Systemic Administration of Arecoline Reduces Ethanol-Induced Sleeping Through Activation of Central Muscarinic Receptor in Mice
Abstract
Background: Epidemiological evidence of co-use of alcohol and areca nuts suggests a potential central interaction between arecoline, a major alkaloid of areca and a muscarinic receptor agonist, and ethanol. Moreover, the central cholinergic system plays an important role in the depressant action of ethanol and barbiturates. The purpose of this study was to investigate the effects of arecoline on pentobarbital- and ethanol-induced hypnosis in mice.
Methods: Male ICR mice were tested for locomotor activity following acute systemic administration of ethanol alone, arecoline alone, or ethanol plus arecoline. For the loss of the righting reflex (LORR) induced by pentobarbital and ethanol, sleep latency and sleeping duration were evaluated in mice treated with arecoline alone or the combination of arecoline and scopolamine or methscopolamine.
Results: Ethanol (1.0 to 3.0 g/kg, i.p.) reduced locomotor activity significantly and a declining trend was observed after treatment with arecoline (0.25 to 1.0 mg/kg, i.p.), but there were no synergistic effects of ethanol and arecoline on locomotor activity. The experiments on LORR demonstrated that arecoline (0.125 to 1.0 mg/kg, s.c.) shortened the duration of sleeping induced by ethanol (4.0 g/kg, i.p.), but not pentobarbital (45 mg/kg, i.p.). In addition, alterations of sleep latency were not obvious in both pentobarbital- and ethanol-induced LORR. Statistical analyses revealed that scopolamine (centrally acting), but not methscopolamine (peripherally acting), could antagonize the effect of arecoline on the duration of ethanol-induced LORR in mice.
Conclusions: These results suggest that central muscarinic receptor is a pharmacological target for the action of arecoline to modulate ethanol-induced hypnosis.
Arecoline (methyl-1, 2, 5, 6-tetrahydro-1-methyl nicotinate) is a major alkaloid of areca, which can activate cholinergic neurotransmission (Cox et al., 2004; Soncrant et al., 1989). A large proportion of the population in Southern China, Taiwan, Tibet, and Southeast Asia chew areca nuts to produce psychoactive and muscarinic effects, including mild euphoria, relaxation, improved concentration, enhanced postprandial satisfaction, vertigo, salivation, miosis, tremor, and bradycardia, which are considered to be largely attributable to the predominant alkaloid arecoline stimulating the sympathetic, parasympathetic, and central nervous systems (Chiou and Kuo, 2008; Ghelardini et al., 2001; Gilani et al., 2004; Giri et al., 2006; Molinengo et al., 1988; Pradhan and Dutta, 1970). Several lines of evidence have shown that both ethanol and areca nuts are among the top 4 most commonly used substances worldwide (Chu, 2001; Norton, 1998; Winstock, 2002). Investigations in humans have reported that more than half of areca nut chewers have an alcohol-drinking habit (Chen et al., 2001; Zhang et al., 2008). Although ethanol and arecoline are well known to have dominant psychoactive effects, the effects of their combination upon the brain have not been documented.
Medium to large doses of ethanol, in view of its depressant effect on the central nervous system, can induce hypolocomotion and sedation/hypnosis in mice and rats (Correa et al., 2001; Popova and Ivanova, 2002). It is a well-known fact that multiple neurotransmitters and receptors participate in this depression. Ethanol-induced hypnosis is associated with its pharmacological profiles to enhance the inhibitory effects of the γ-aminobutyric acid (GABAergic) system (Ariwodola and Weiner, 2004; Wallner et al., 2003) and alleviate the excitatory activity of N-methyl-d-aspartate receptor (Honse et al., 2004; Sato et al., 2006). Naloxone, an opioid receptor antagonist, can decrease, whereas haloperidol, dopamine D2 receptor antagonist, can increase the hypnotic effects of ethanol (Cohen et al., 1997; Devoto et al., 1994). Moreover, previous studies have indicated that cholinergic neurotransmission plays a role in physical dependence, preference, behavioral sensitization, and sleeping induced by ethanol (Imperato et al., 1998; Jamal et al., 2007; Larsson and Engel, 2004;Nordberg and Wahlström, 1982; Rothberg et al., 1993; Stancampiano et al., 2004), suggesting the possibility of an interaction between ethanol and arecoline in the central nervous system.
