CB1 Receptor Blockade Decreases Ethanol Intake and Associated Neurochemical Changes in Fawn-Hooded Rats
Abstract
Background: This study was undertaken to identify the neurochemical changes underlying the attenuation of voluntary ethanol intake induced by the cannabinoid CB1 receptor antagonist AM251 in fawn-hooded rats.
Methods: Rats were exposed to the 2-bottle-choice paradigm (ethanol 10% v/v or water) for 15 days. After this period, rats received AM251 (3 to 6 mg/kg, i.p.) or vehicle.
Results: Voluntary ethanol intake decreased (30%) with the administration of incremental dosages of AM251 (3 mg/kg, 5 days and 6 mg/kg, 5 days) in rats with acquired high preferring ethanol consumption (>3.5 g of ethanol/kg/d). Ethanol intake significantly decreased proopiomelanocortin expression in the arcuate nucleus (38.31%) and μ-opioid-DAMGO-stimulated [35S]-GTPγ binding in the caudate-putamen (40%), nucleus accumbens core (AccC) (32.87%), and shell (AccS) (34.21%). Moreover, ethanol intake increased tyrosine hydroxylase (TH) gene expression in the substantia nigra (24%) and ventral tegmental area (23%) and corticotrophin-releasing gene expression in the paraventricular hypothalamic nucleus (41.6%). The reduction of ethanol intake induced by AM251 was associated with blockade or significant reduction of the changes produced by ethanol in the expression of these genes in key regions related to drug dependence. Interestingly, treatment with AM251 reduced (20%) TH gene expression in rats drinking only water. In this respect, the action of AM251 in reducing TH gene expression may not be specific.
Conclusion: Taken together, these results revealed that blockade of cannabinoid CB1 receptors (CB1r) decreased voluntary ethanol intake in ethanol-habituated rats by normalizing the neurochemical alterations induced by ethanol.
Different Psychosocial and psychiatric conditions and/or alterations of neurochemical or genetic traits in the brain contribute to the development of problems related with ethanol consumption and alcohol dependence. Clinical studies using neuroimaging techniques and preclinical studies using different types of animal models of problems related to ethanol consumption have improved our knowledge of the neurochemical mechanisms involved in the development and treatment of ethanol dependence.
In recent years, evidence has accumulated that suggests a close relationship between the endogenous cannabinoid system and the actions of ethanol in the brain. Electrophysiological findings reveal that ethanol-induced effects on the mesolimbic dopaminergic system require CB1 stimulation by endogenous agonists (Perra et al., 2005). Ethanol increases the release of the endogenous cannabinoid ligands arachidonylethanolamide (AEA) and 2-arachidonylglycerol (2-AG) in neuronal cells (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2000, 2003). Further studies suggest that cannabinoid CB1 receptors are involved in ethanol preference (Hungund et al., 2003; Wang et al., 2003) and that CB1 receptor density decreases after administration of the cannabinoid CB1 receptor antagonist SR141716A in the ethanol free-choice paradigm (Lallemand and De Witte, 2006; Lallemand et al., 2001). An operant self-administration study revealed that SR141716A reduced operant response for ethanol in Wistar rats that had previously experienced 14 days of exposure to ethanol in vapor chambers, but not in rats that were not exposed to ethanol vapor (Rodriguez de Fonseca et al., 1999). In contrast, acute administration of the cannabinoid CB1 receptor agonist CP-55,940 increased the motivation to consume ethanol in Wistar rats and significantly stimulated voluntary ethanol consumption in ethanol-preferring sP rats. In both strains of rats, increases in ethanol consumption were prevented by pretreatment with SR141716A (Colombo et al., 1998; Gallate and McGregor, 1999; Gallate et al., 1999; Serra et al., 2001, 2002; Vacca et al., 2002).
Voluntary ethanol consumption also is reduced by SR141716A in ethanol-preferring C57BL/6 mice (Arnone et al., 1997) and in ethanol self-administering Long Evans rats (Freedland et al., 2001). Moreover, SR141716A can also reduce ethanol-seeking behavior in a rat model of relapse (Cippitelli et al., 2005).
While these reports reflected behavioral modifications after treatment with the cannabinoid CB1 receptor antagonist SR141716A, the neurochemical alterations associated with these changes remain to be determined. Previous studies reveal that the decrease in ethanol consumption after treatment with naltrexone is accompanied by a full blockade or a high tendency to return to normal values several alterations previously induced by ethanol, which include the μ-opioid receptor, tyrosine hydroxylase (TH), proenkephalin (PENK), corticotrophin-releasing factor (CRF), cannabinoid CB1 receptors, and serotonin transporter (5-HHT) in specific brain regions (Oliva and Manzanares, 2007). Considering these previous studies with naltrexone, it is tempting to hypothesize that the reduction of ethanol consumption by the cannabinoid CB1 antagonist SR141716A may be mediated, at least in part, by its ability to return to normal values several alterations induced by ethanol. The identification of these “adaptations” in animals that have previously shown high consumption of ethanol and in which the treatment with the cannabinoid CB1 receptor antagonist has decreased their consumption may identify potential pharmacological targets for treating alcohol dependence more effectively.
