be higher than that of products, and by further demanding that the fuel used in the production process be a material, they limit their analysis quite severely. Processes not included in their analysis are, for example, those which run on non-material energy sources (e.g., photovoltaic and wind energy), those in which the products have a higher specific entropy than the raw materials (e.g., processes of mixing or alloying), and those in which the products have a lower specific exergy than the raw materials (e.g., PVC production from ethylene or natural gas). Starting out from a description of regular industrial processes in terms of entropy, the authors then switch to exergy for their analysis of necessary and inefficiency-based waste production.6
Another application of the exergy concept is in finding a general measure for environmental impacts. Ayres (as explained here previously) was one of the first to introduce (specific) exergy as an indicator for the harmfulness of a substance released into the environment. In an effort to find a common measure for environmental impacts of emissions, Seager and Theis have instrumentalized the exergy concept by using the exergy of mixing as an indicator for potential environmental damage (Seager and Theis 2002). The exergy of mixing is the contribution to the exergy of an emission that arises from concentration differences between emitted flow and the corresponding part of the environment. It is numerically equivalent to the entropy of mixing. It should not be confused with the exergy (loss) of mixing that occurs within the process being studied and which is a cause for resource consumption as described in this article. Seager and Theis's reasoning is based on the fact that the exergy of mixing is high for chemical species with a low background in the environment (which are therefore alien to surrounding ecosystems). If the exergy of mixing is further integrated over time (to account for different lifetimes of species), this measure will also accentuate the potential problems arising from very persistent substances. In my view, the exergy of mixing can only be a first order approximation to environmental impacts, since it misses some important aspects like toxicity, bioaccumulation, reversibility of effects, and spatial scope of effects (global versus local). This approach is only marginally related to the entropy approach described in this article, since it deals with impact issues, which are located outside the process boundaries. Still, it nicely illustrates the scope of the concepts based on the second law of thermodynamics.
Summarizing, one can say that entropy and exergy are closely related, almost like two sides of the same coin. While exergy is defined so as to measure the availability of energy, entropy rather measures its nonavailability. The main conceptual difference, however, lies in the respective reference states. In general, energy is always only defined relative to an arbitrary constant. For exergy, this constant is chosen such that the system under investigation has zero exergy when it is in thermodynamic equilibrium with the so-called reference environment. This equilibrium state is also referred to as the dead state. For entropy, the dead state for each substance (and for each system) is the state at absolute zero temperature. This reference state, however, is not arbitrary. It is rather an empirical fact that the entropy of a system is zero at absolute zero temperature. Following from the discussion above and the last point, in my view, the definition of exergy brings with it four problematic conceptual properties which limit the explanative power of the concept and which made me choose entropy instead as a measure for resource consumption:
- 1
The reference environment is a basic prerequisite for the interpretation of exergy as available energy. The reference environment is usually chosen so as to approximate the actual natural environment as an end point for all transformations of materials and energy. The assumption that the system under investigation can be brought into equilibrium with the reference environment assumes that the reference environment is itself in equilibrium. This is not the case for the natural environment, which makes the choice for a specific reference environment highly questionable. The natural environment is rather to be described as being far from equilibrium with large spatial and temporal variations of thermodynamic properties. This would (at least) call for an adjustment of the exergy reference environment with regard to space and time, which is usually not done in exergy analysis. As an extreme example, the exergy calculations for processes in interstellar space would have to be done on the basis of a reference environment very different from the one currently adopted. As a less extreme example, consider the reference state for water. In its fluid state, there exist several forms to be found in the natural environment (which should be the basis for a reference environment): sweet water in lakes and rivers, ground water, and sea water. Each form is in constant transformation into one of the other forms and into gaseous water in the atmosphere. This makes the definition of a reference state for liquid water highly ambiguous. These ambiguities can lead to definitions of reference environments that make the interpretation of exergy as the maximum useful work obtainable from a flow no longer valid. See for example Dincer and Cengel (2001) for a discussion of different choices of reference environments.
- 2
The interpretation of exergy as available energy suggests that the exergy of material and energy flows is, in principle, available to be used by humans. This could only be true if the calculation of exergy would take into account the actual environment the potential user of the material and energy flows is operating in, which is usually not the case (see previous point). In addition, this interpretation quietly assumes that the limit for the technical utilization for resource flows is reversible processes, which is far from the truth and therefore highly theoretical. It has to be noted, though, that this goes for any attempt to evaluate the thermodynamic perfection of industrial processes without taking into account the intrinsically irreversible nature of all processes. Thus, exergy is a measure for the theoretically available energy of matter and energy flows. How much of this energy could practically be used depends very much on the technical, spatial, and temporal limitations of the process in question. Much scientific work has been done on this subject in the fields of entropy generation minimization (see e.g., Bejan 1996) and finite time thermodynamics (see e.g., Andresen 1984), which both approach this problem from a process perspective, not from a substance perspective. In conclusion, the interpretation of exergy as available energy can be quite misleading, because the availability of a substance depends very much on the context of its uses and the available processes.
- 3
On another note, the interpretation of exergy as a measure of a substance's energetic value or the inherent driving force only makes sense for substances that are somehow used to drive a process. In view of the above example of the exergy of pure copper and iron, I conclude that the absolute exergy value of a substance only has relevance for substances that are used energetically (which includes, for example, driving a chemical reaction). The meaning of their exergy content is then the amount of useful energy that can be harvested from these substances. For substances that are used nonenergetically (e.g., by providing structure or by conducting heat and electricity), their exergy content has no practical relevance. For substances or materials that are used in a cascading way, the relevance of their exergy content might, however, come into play in a later use phase.
- 4
In most exergy analyses, it is not the absolute exergy content of material and energy flows that is analyzed, but rather the differences between input and output. The exergy loss is then interpreted as a measure for the actual resource consumption. Under these circumstances, the definition of a reference environment becomes irrelevant, since it cancels out in the calculation. On the other hand, the loss of exergy is directly proportional to the accompanying production of entropy (in many cases the Gouy-Stodola equation provides the correct relation: ΔS·T0 = ΔEx). Thus, by using entropy production instead of exergy loss, the definition of a reference environment, with all the mentioned difficulties, can be avoided.
Conclusion
In this article, I have argued that physical resource consumption (in the meaning of decreasing potential utility) can be approximated by measuring entropy production. It was shown that entropy production measures the actual transformations of matter and energy for the most relevant transformation types occurring in industrial processes. Furthermore, as a measure for resource consumption, it is superior to throughput-oriented measures (energy, mass), as it combines matter and energy transformations and focuses also on the qualitative, not only on the quantitative, changes. In addition, analyzing entropy production shifts the focus towards the internal flows and transformations within the technosphere, enhancing the knowledge of how resource consumption comes about and how it can be minimized.
As a general rule, entropy analysis always reveals the thermodynamic inefficiencies of the process under investigation, regardless of the nature of the inefficiency. Combined with a technological (and possibly an economic) feasibility study, it has to be decided, whether these inefficiencies can be eliminated and at what cost. Used in this manner, entropy analysis serves another purpose, despite being a measure for resource use. It locates theoretical starting points for optimization.
Acknowledgments
The author is grateful for the critical remarks of three anonymous reviewers, which helped to sharpen and clarify the ideas presented. This work was supported in parts by the German Federal Environmental Foundation (DBU).
Notes
References
About the Author
Stefan Gößling-Reisemann is an assistant professor at the University of Bremen, Faculty of Production Engineering, in Bremen, Germany.