Acute administration of pentobarbital can result in sedation, anticonvulsant activity and sleeping or anesthesia (Dalal et al., 2006; Nelson et al., 2002; Uribe-Escamilla et al., 2007). Similar to ethanol, pentobarbital-induced sleeping behavior appears as a loss of the righting reflex (LORR) in mice (Kralic et al., 2003). As far as we are aware, a major molecular target of pentobarbital action is thought to be the GABAA receptor complex (Kralic et al., 2003; Nelson et al., 2002). In addition, the central cholinergic system has been implicated in modulating the depressant action of pentobarbital. In vivo studies indicate that acute treatment with pentobarbital can inhibit Ach release and decrease muscarinic receptor binding in the frontal cortex, striatum and hippocampus of rats and mice (Inoue et al., 1992; Kikuchi et al., 1997; Sato et al., 1996). Intra-septal microinjection of arecoline is reported to significantly shorten the duration of pentobarbital-induced LORR (Zucker et al., 1987). Presumably systemic administration of arecoline may or may not affect the hypnotic effect of pentobarbital.
Our present study was designed to investigate the interaction of arecoline and ethanol on locomotor activity in mice. The LORR mouse model was used to compare differential effects of systemic treatment with arecoline on ethanol- and pentobarbital-induced hypnotic behaviors. Furthermore, 2 anti-muscarinic agents scopolamine (centrally acting) and methscopolamine (peripherally acting) were used to determine the possible mechanisms underlying the modulation of arecoline on the alteration of LORR induced by ethanol or pentobarbital in mice.
Material and Methods
Animals
Male ICR mice, initially weighing 18 to 22 g, were purchased from the Department of Laboratory Animal Science, Peking University Health Science Center (Beijing, China). The animals were housed in groups of 5 to 6 in clear plastic cages with free access to water and food in a room that was kept at a constant temperature (22 ± 2°C) and humidity (50 ± 20%) and on a 12/12 h light/dark lighting schedule (light on at 08:00 h). Mice were acclimatized to laboratory conditions for at least 3 days before the test. Experiments were performed during the daytime, and each animal was tested only once. All experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals (NIH publications no. 80-23, revised 1996). The experimental procedures were approved by the local Committee on Animal Care and Use.
Drugs
Arecoline hydrobromide, scopolamine hydrobromide, and methscopolamine bromide were purchased from Sigma Chemicals (St Louis, MO). Sodium pentobarbital was purchased from Fushan Manufactory of Chemical Technology (Fushan City, China). These drugs were dissolved in saline (0.9% NaCl) and kept in light-resistant containers. Ethanol (≥99.7% w/v; Beijing Beihua Fine Chemicals Co., Ltd., Beijing, China) was diluted by saline. Arecoline, pentobarbital, and ethanol were given i.p., while scopolamine and methscopolamine were administered s.c. All drugs were injected in a volume of 0.1 ml per 10 g of body weight, except that ethanol was injected in a volume of 0.2 ml per 10 g of body weight. These solutions were prepared prior to every experimental trial.
Locomotor Activity Test
Locomotor activity was measured by an ambulometer with 5 activity chambers (JZZ98, Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing, China). Each activity chamber of 26 × 14 × 15 cm (length × width × height) consisted of white opaque Perspex walls, a transparent Perspex lid and a floor. The floors were composed of 25 parallel copper bars with a fixed interval of 1 cm between the adjacent bars. The odd bars were grounded and the even bars were active and connected with micro-amper energy sources. The paws of the mice contacted or disconnected the active bars producing random configurations that were converted into pulses. The pulses, which were proportional to the locomotor activity of the mice, were recorded as the cumulative total counts of locomotor activity for a predetermined period of minutes (Li et al., 2004).
Before drug administration, the locomotor activity of each animal was measured for 10 minutes as the baseline. To assess effects of ethanol or arecoline on locomotor activity, animals were treated with saline, ethanol (1.0, 2.0, and 3.0 g/kg, i.p.) or arecoline (0.25, 0.5, and 1.0 mg/kg, i.p.); to examine effects of arecoline on ethanol-induced hypoactivity, animals were treated with arecoline (0.25, 0.5, and 1.0 mg/kg, i.p.) 5 minutes prior to ethanol injection (2.0 g/kg, i.p.). After the last injection, animals were immediately put into the same test chambers to record their locomotor activity every 10 minutes for 90 minutes.