The purpose of this study was to identify: (i) the changes in the expression of various genes and in the functional activity of the μ-opioid receptor involved in the initiation or maintenance of ethanol dependence that are modified after treatment with the cannabinoid CB1 receptor antagonist AM251 and (ii) the specific brain regions of the rat in which these alterations occur. To this end, proopiomelanocortin (POMC), CRF, and TH gene expression and μ-opioid receptor functional autoradiography in selected brain regions were examined after AM251 treatment of fawn-hooded rats that had been habituated to ethanol preference.
Materials and Methods
Animals
Adult male fawn-hooded rats (2 to 3 months, 275 to 300 g body weight) obtained from Janvier (France) were maintained in an environment of temperature and light control (23 ± 1°C, light on between 8:00 am and 8:00 pm). Food and tap water were provided ad libitum. Animals were immediately singly housed upon arrival and stayed in individual cages during the experiment. They were allowed to acclimate to their colony room individually caged 1 week before the experimental procedure. All experiments were performed following the standards of animal care established by national and international laws (Spain and the European Union) for the care and use of laboratory animals.
Ethanol Intake Protocol
Ethanol intake protocol was conducted as described previously (Oliva and Manzanares, 2007). The experiment was divided into 3 phases: acquisition, maintenance, and treatment. The 2-bottle-choice paradigm of ethanol consumption was used throughout the entire process. Rats initially were divided randomly into 2 groups: (i) rats allowed unlimited access to 2 bottles of water (n = 20 rats) and (ii) rats constantly exposed to 2 bottles, 1 bottle containing water and the other containing an ethanol solution (n = 40 rats). Drinking bottles were calibrated by using a test tube (50 ± 0.75 ml). During the acquisition phase (12 days), in which animals acquired ethanol-intake behavior, the concentration of the ethanol solution was gradually increased (2.5, 5, 7.5, and 10% v/v) every 3 days in the ethanol group. In the maintenance (15 days) and treatment (10 days) phases, the concentration of the ethanol solution was kept constant at 10%. At the end of the maintenance phase, the animals that met the minimum consumption criterion of >3.5 g of ethanol/kg/d (approximately 70% of rats) were selected. From all of those, 20 rats were selected based on the more consistent level of consumption during the total time of exposure. These rats were randomly divided into 2 groups to evaluate the effectiveness of AM251 in reducing ethanol consumption (ethanol-AM251 and ethanol-vehicle groups). Rats allowed unlimited access to water were also randomly divided into 2 groups (n = 10/group) to evaluate the effects of AM251 (water-AM251 and water-vehicle groups). In order to avoid positional preference for ethanol intake, the position of the bottles in the cage was changed every day. The intake of water and ethanol contained in special bottles with caps fitted with a small ball at the tip that minimized spilling was measured in milliliters every day. Rats were weighed 3 times a week to obtain grams of ethanol/kg/d data.
AM251 Treatment Protocol
Rats previously selected at the end of the maintenance phase of the ethanol intake protocol were divided randomly into 2 groups (10 rats per group). One group received vehicle (DMSO, Tween80 and saline, 1:1:8, i.p) and the other group received a single i.p administration of the commercially available CB1 receptor antagonist AM251 diluted in vehicle (Tocris, Bristol, U.K.). AM251 is structurally related to SR141716A but may be more potent and have more selectivity for the CB1 receptor (Lan et al., 1999). During the first 5 days, the AM251 dosage was 3 mg/kg. In the following 5 days, the AM251 dosage was 6 mg/kg. These doses were used previously to antagonize other actions of the CB1 receptor agonists (Maćkowiak et al., 2009; Resstel et al., 2008; Zarrindast et al., 2008). This is the first study that uses AM251 to reduce ethanol intake and compare the neurochemical alterations under this experimental design. This pattern of treatment was used following the same design as another study with naltrexone using Wistar rats (Oliva and Manzanares, 2007). Treatments were carried out daily 30 minutes before the dark phase started (8:00 pm). Twenty-four hours after the last AM251 treatment, when the effects of the last dose had been eliminated, animals were killed by decapitation and their brains were quickly removed, frozen on dry ice, and stored at −80°C.
In Situ Hybridization Histochemistry
Brain sections, 12-μm thick, were made at 3 different levels containing regions of interest for their important roles in ethanol reward and reinforcement behavior, including ethanol consumption. The first level contained the paraventricular nucleus (PVN); the second level included the arcuate nucleus (ARC), and the third level contained the ventral tegmental area (VTA) and substantia nigra (SNc). All these sections were obtained according to the Paxinos and Franklin Atlas (Paxinos and Watson, 2005) and were mounted on gelatin-coated slides and stored at −80°C until the day of the assay.
In situ hybridization histochemistry was performed as described previously (Young et al., 1986) using synthetic oligonucleotide probes complementary to TH (bases 1223 to 1252; Perkin Elmer, Pacisa-Giralt, Madrid, Spain), CRF (bases 496 to 543; Perkin Elmer), and POMC (bases 96 to 134; Advanced Biotechnology Center, Charing Cross and Westminster Medical School, London, England). Oligonucleotide probes were labeled using terminal deoxytransferase (Boehringer, Madrid, Spain) to add a 35S-labeled deoxyATP (1000 Ci/mmol; Amersham, Madrid, Spain) tail to the 3′-end of the probes. The probe (in 50 μl of hybridization buffer) was applied to each section and left overnight at 37°C for hybridization. Following hybridization, the sections were washed 4 times, 15 minutes each time, in 0.15 M NaCl, 0.015 M sodium citrate, pH 7.2 (1X saline sodium citrate, SSC) at 55°C, followed by two 30-minute washes in 1X SSC at room temperature and one brief water dip, and then were blow-dried with air. To control for imaging enhancement variables, each set of slides was apposed to the same film (Kodak BioMax MR-1; Amersham) in individualized cassettes.