Loss of the Righting Reflex Test
Animals were treated with saline or arecoline (0.125, 0.25, 0.5, and 1.0 mg/kg, i.p.) 5 minutes prior to the injection of pentobarbital (45 mg/kg, i.p.) or ethanol (4.0 g/kg, i.p.) and the latency and duration of LORR (sleep time) were measured. Latency to LORR was calculated as the time between ethanol or pentobarbital injection and LORR. LORR was defined as the inability of an animal to right itself within 30 s. Upon LORR, animals were isolated in small individual clear plastic cages (28 × 18 × 12 cm) and the time to recover righting reflex was recorded. Recovery of the righting reflex was defined as the ability of the animal to re-right itself 3 times within 60 seconds after being placed on its back. Duration of LORR was evaluated by measuring the time interval between loss and recovery of the righting reflex (Wu et al., 2000).
Muscarinic receptor antagonists (scopolamine and methscopolamine) were further tested to examine their effects on ethanol-induced LORR. In the interaction study to confirm muscarinic receptor involvement, animals received saline (s.c.), scopolamine (0.125, 0.25, and 0.5 mg/kg, s.c.) or methscopolamine (0.25 and 1.0 mg/kg, s.c.) 15 minutes before i.p. injection of saline (i.p.) or arecoline (0.5 mg/kg, i.p.). Ethanol (4.0 g/kg, i.p.) was administered 5 minutes after the second administration. Then the duration of LORR induced by ethanol was recorded.
Statistical Analysis
The data are expressed as mean ± SEM. In 1-3, the locomotor activity was analyzed using two-factor repeated measures analysis of variance (ANOVA) for time block and treatment. In the others, statistical analyses were performed by one-way ANOVA. The LSD multiple comparisons were used in all experiments at a minimum significance level of p < 0.05.
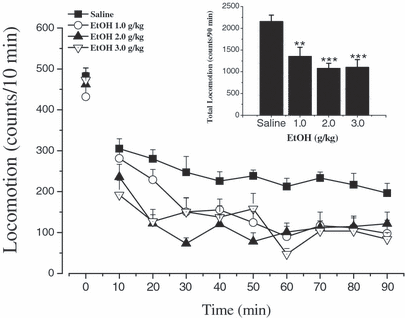
Acute administration of ethanol reduced locomotor activity in mice. Mice were put into the test chambers to measure the baseline of their locomotor activity for 10 minutes. Then they were given saline or ethanol (1.0, 2.0, and 3.0 g/kg, i.p.) and the locomotor activity was recorded immediately every 10 minutes for 90 minutes. Data are expressed as mean ± SEM (n = 11 to 12 per group). Inset shows the mean ± SEM of cumulative locomotor activity; **p < 0.01 and ***p < 0.001 compared with saline group. EtOH = ethanol.
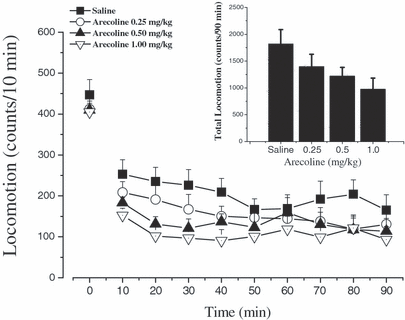
Effects of acute administration of arecoline on locomotor activity in mice. Mice were put into the test chambers to measure the baseline of their locomotor activity for 10 minutes. Then mice received 2 injections at 5-minute intervals, the first one was saline or arecoline (0.25, 0.5, and 1.0 mg/kg, i.p.) and the second was saline. Locomotor activity was monitored immediately every 10 minutes for 90 minutes after the second injection. Data are expressed as mean ± SEM (n = 12 per group). Inset shows the mean ± SEM of cumulative locomotor activity.
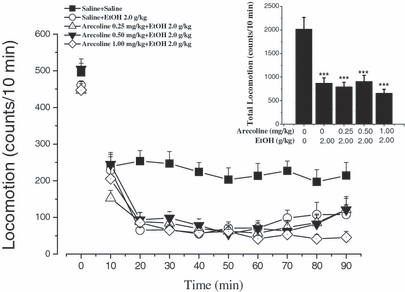
Effects of arecoline on ethanol-induced locomotor suppression in mice. Mice were put into the test chambers to measure the baseline of their locomotor activity for 10 minutes. Then the mice were given saline or arecoline (0.25, 0.5, and 1.0 mg/kg), 5 minutes later, they were given saline or ethanol 2.0 g/kg and locomotor activity was recorded immediately every 10 minutes for 90 minutes. Data are expressed as mean ± SEM (n = 12 per group). Inset shows the mean ± SEM of cumulative locomotor activity; ***p < 0.001 compared with saline + saline group. EtOH = ethanol.
Results
Effects of Systemic Administration of Ethanol on Locomotor Activity in Mice
Inset of Fig. 1 shows that the effects of a series of ethanol doses on total locomotor activity in mice. One-way ANOVA [F(3,43) = 9.456, p < 0.001] and post hoc LSD test revealed that systemic administration of ethanol (1.0, 2.0, 3.0 g/kg) reduced locomotor activity, but there were no significant differences in baseline activity between mice before ethanol administration [F(3,43) = 0.685, p > 0.05]. Further, two-factor repeated measures ANOVA was used to analyze time-course effects of ethanol on locomotor activity in mice, which demonstrated that there were significant main effects of ethanol [F(treatment)(3,43) = 9.456, p < 0.001], time effect [F(time)(8,3447) = 16.787, p < 0.001], and an ethanol × time interaction [F(treatment × time)(24,344) = 1.880, p < 0.01], indicating that the effect of ethanol on locomotor activity was dose- and time-dependent. The suppression of locomotor activity in all the ethanol-treated groups persisted for at least 90 minutes (Fig. 1), but we did not observe that any mouse was in LORR following systemic administration of ethanol at the doses of 1.0 to 3.0 g/kg.
Effects of Systemic Administration of Arecoline on Locomotor Activity in Mice
To characterize overall effects of systemic administration of arecoline, we examined the responsiveness of mice to different doses of arecoline (0.25, 0.5, and 1.0 mg/kg, i.p.) in the locomotor activity test. As shown in Fig. 2, the trend in suppression of locomotor activity was observed after i.p. injection of arecoline, but two-factor repeated measures ANOVA demonstrated that the main effect of treatment with arecoline was statistically insignificant [F(treatment)(3,44) = 2.612, p > 0.05] and there was no significant interaction of arecoline treatment and time of drug action [F(treatment × time)(24,352) = 0.755, p > 0.05]. One-way ANOVA was used to further analyze the total locomotor activity of 90 minutes, revealing that the effect of systemic administration of arecoline on locomotor activity was still not statistically obvious [F(3,44) = 2.612, p > 0.05] (inset of Fig. 2). Similar to Fig. 1, one-way ANOVA excluded any basal differences in locomotor activity between the groups before drug administration [F(3,44) = 0.424, p > 0.05]. The mice of all arecoline groups did not show any cholinergic responses, such as sialorrhea or tremor. There were some loose stools occasionally in the mice systemically treated with 1.0 mg/kg arecoline. During monitoring locomotor activity, 0.25 to 1.0 mg/kg of arecoline did not cause LORR in mice.
Effects of Systemic Administration Arecoline on Ethanol-Induced Hypoactivity in Mice
Figure 3 shows the effects of arecoline on the locomotor suppression following a 2.0 g/kg dose of ethanol. Two-factor repeated measures ANOVA and post hoc LSD test demonstrated that treatment with ethanol 2.0 g/kg produced obvious main effects of inhibiting locomotor activity in mice [F(treatment)(4,55) = 13.907, p < 0.001] and there was a significant interaction between ethanol treatment and time [F(treatment × time)(32,440) = 2.137, p < 0.001]. This result was in accord with Fig. 1. However, the post hoc LSD test revealed that pretreatment with arecoline (0.25, 0.5, 1.0 mg/kg, i.p.) had no significant effect on the locomotor suppression induced by ethanol when compared with saline + ethanol (2.0 g/kg) group (p > 0.05).