DAMGO-Stimulated [35S]GTPγS Binding Autoradiography
μ-Opioid receptor [35S]GTPγS binding was performed as previously described (Sim et al., 1996). Twelve-micron brain sections that were obtained at the level of caudate-putamen (CPu) (Paxinos plate 19 to 20), and contained cingulate cortex (Cg), nucleus accumbens shell (AccS) and nucleus accumbens core (AccC) (Paxinos and Watson, 2005), were mounted on gelatin-coated slides and rinsed in assay buffer (50 mM TRIS, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4) at 25°C for 10 min, then incubated with 2 mM GDP in assay buffer at 25°C for 30 min. Sections were incubated for 2 hours at 25°C in assay buffer with 0.04 nM [35S]-GTPγS, 2 mM GDP and the μ-opioid receptor agonist 7.5 μM DAMGO. Baseline activity was assessed in the absence of agonist, whereas nonspecific binding was measured in the presence of 10 μM unlabeled GTPγS. Additional brain sections of the ethanol-naive rats were incubated with 0.04 nM [35S]GTPγS, 2 mM GDP, DAMGO, and naltrexone 0.3 μM to test for the specificity of the agonist receptor activation. After incubation, slides were rinsed twice in 50 mM TRIS buffer, pH 7.4, and once in cold deionized water, then air dried and exposed to film (Kodak BioMax MR-1; Amersham Iberica) for 36 to 48 hours.
Image Analysis Quantification
In the autoradiograms from μ-opioid-agonist-stimulated [35S]GTPγS autoradiography, optical densities were calculated by subtracting from each “stimulated” measurement the corresponding “baseline” value, as previously shown (Corchero et al., 1999b). The addition of naltrexone completely blocked the signal induced by DAMGO, indicating that its effects under these experimental conditions are specific (data not shown).
Autoradiograms from in situ hybridization studies (2 slides per level [2 slices/slide; 2 measurements/slice] from each animal) were analyzed with a PC computer using the public-domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Previous experiments in our laboratory have found that the selected film exposure times in these brain regions under our experimental conditions (oligonucleotide probe, radioactivity added to each slide, incubation conditions, and type of film selected) render a hybridization signal whose gray levels are linear with the optical density, according to the NIH Image Program. Therefore, optical densities were calculated from the uncalibrated mode of the Image Program by subtracting the corresponding background from each measurement and were expressed in gray-scale values. The background measurement was obtained from an area of the slice with the lowest nonspecific hybridization signal and was subtracted from the hybridization signal measurement obtained in the same slice. The measurements from the brain sections were pooled and the values were averaged. Mean control values were considered to be 100% for the presentation of results. Additional brain sections were co-hybridized with a 100-fold excess of cold probes or with RNAse to assert the specificity of the signal. As expected, no hybridization signal was detected in these sections (data not shown).
Statistical Analyses
Drinking behavior was analyzed using the Mann–Whitney rank sum test or 2-way analysis of variance (ANOVA) with repeated measures. The factors of variation were treatment and days of treatment as independent variables. Two-way ANOVA followed by Student–Newman–Keul’s test was performed for μ-opioid binding autoradiography and in situ hybridization studies. Differences were considered significant when the probability of incorrectly rejecting the null hypothesis was less than 5%. SigmaStat 3.1 software was used for all statistical analyses.
Results
The behavioral data on ethanol intake were divided into 3 consecutive phases. At the beginning of the induction phase, the animals drank about 2.7 g of ethanol/kg/d at ethanol concentrations of 2.5, 5, and 7.5% (data not shown). However, when the concentration of the ethanol solution was raised to 10%, animals reached intake levels around or more than 3.5 g ethanol/kg/d (Fig. 1A). In the maintenance phase, rats were randomly selected from among the animals in which alcohol intake averaged approximately 3.5 g of ethanol/kg/d (see progression of the alcohol consumption of these rats in the supplementary Figure S1) and divided in 2 groups to enter the third phase. In this phase, water intake also was measured. The rats selected for the following phase presented high ethanol preference (>70%) for 10% ethanol (data not shown). The third phase corresponded to treatment with AM251, which was administered using an incremental dosage, 3 mg/kg/d (5 days) followed by a dose of 6 mg/kg/d (5 days). As shown in Fig. 1B, the administration of AM251 was effective in reducing ethanol intake (30%) compared to the vehicle group, decreasing consumption to an average of 2.940 g ethanol/kg/d (Mann–Whitney rank sum test; T = 7293,000; p < 0.001) without significant changes in the body weight of the rats during the 10 days of treatment. Two-way ANOVA with repeated measures was performed to evaluate differences in alcohol intake between the ethanol-AM251 and ethanol-vehicle groups every day during AM251 administration. The statistical analyses showed that the AM251 factor was significant; AM251 decreased ethanol intake (AM251 F1,199 = 38.340, p < 0.001). However, the factor Day and the interaction between AM251 Treatment and Day were not significant (Days F9,199 = 1.902, p = 0.063; AM251 × Days F9,199 = 0.825, p = 0.595) (Fig. 1B). These results suggest that the effect of AM251 treatment on ethanol consumption was not cumulative. Although the Day × Treatment interaction was not significant, posthoc analyses contrasting the means (AM251 vs. vehicle within each day of treatment) were performed because it was of a priori interest to evaluate day-specific drug effects. These analyses revealed that AM251 treatment was significantly effective on days 3 and 4 for the 3 mg/kg dose and on days 1 to 5 for the 6 mg/kg dose (p < 0.05, Student–Newman–Keuls). There was no effect of AM251 treatment in the water group rats (data not shown).