Effects of Systemic Administration of Arecoline on Pentobarbital- or Ethanol-Induced LORR in Mice
Pentobarbital and ethanol both can induce LORR. We investigated the effect of arecoline on pentobarbital- and ethanol-induced LORR in mice. As shown in Fig. 4, arecoline, at the doses used, did not affect the latency [F(4,53) = 0.989, p > 0.05] or duration [F(4,53) = 0.888, p > 0.05] of LORR elicited by pentobarbital (45 mg/kg). However, one-way ANOVA and post hoc LSD test revealed that systemic administration of arecoline (0.25, 0.5, 1.0 mg/kg) significantly shortened the duration of LORR in mice treated with ethanol (4.0 g/kg) [F(4,52) = 2.588, p < 0.05], while no noticeable difference was found in the latency to LORR [F(4,52) = 0.523, p > 0.05].
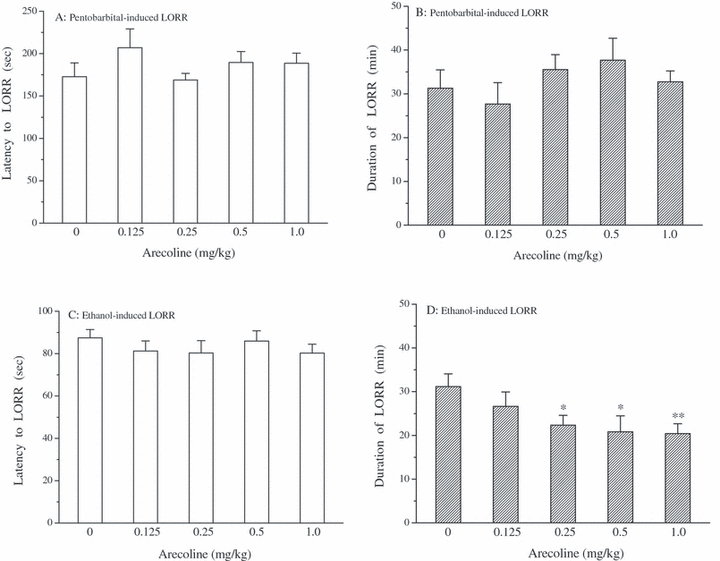
Effects of arecoline on pentobarbital- and ethanol-induced LORR in mice. Mice were treated with saline or arecoline (0.125, 0.25, 0.5, and 1.0 mg/kg, i.p.) 5 minutes prior to receiving pentobarbital (45 mg/kg, i.p.) or ethanol (4.0 g/kg, i.p.). Then the latency and duration of LORR induced by pentobarbital (upper panel; A: latency and B: duration) or ethanol (lower panel; C: latency and D: duration) was recorded. Data are expressed as mean ± SEM (n = 11 to 12 per group); *p < 0.05 and **p < 0.01 compared with saline (0 mg/kg) group.
Effects of Arecoline Plus Scopolamine or Methscopolamine on the Duration of Ethanol-Induced LORR in Mice
Scopolamine and methscopolamine can antagonize muscarinic neurotransmission. Pretreatment with scopolamine or methscopolamine alone were tested to determine the effects on the duration of ethanol-induced LORR. Figure 5A shows that systemic treatment with scopolamine (0.125, 0.25, 0.5 mg/kg, s.c.) did not affect the duration of ethanol-induced LORR in mice [F(3,41) = 1.291, p > 0.05]. Similarly, as shown in Fig. 5B, there was no significant difference in the effect of methscopolamine (0.25, 1.0 mg/kg, s.c.) on the duration of LORR induced by ethanol in mice [F(2,31) = 1.326, p > 0.05].
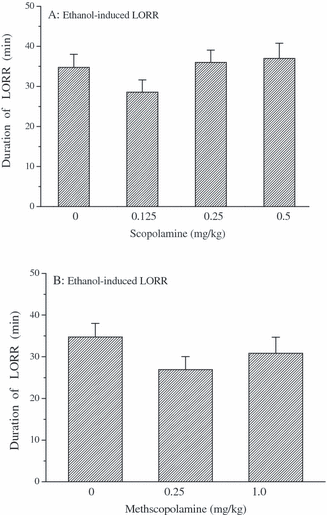
Effects of scopolamine (A) or methscopolamine (B) on the duration of ethanol-induced LORR in mice. Mice were treated with saline (s.c.) or scopolamine (0.125, 0.25, and 0.5 mg/kg, s.c.) or methscopolamine (0.25 and 1.0 mg/kg, s.c.) 15 minutes before i.p. injection of saline. Ethanol (4.0 g/kg, i.p.) was administered 5 minutes after the second administration. Then the duration of LORR induced by ethanol was recorded. Data are expressed as mean ± SEM (n = 11 to 12 per group).
As shown in Fig. 6, systemic administration of arecoline (0.5 mg/kg) alone could significantly reduce the duration of the ethanol-induced LORR, previously shown in Fig. 4D. The centrally acting muscarinic antagonist scopolamine (0.125, 0.25, and 0.5 mg/kg) dose-dependently reversed the effect of arecoline on the duration of ethanol-induced LORR [F(4,53) = 2.651, p < 0.05] (Fig. 6A). Moreover, one-way ANOVA demonstrated that there were differences for all the groups in Fig. 6B [F(3,64) = 3.409, p < 0.05], but post hoc LSD test revealed that the peripherally acting muscarinic antagonist methscopolamine (0.25, 1.0 mg/kg, s.c.) did not affect the effect of arecoline on the ethanol-induced LORR when compared with saline + arecoline (0.5 mg/kg) group (p > 0.05).
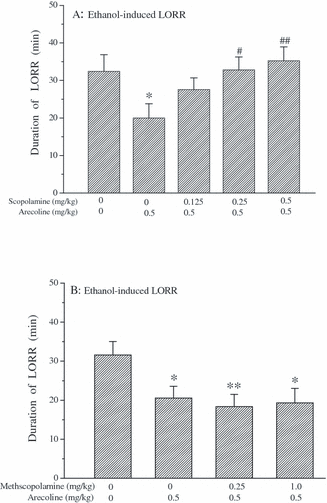
Effects of arecoline plus scopolamine (A) or methscopolamine (B) on the duration of ethanol-induced LORR in mice. Mice were treated with saline (s.c.) or scopolamine (0.125, 0.25, and 0.5 mg/kg, s.c.) or methscopolamine (0.25 and 1.0 mg/kg, s.c.) 15 minutes before i.p. injection of saline or arecoline (0.5 mg/kg). Ethanol (4.0 g/kg, i.p.) was administered 5 minutes after the second administration. Then the duration of LORR induced by ethanol was recorded. Data are expressed as mean ± SEM (n = 11 to 12 per group in A and n = 16 to 18 per group in B); *p < 0.05 and **p < 0.01 compared with saline + saline group; and #p < 0.05 and ##p < 0.01 compared with saline + arecoline group.
Discussion
Ethanol and arecoline have been shown to produce various psychoactive effects. One interesting belief is that there may be an interaction of ethanol and arecoline on the central nervous system. Therefore, the main purpose of this study was to investigate a possible influence of arecoline on the acute sedation and hypnosis mediated by ethanol. This was achieved by examining the effect of arecoline on hypolocomotion and LORR induced by ethanol in mice. This study is, to our knowledge, the first to focus on the issue of co-abusing ethanol and areca nuts through characterizing the pharmacological profiles of their interaction in mice.
In some rodents, the biphasic effects of ethanol on locomotor activity are well established. Generally, low-to-moderate doses of ethanol may produce locomotor stimulant effects resulting from the enhancement of dopaminergic neurotransmission, which seems to be of great relevance to its positive reinforcing properties (de Araujo et al., 2009; Boerngen-Lacerda and Souza-Formigoni, 2000; Wise and Bozarth, 1987), whereas exposure to higher ethanol doses can result in the inhibition of locomotor activity and LORR. The depressant action of ethanol on the central nervous system has been thought to be involved in its negative reinforcing properties related to alleviating some negative events (e.g., anxiety or stress) and/or preventing ethanol withdrawal symptoms (Colombo et al., 1995; Eckardt et al., 1998). In this study, however, we examined the effects of a wide range of sub-hypnotic doses of ethanol (1.0 to 3.0 g/kg, i.p.) on locomotor activity and found only its depression, but not activation, in ICR mice. The previous studies have demonstrated the variation of the mouse strains in responses to ethanol. In NMRI mice, ethanol increased locomotor activity at a low dose (2.25 g/kg, i.p.) and decreased at a higher dose (4.5 g/kg, i.p.), while both 2.25 and 4.5 g/kg of ethanol produced only locomotor depressant effects in C57BL and CBA mice (Liljequist and Karcz-Kubicha, 1993). Dudek and Phillips (1990) investigated different aspects of behavioral action of ethanol in a battery of 7 inbred strains and in the selectively bred Long-Sleep (LS) and Short-Sleep (SS) mice, their data showed that exposure to ethanol at doses ranging from 1.0 to 3.0 g/kg (i.p.) can cause dual reactivity of locomotor stimulation and depression in AU/SsAbg, MOLD/RkAbg, SK/RkAbg, DBA/2Abg, BALB/cAbg, BALB/cByAbg, LS, and SS mice. However, only locomotor depressant activity was observed in C57BL/6Abg treated with either low or high doses. Corollary, the biphasic effects of ethanol on locomotor activity are strongly strain-dependent and related to genetic background of animals, which provides an explanation for the observed inhibitory, but not excitatory, effect of ethanol in ICR mice.