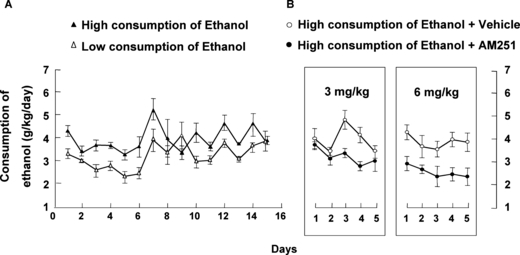
(A) Levels of ethanol intake measured in rats during the maintenance phase (volume of ethanol intake is represented as g/kg/d) (n = 40 rats). The 2-bottle-choice paradigm of ethanol consumption was used: high consumption of ethanol, low consumption of ethanol. (B) Levels of ethanol intake measured in rats during the treatment phase (n = 20 rats). At the end of the maintenance phase, the animals that voluntarily consumed >3.5 g ethanol/kg/d were randomly divided into 2 groups (n = 8 to 10 rats per group). One group was treated with vehicle (DMSO: Tween 80: saline; 1:1:8; i.p.) and the other group was treated with AM251 3 mg/kg (i.p.; 5 days) followed by AM251 6 mg/kg (i.p.; 5 days) every day, 30 minutes before the end of the light phase (see Methods). High consumption of ethanol + vehicle, high consumption of ethanol + AM251. Significance symbols are excluded from the figure for individual mean comparisons because only the main effect of AM251 treatment was significant and did not interact with Day (see text).
Two-way ANOVA revealed that CRF gene expression in the PVN significantly increased after chronic and voluntary ethanol consumption (41.6%), and this effect was blocked after treatment with AM251 (ethanol F1,38 = 13.666, p < 0.001; treatment F1,38 = 6.653, p = 0.014; ethanol × treatment interaction F1,38 = 12.006, p < 0.001). The lower panels of Fig. 2 show representative autoradiograms of coronal brain sections at the level of the PVN in which differences can be appreciated in the hybridization signal of CRF gene expression induced by chronic ethanol intake and treatment with AM251.
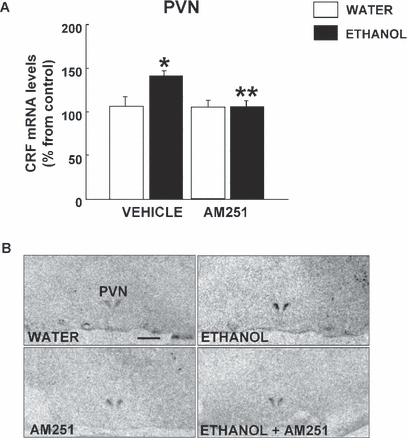
(A) Effects of AM251 and ethanol intake on corticotrophin-releasing factor (CRF) gene expression in the paraventricular nucleus (PVN) of fawn-hooded rats. The columns represent the means and the vertical lines mark the SEM of CRF mRNA levels in 8 to 10 rats per group. *Values of the ethanol-vehicle group that differed significantly (p < 0.05) from the water-vehicle group. **Values from ethanol-AM251-treated rats that differed significantly (p < 0.05) from those of the vehicle-ethanol group. (B) Representative autoradiograms of coronal brain sections obtained at the level of the PVN in rats treated with ethanol, AM251, or vehicle. Bar represents 1 mm.
The alterations induced in POMC gene expression at the level of ARC by ethanol consumption and treatment with AM251 are illustrated in Fig. 3. The results of two-way ANOVA revealed a decrease in POMC gene expression levels (38.31%) that was blocked by the cannabinoid antagonist AM251 (ethanol F1,37 = 10.23, p = 0.003; treatment F1,37 = 0.791, p = 0.380; ethanol × treatment interaction F1,37 = 10.948, p = 0.002). Interestingly, the administration of AM251 produced a moderate but significant reduction in POMC gene expression. The lower panels of Fig. 3 show the representative autoradiograms of coronal brain sections at the level of the ARC in rats that consumed vehicle, voluntarily consumed ethanol, and were treated with AM251.