In this study, systemic administration with arecoline at the doses of 0.25 to 1.0 mg/kg showed a dose-related tendency to reduce locomotor activity in ICR mice. The effects resemble the results reported by Cassone and colleagues (1990). There are however some inconsistencies regarding the effects of arecoline on locomotor activity in rodents. One investigation has reported that arecoline at the low doses ranging from 0.25 to 1.0 mg/kg can lead to slight enhancement in locomotor activity in rats (Pradhan and Dutta, 1970), and another has demonstrated that 0.64 to 2.5 mg/kg of arecoline dose-dependently decreased, whereas 5.0 to 20.0 mg/kg increased locomotor activity in C57BL/6Nnia mice (Retz et al., 1987). Although the LD50 for an acute administration (i.p.) in mice is between 84 and 68 mg/kg (Molinengo and Orsetti, 1986), more than 1.0 mg/kg of arecoline was not used in this study. In our primary experiments, severe cholinergic responses, including diarrhea, sialorrhea, and tremor have been observed in mice systemically treated with above 1.0 mg/kg of arecoline. Taken together, similar to ethanol, systemic treatment with low doses of arecoline alone can inhibit locomotor activity in mice. However, further investigation in this study has indicated that co-administration of ethanol and arecoline could not significantly cause synergistic inhibitory effects on locomotor activity in mice, suggesting the involvement of different mechanisms underlying their suppression of locomotor activity.
Several lines of evidence indicate that ethanol and pentobarbital appear to share some pharmacological effects that are mediated through GABAA receptors (Mehta and Ticku, 1999). At the behavioral level, the GABAA receptor antagonists, pentylenetetrazol, bicuculline, and picrotoxin, have been reported to reduce the duration of ethanol-induced sleep in rats and mice (Beleslin et al., 1997; Liljequist and Engel, 1982). Similarly, muscimol acts as a GABAA receptor agonist to enhance, whereas bicuculline and picrotoxin as GABAA receptor antagonists to reverse pentobarbital sleeping time in mice (Sivam et al., 1982). Electrophysiologically, ethanol produces allosteric modulation of GABAA receptors to potentiate GABA-mediated Cl− current (Koob, 2004). Pentobarbital can directly activate the GABAA receptor and increase the opening duration and number of Cl− channels, which is independent of the presence of GABA (Korpi et al., 2002). Accordingly, these findings implicate the GABAA receptor complex in the same pharmacological effects of ethanol and pentobarbital on the central nervous system. For a better understanding of mechanisms by which arecoline would alter ethanol-induced hypnosis, the effects of arecoline on pentobarbital-induced hypnosis were also investigated. The results in this study have indicated that, in ICR mice, arecoline at the doses of 0.125, 0.25, 0.5, and 1.0 mg/kg reduces the duration of behavioral sleeping induced by ethanol (4 g/kg, i.p.), but not pentobarbital (45 mg/kg, i.p.). Two aspects of the possible explanations should be considered for differential effects of arecoline on ethanol- and pentobarbital-induced hypnosis in mice. First, differences in the efficacy of arecoline are related to the dose and the route of drug administration. As mentioned earlier, Zucker and colleagues (1987) reported that intraseptal administration of arecoline at the dose of 2 μg per mouse can produce obvious reversal of the sleeping duration in pentobarbital-induced LORR. Therefore, it is suggested that systemic administration with arecoline at the highest dose of 1.0 mg/kg used in this study might not reach the effective concentrations in the central nervous system to produce the obvious pharmacological profiles of inhibiting pentobarbital sleeping in mice. Nevertheless, severe cholinergic responses set a limit to higher doses of arecoline used in this study; second, the asymmetrical effects of arecoline suggest the different potency of ethanol and pentobarbital in the mouse behavioral sleeping test, attributing to the existence of a degree of specificity in the sites of their action (Koob, 2004; Korpi et al., 2002; Mehta and Ticku, 1999). Certainly, the exact differences in mechanisms underlying ethanol- and pentobarbital-induced sleeping require further investigation.
In view of the clear changes in central cholinergic function induced by ethanol, in vivo studies have demonstrated that ethanol decreases the release of acetylcholine (ACh) from cortex and reticular formation in rabbits (Erickson and Graham, 1973), hippocampus (Henn et al., 1998), prefrontal cortex (Stancampiano et al., 2004), medial frontal cortex (Jamal et al., 2005) in rats. Under the condition of electrical or scopolamine stimulation, the increases in the release of ACh from in vitro cortical slices and in vivo hippocampus in rats can be antagonized by ethanol (Carmichael and Israel, 1975; Henn et al., 1998). Choline acetyltransferase (ChAT) catalyzes the biosynthesis of ACh in the cytoplasm of presynaptic terminals and indicates the functional state of central cholinergic system. Jamal and colleagues (2007) reported that ethanol and its metabolite acetaldehyde can reduce ChAT expression in the frontal cortex and hippocampus of rats, which is related to suppression of central ACh release induced by ethanol. Taken together, there is extensive evidence to suggest that acute administration of ethanol, especially at higher doses, causes central cholinergic hypofunction, strongly contributing to ethanol-induced hypnosis. Moreover, a previous study has shown that arecoline increases the ACh levels in subcortical regions in mice, although the higher doses were used (Molinengo et al., 1988). At neurotransmitter level, therefore, the activation of central cholinergic system may be involved in the mechanisms underlying antagonism of arecoline against the duration of sleep induced by ethanol in mice.
As mentioned above, arecoline is a muscarinic receptor agonist. Actually, the depressant effect of arecoline on locomotor activity can be antagonized by anticholinergic drugs such as scopolamine (Pradhan and Dutta, 1970). In this study, the decreases of arecoline in ethanol sleeping duration were reversed by the muscarinic antagonist scopolamine. Since scopolamine itself has no effects on ethanol-induced behavioral hypnosis at the doses used (0.125 to 0.5 mg/kg), such antagonism appears to be pharmacologically specific. However, methscopolamine failed to produce the same reversal actions as scopolamine in ethanol-induced hypnosis, because it cannot pass through the blood-brain barrier. On the reduction of ethanol-induced hypnosis, thus, arecoline may exert the activation of central muscarinic receptor in mice.
Like arecoline, physostigmine, a cholinesterase inhibitor, has been reported to shorten the sleep time in mice treated with 4.5 g/kg of ethanol (Deitrich et al., 1989). However, there is great difference in the pharmacological profiles of arecoline and physostigmine. The duration of action of arecoline is about 15 to 20 minutes (Pradhan and Dutta, 1970), suggestive of rapid drug elimination. Obviously, short-term central muscarinic agonists could be advantageous for alleviating central depression and intoxication from ethanol. In this regard, arecoline may represent a lead compound for research and development into drugs to reverse acute intoxication. On another hand, the experiences from co-users of alcohol and areca nut verify that they prefer chewing areca nut and feel more comfortable following drinking alcohol, although there is the lack of the exact investigations. Accordingly, arecoline removing central depression of alcohol may be one of the mechanisms underlying co-use of alcohol and areca nut in certain population. Overall, our data provide the first evidence to show an interaction between arecoline, a major alkaloid of areca nut, and alcohol.
Acknowledgments
This work was supported by grants from National Nature Science Foundation of China (30570653, 30870894), National Basic Research Program of China (2003CB515400, 2009CB522000), and 985 Program of China Ministry of Education (State Key Laboratory of Natural and Biomimetic Drugs, Peking University).