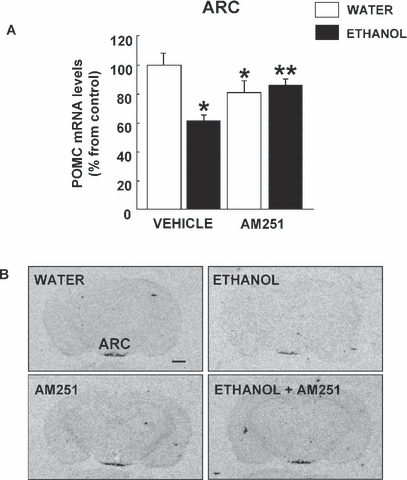
(A) Effects of AM251 and chronic ethanol administration on proopiomelanocortin (POMC) gene expression in the ARC of fawn-hooded rats. The columns represent the means and the vertical lines mark the SEM of POMC mRNA levels in 8 to 10 rats per group. *Values from the ethanol or AM251 groups that differed significantly (p < 0.05) from those of the water-vehicle group. **Values from ethanol-AM251 treated rats that differed significantly (p < 0.05) from those of the ethanol-vehicle group. (B) Representative autoradiograms of coronal brain sections at the level of the ARC in rats treated with ethanol, AM251, or vehicle. Bar represents 1 mm.
The effects of chronic ethanol intake and AM251 treatment on TH gene expression in the VTA and SNc are shown in Fig. 4. Two-way ANOVA revealed an increase in TH mRNA levels after chronic and voluntary ethanol consumption in VTA (23%) and SNc (24%). These effects were blocked by treatment with AM251. Interestingly, AM251 treatment in rats that consumed water and did not consume ethanol decreased TH gene expression in the VTA (20%) (ethanol F1,39 = 11.314, p < 0.05; treatment F1,39 = 5.494, p < 0.05; ethanol × treatment interaction F1,39 = 0.006, p = 0.997) and SNc (22%) (ethanol F1,43 = 36.169, p < 0.05; treatment F1,43 = 18.23, p < 0.05; ethanol × treatment interaction F1,43 = 0.099, p = 0.754). In the lower part of Fig. 4, representative autoradiograms of coronal brain sections of the vehicle, ethanol and AM251 groups show the differences in TH gene expression in VTA and SNc.
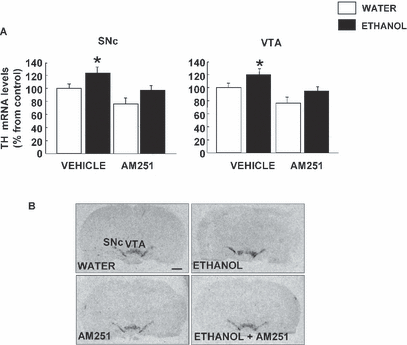
(A) Effects of AM251 and chronic ethanol administration on tyrosine hydroxylase (TH) gene expression in the ventral tegmental area (VTA) and SNc of fawn-hooded rats. The columns represent the means and the vertical lines mark the SEM of TH mRNA levels in 8 to 10 rats per group. *When ethanol was effective to modify significantly the TH gene expression from vehicle group (p < 0.05). (B) Representative autoradiograms of coronal brain sections at the level of the SNc and VTA in rats treated with ethanol, AM251, or vehicle. Bar represents 1 mm.
The analysis of μ-opioid receptor function in several brain regions after ethanol intake and AM251 treatment of fawn-hooded rats is shown in Fig. 5. Two-way ANOVA results revealed that ethanol consumption significantly reduced DAMGO-stimulated [35S]-GTPγS binding levels in the CPu (40%) (ethanol F1,24 = 6.904, p = 0.015; treatment F1,24 = 2.255, p = 0.148; ethanol × treatment F1,24 = 1.698, p < 0.012), AccC (32.87%) (ethanol F1,24 = 8.177, p < 0.009; treatment F1,24 = 7.263, p = 0.014; ethanol × treatment interaction F1,24 = 20.916, p < 0.001), and AccS (34.21%) (ethanol F1,24 = 16.069, p < 0.001; treatment F1,24 = 13.970, p < 0.001; ethanol × treatment interaction F1,24 = 16.869, p < 0.001), but did not have effects on the Cg (ethanol F1,24 = 3.25, p = 0.068; treatment F1,24 = 1.158, p = 0.197; ethanol × treatment interaction F1,24 = 4.597, p < 0.876). Treatment with AM251 fully blocked the decreases in μ-opioid receptor function induced by voluntary consumption of ethanol. In the AccC, a small but statistical decrease in DAMGO-stimulated [35S]-GTPγS binding levels was found after treatment with AM251. The autoradiograms in the lower part of Fig. 5 show the differences in μ-opioid receptor functional activity of coronal brain sections in the vehicle, ethanol, and AM251 groups.
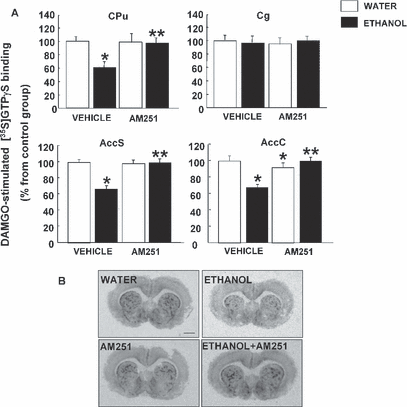
(A) Effects of AM251 and chronic ethanol intake on μ-opioid receptor function in CPu, Cg, AccS, and AccC. The columns represent the means and the vertical lines mark the SEM of DAMGO-stimulated-[35S]GTPγS binding in 8 to 10 rats per group. *Values from ethanol or AM251 groups that differed significantly (p < 0.05) from those of the water-vehicle group. **Values from ethanol-AM251 treated rats that differ significantly (p < 0.05) from those of the vehicle-ethanol group. (B) Representative autoradiograms of coronal brain sections at the level of the CPu in rats treated with ethanol, AM251, or vehicle. Bar represents 1 mm.
Discussion
The results of this study provide evidence for the first time of the “neurochemical adaptations” that occur with ethanol intake and that are modified by treatment with the cannabinoid receptor antagonist AM251 of fawn-hooded rats previously exposed to ethanol consumption. Furthermore, these results point to specific key proteins closely involved in the “normalization” of the activity of brain regions altered by prolonged ethanol consumption. The experimental design of the “2-bottle-choice paradigm” was similar to a design used previously in our laboratory (Oliva and Manzanares, 2007). A 12-day acquisition period, in which solutions of progressively higher ethanol concentration were consumed, was followed by a 15-day maintenance period (10% ethanol v/v). In this phase, fawn-hooded rats exposed to ethanol intake that consumed >3.5 g/kg/d were selected from the ethanol-preferring rats (approximately 70%). AM251 treatment (increasing dosage, 3 mg/kg/d for 5 days followed by 6 mg/kg/d for 5 days) significantly reduced (by approximately 30%) voluntary ethanol intake.
Blood ethanol levels were not measured in this study. Rats drink during the dark phase mainly in 2 peaks. In our experiment, animals had free access to ethanol throughout the 24 hours of the day. Measurements of blood ethanol levels immediately after the dark phase cannot be made accurately due to obvious differences in intake times from rat to rat. Only in experimental designs where ethanol intake is restricted to a discrete period of time can ethanol be measured properly in blood samples of rats. In an effort to simulate fully voluntary ethanol intake (as a potential animal model of problems related to excessive alcohol consumption), ethanol exposure was not restricted to a certain time period. Measurement of blood ethanol levels under this experimental design would have yielded inconsistent values. A similar experimental design also was used in previous studies in our laboratory to evaluate the effects of naltrexone on gene expression and functional autoradiography in Wistar rats (Oliva and Manzanares, 2007).
The finding that the blockade of cannabinoid CB1 receptors reduces ethanol intake coincides with previous reports in several animal models (Arnone et al., 1997; Colombo et al., 1998; Freedland et al., 2001; Gallate and McGregor, 1999; Lallemand and De Witte, 2006; Lallemand et al., 2001; Rodriguez de Fonseca et al., 1999; Serra et al., 2001, 2002; Vacca et al., 2002). It is important to consider that the magnitude of the reduction in ethanol consumption varies and may depend on the experimental design, strain of rat, duration and dose of treatment employed, or the length of the period that rats have been drinking ethanol before initiating treatment. In this study, the administration of AM251 did not alter food or water intake, or the weight of the rats during the development of the experiment. This is in contrast with several reports (McLaughlin et al., 2003; Shearman et al., 2003; Tallett et al., 2007) that suggest that AM251 reduces food intake. Possible reasons that may account for these discrepancies are: (i) strain and species differences (fawn-hooded rats, Sprague–Dawley rats, 129/SVE and C57BL/6 mice) or (ii) different routes of administration, distinct doses (2 to 30 mg/kg), or different duration or pattern of administration of the drug.
In addition, AM251 alone failed to produce any alteration of locomotor activity at any of the doses studied (3 and 6 mg/kg, i.p.). Controversial results have been obtained about locomotor effects of AM251 (Zarrindast et al., 2008). Low doses of AM251 did not alter locomotor activity. However, high doses resulted in diminished locomotor activity. In contrast, AM251 alone (1 and 5 mg/kg, i.p.) failed to produce any alteration in locomotor activity (Bhatti et al., 2009). These discrepancies may be due to: (i) individual and species differences between the strains used (fawn-hooded rats, Sprague–Dawley rats) or (ii) the route of administration, distinct doses (3 to 6 mg/kg) or different duration or pattern of administration of the drug. In the particular conditions of this study, the reduction of ethanol intake was associated with specific alterations in the plasticity of several genes involved in the regulation of stress and the hypothalamic–pituitary–adrenal axis (CRF and POMC), in the homeostatic regulation of mesolimbic and nigrostriatal dopaminergic activity (TH), and in the functional activity of μ-opioid receptors in the CPu, AccC, AccS, and Cg (areas closely related to the development of drug dependence).
Stress has been identified as one of the most important factors that determines increased vulnerability to the consumption of substances of abuse, the development of dependence, and the high risk of relapse (Piazza and Le Moal, 1996). In this study, voluntary ethanol consumption significantly increased CRF gene expression in the PVN of the hypothalamus, an effect that was completely blocked by treatment with AM251, suggesting that “normalization” of CRF gene expression in the PVN of the hypothalamus is associated with a decrease in ethanol consumption. The increase of CRF mRNA levels in the PVN is consistent with previous reports showing upregulation of this gene after acute or chronic ethanol intake (Heilig and Koob, 2007; Lack et al., 2005; Li et al., 2005; Liu and Weiss, 2002; Rivier and Lee, 1996; Rivier et al., 1990, 1996). The molecular mechanisms by which the blockade of the CB1 cannabinoid receptor may regulate CRF expression remain to be determined. Several studies show that ethanol exposure increases the release of the endogenous cannabinoid ligands AEA and 2-AG in neuronal cells (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2000, 2003). This increase in endogenous cannabinoids could stimulate the secretion of hypothalamic–pituitary axis hormones and, therefore, the expression of the precursor gene CRF, as has been shown with cannabinoid receptor agonists (Corchero et al., 1999a; Manzanares et al., 1999a). AM251 treatment may block the actions of endogenous cannabinoid ligands, thus reducing CRF expression. These results further suggest that CRF may be an interesting target for controlling ethanol dependence. Indeed, recent reports have suggested that the blockade of CRF receptors reduces acquisition, sensitization, excessive ethanol drinking, and ethanol self-administration (Funk and Koob, 2007; Funk et al., 2007; Heilig and Koob, 2007; Pastor et al., 2008).
Exposure to ethanol significantly decreased (38% of the vehicle group) POMC gene expression in the ARC of the hypothalamus. This finding is consistent with previous studies showing a reduction in POMC gene expression (Rasmussen et al., 2002; Scanlon et al., 1992) and decreased immunoreactivity of the melanocortin neuropeptide alpha-melanocyte-stimulating hormone (Navarro et al., 2008) after chronic ethanol intake. Administration of AM251 blocked the reduction in POMC gene expression induced by ethanol consumption. This indicates that the cannabinoid CB1 receptor is an important component of this opiodergic circuit. Interestingly, in this study the blockade of cannabinoid CB1 receptors slightly decreased POMC mRNA levels in the ARC. This may be due to disruption of a stimulatory endogenous cannabinoid tone in the POMC gene of the hypothalamus. Therefore, blockade of this endogenous activation might result in a slight decrease in POMC gene expression of the hypothalamus. It is important to note that in this particular strain of rats, which is highly vulnerable to ethanol consumption, diminished POMC gene expression compared to Wistar rats under baseline conditions has already been reported (Manzanares et al., 2005). This genetic alteration may be caused by failure in the regulatory feedback mechanisms that maintain steady levels of POMC. Further studies are needed to determine the precise effect of the CB1 cannabinoid receptor on hypothalamic POMC expression.
The mesolimbic dopaminergic system has long been considered to be the circuit most closely involved in the positive reinforcing properties of natural reward stimuli and substances of abuse, such as ethanol (Robbins and Everitt, 1996; Robbins et al., 1989; Spanagel and Weiss, 1999). Indeed, ethanol enhances the firing rate of DA neurons in the VTA and SNc (Brodie and Appel, 1998; Brodie et al., 1990; Gessa et al., 1985; Koyama et al., 2007; Mereu et al., 1984), producing a significant increase in DA release in the dorsal striatum, nucleus accumbens, ventral pallidum, and Cg (Di Chiara and Imperato, 1985, 1986, 1988; Imperato and Di Chiara, 1986; Melendez et al., 2003; Yim and Gonzales, 2000; Yoshimoto et al., 2000). Our results revealed that TH gene expression significantly increased in the VTA (23%) and SNc (24%) after chronic and voluntary ethanol consumption and these effects were fully blocked by AM251 treatment. These results suggest that the blockade of CB1 receptors may antagonize the ethanol-induced neurochemical changes that in turn decrease voluntary ethanol consumption. The increases of TH gene expression in the VTA or SNc found in this study after ethanol consumption are consistent with previous reports (Lee et al., 2005; Oliva and Manzanares, 2007; Oliva et al., 2008). Interestingly, AM251 treatment of rats drinking only water resulted in a reduction (20%) in TH gene expression. Despite this effect, AM251 rendered to normal values the ethanol induced increase of this gene in the VTA and SNc. It can be hypothesized that constitutively active CB1 receptors stimulate TH gene expression in the cell bodies of the nigrostriatal and mesolimbic dopaminergic systems. Blockade of this stimulatory endogenous basal activity would result in decreased TH mRNA levels in VTA and SNc. Therefore, one possible interpretation is that AM251 administration is not blocking the action of ethanol but, instead, is reducing TH expression. In this respect, the action of AM251 in reducing TH gene expression may not be specific. Alternatively, AM251 may be considered to act in a different manner depending on the level of neuronal activity. This means that this cannabinoid antagonist may block the “physiological stimulatory tone” under baseline conditions, but when the dopaminergic neuronal activity is abnormally elevated, as it may be after chronic ethanol exposure, AM251 treatment may tend to stabilize TH expression “pharmacologically.” Further studies of the effects of different dosages of AM251 on the release of DA and endogenous ligands in terminals of the mesolimbic and nigrostriatal systems are needed to identify the exact neurochemical mechanisms that regulate TH gene expression.
In recent years, evidence has accumulated from animal and human studies that the opioid endogenous systems and, in particular, the μ-opioid receptor, are the most relevant elements involved in differences in vulnerability to ethanol consumption (Barr et al., 2007; Cowen et al., 1999; Fadda et al., 1999; Heinz et al., 2005; Ortiz et al., 2004b; Ray and Hutchison, 2004; Roberts et al., 2000; Sim-Selley et al., 2002) and the development of ethanol dependence and relapse. Differences in μ-opioid receptor densities in mesolimbic areas, such as nucleus accumbens, AccS, AccC, and Cg (Cowen et al., 1999; Fadda et al., 1999; Sim-Selley et al., 2002) were reported in rats presenting high and low preference for ethanol consumption. Consequently, they may be some of the most useful targets for regulating ethanol dependence and craving (Benjamin et al., 1993; Bienkowski et al., 1999; Davidson and Amit, 1997; Goodwin et al., 2001; Manzanares et al., 2005; Myers and Lankford, 1996; Oliva and Manzanares, 2007; Phillips et al., 1997; Stromberg et al., 2001, 2002). In this study, ethanol exposure significantly reduced μ-opioid functional activity in the CPu, AccC, and AccS. The reduction in μ-opioid-stimulated [35S]GTPγ binding in the brain regions closely involved in reward responses after prolonged ethanol exposure is consistent with previous findings (Chen and Lawrence, 2000; Oliva and Manzanares, 2007; Saland et al., 2004; Sim-Selley et al., 2002). Treatment with AM251 fully blocked the effects of voluntary ethanol consumption on μ-opioid receptors in the brain regions examined. Previous studies reveal that the μ-opioid antagonist, naltrexone, completely blocks the decrease of μ-opioid receptor functional activity induced by ethanol in the AccS (Manzanares et al., 2005). The mechanism by which the blockade of cannabinoid receptors “normalized”μ-opioid functional activity after ethanol exposure remains to be seen. Exposure to ethanol increases the release of endogenous cannabinoid ligands (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2000, 2003) and, as a consequence, decreases WIN-55,212-stimulated [35S]GTPγ binding and CB1 receptor gene expression in several brain regions (Manzanares et al., 1999b, 2005; Ortiz et al., 2004a). Furthermore, the consumption of ethanol or the administration of cannabinoid agonists increases the release of endogenous opioids (Manzanares et al., 1999b, 2005). AM251 treatment may indirectly tend to normalize the activity levels of the μ-opioid receptor by blocking, through a still unknown mechanism, the effects of cannabinoid and/or opioid ligands (in this case β-endorphin) released by ethanol exposure.
Our group has recently proposed the idea that ethanol exposure produces a number of “neurochemical adaptations” (reflected in alterations in the expression of certain key genes, such as TH, proenkephalin, CB1 receptor, and serotonin transporter) and that the decrease in ethanol consumption produced by certain drugs requires “readaptation” of the genes that have been modified by ethanol (Oliva and Manzanares, 2007). The results of this study confirm this hypothesis by showing that treatment with AM251 decreased ethanol consumption and “normalized” the neurochemical changes that were produced. Furthermore, the findings of this study support the relevance of μ-opioid receptor, CRF, POMC, and TH gene expression among the main markers of ethanol consumption and, in particular, in the mechanisms underlying the decrease in ethanol intake after drug treatment.
The search for drugs designed to “stabilize” these genes may be worthwhile in the treatment of ethanol dependence. As different drugs may share similar mechanisms for stabilizing the functional activity of specific markers related to the decrease in ethanol consumption, it may be advantageous to consider associating these drugs to treat ethanol dependence more efficiently. Indeed, preclinical studies have shown that concurrent treatment with opioid and cannabinoid receptor antagonists has a synergistic effect in reducing ethanol consumption (Gallate et al., 2004). Recently, Soyka and colleagues (2008), in a controlled study to assess the efficacy of SR141716A in the treatment of alcohol-dependent subjects, showed that a dosage of 20 mg/d in recently detoxified alcohol-dependent patients did not significantly increase the time to the first drink compared to placebo. The difference (8% better outcome) was more marked in patients who relapsed to “heavy drinking.” However, the lack of efficacy in this study may be due to a high response rate and relatively short treatment duration in the placebo group.
There are a number of limitations to the translation of the findings of this animal model to human alcohol dependence. Nonetheless, the results of this study strongly suggest that the CB1 receptor plays an important role in decreasing alcohol consumption and CB1 antagonists should be considered as complementary drugs for the treatment of alcohol dependence, at least in certain alcoholic patient subtypes (Hertling et al., 2005; Hesselbrock and Hesselbrock, 2006; Leggio et al., 2009; Walter et al., 2008). In this respect, considering the behavioral and neurochemical features of fawn-hooded rats, it is tempting to speculate that the subtype of alcoholic patient most amenable to treatment with CB1 receptor antagonists might include subjects with affective disorders, a family history of alcohol dependence, and affective disorders and heavy drinking (Lahmame et al., 1996; Overstreet et al., 2007; Rezvani et al., 2007). Controlled clinical trials using different dosages and patterns of administration of CB1 receptor antagonists should be carried out to test the potential usefulness of these drugs in the treatment of alcohol dependence.
Acknowledgments
This research was funded by Red Temática de Investigación Cooperativa en Salud (RETICS, Instituto de Salud Carlos III, MICINN and FEDER, Madrid, Spain: Red de Trastornos Adictivos) RD06/0001/1004 to J.M. MSG-G was supported by a fellowship from the Spanish Ministry of Health. We thank Patricia Rodríguez and Analía Rico for excellent technical assistance. P.R. and A.R. are technicians supported by “Fundación para la Salud en Castilla La Mancha” (FISCAM) and “RETICS,” respectively.




