Glycine Receptors in the Nucleus Accumbens Involved in the Ethanol Intake-Reducing Effect of Acamprosate
Abstract
Background: We have previously demonstrated that strychnine-sensitive glycine receptors (GlyRs) in the nucleus accumbens (nAc) and nicotinic acetylcholine receptors (nAChRs) in the ventral tegmental area are involved in mediating ethanol (EtOH)-induced elevation of dopamine in the rat mesolimbic dopamine system. This neuronal circuitry was also demonstrated to mediate dopamine elevation in the nAc after both taurine, an endogenous agonist of GlyRs, and acamprosate, a synthetic derivate of homotaurine. The aim of this study was to investigate whether the EtOH intake-reducing effect of acamprosate involves accumbal GlyRs.
Methods: For this purpose, we used a voluntary EtOH consumption model where EtOH medium- and high-preferring rats were implanted with guide cannulae in the nAc. The animals received daily injections of acamprosate or 0.9% NaCl before accessing a bottle of 6% EtOH and a bottle of water. After 2 days, a microinjection of strychnine or vehicle preceded the daily systemic injection and bottle-access period.
Results: Acamprosate, but not saline, decreased EtOH intake. Pretreatment with Ringer in the nAc did not influence EtOH intake in saline or acamprosate-treated animals. Pretreatment with strychnine had no effect on EtOH intake in saline-treated animals, whereas it completely reversed the EtOH intake-reducing effect of acamprosate.
Conclusions: Based on current and previous results, we suggest that acamprosate primarily interacts with accumbal GlyRs and secondarily with ventral tegmental nAChRs, in a similar manner to that previously observed with EtOH and taurine. The interaction between acamprosate and GlyRs does not only influence dopamine output in the nAc but also EtOH consumption, giving further support for our hypothesis that GlyRs are of importance in EtOH reinforcement.
Alcohol addiction is a leading cause of morbidity and mortality and a major contributor to healthcare costs all over the world. One of the substances currently used in treatment of alcoholism is acamprosate (Campral®, Merck, Darmstadt, Germany). Acamprosate is a synthetic derivative of homotaurine and has been reported to prevent relapse in detoxified alcoholics (Littleton, 1995; Mason, 2005; Terenius, 1998), as well as to decrease alcohol consumption in rats (Boismare et al., 1984; Czachowski et al., 2001; Heyser et al., 1998; Olive et al., 2002).
The mesolimbic dopamine system, originating in the ventral tegmental area (VTA) and projecting mainly to the nucleus accumbens (nAc), has been implicated in alcohol addiction/dependence. It is well known that drugs of abuse, including ethanol (EtOH), activate the mesolimbic dopamine system and increase extracellular dopamine levels in the terminal region (Koob and Nestler, 1997; Spanagel and Weiss, 1999).
Our research group has previously suggested that glycine receptors (GlyRs) are involved in both basic regulation of dopamine levels in the nAc and in accumbal dopamine output in response to EtOH (Molander and Söderpalm, 2005a,b). We hypothesize that GlyRs regulate dopamine levels in the nAc via a neuronal circuitry, involving gamma-amino-butyric acid (GABA) neurons projecting from the nAc to the VTA, and nicotinic acetylcholine receptors (nAChRs) in the VTA (Ericson et al., 2003, 2008; Löf et al., 2007; for review see Söderpalm et al., 2009; see also Fig. 6). These 2 receptor populations were identified after local administrations of strychnine (a competitive antagonist for GlyRs) and mecamylamine (an unspecific antagonist for nAChRs) which both inhibited the dopamine elevation observed after local application of EtOH in the nAc (Ericson et al., 2003; Molander and Söderpalm, 2005b). These 2 types of receptors were also demonstrated to be important at a functional level when investigating rats in a voluntary EtOH preference paradigm. EtOH high-preferring animals receiving either glycine locally in the nAc or mecamylamine locally in the VTA decreased their voluntary EtOH intake significantly (Ericson et al., 1998; Molander et al., 2005). Further studies using systemic administration of a glycine transporter-1 inhibitor (ORG 25935) also revealed a robust decrease in EtOH intake (Molander et al., 2007), indicating that glycine has an important role in modulating EtOH intake in the rat, possibly via activation of GlyRs.
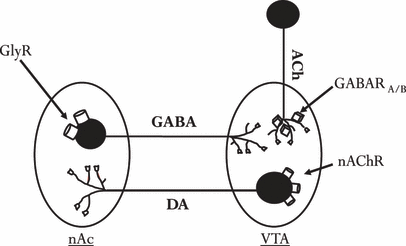
Schematic and simplified figure of a hypothetical neuronal circuitry involved in tonic regulation of accumbal dopamine (DA) output. Activation of glycine receptors (GlyRs) located in the nucleus accumbens (nAc) suppresses inhibitory GABAergic feedback neurons, resulting in enhanced acetylcholine (ACh) output in the ventral tegmental area (VTA).
In an accompanying article, the effects of local and systemic application of acamprosate on dopamine output in the nAc were investigated. Acamprosate was demonstrated to modulate extracellular dopamine levels in the nAc, via both accumbal GlyRs and ventral tegmental nAChRs, in a similar manner as previously observed with both EtOH and taurine (Blomqvist et al., 1997; Ericson et al., 2006; Molander and Söderpalm, 2005a,b). In this study, we further investigated the functional relevance of GlyRs in the nAc in relation to the EtOH intake-reducing effects of acamprosate, by examining the influence of bilateral nAc infusions of strychnine or Ringer on EtOH intake in EtOH medium- and high-preferring rats treated with acamprosate or NaCl.
Materials and Methods
Animals
Male Wistar rats (B&K Universal AB, Sollentuna, Sweden) weighing 290 to 330 g, were used for the experiments. Upon arrival, the rats were housed in groups of 5 in a humidity (65%) and temperature (22°C) controlled room on a 12/12 hour reversed light/dark cycle (on 20:00 off 08:00), with free access to rat food (Harlan Teklad, UK) and tap water. Animals were allowed to adapt for at least 7 days to the animal maintenance facilities of the department prior to the EtOH preference screening (for details, see below). All experiments were conducted according to the Declaration of Helsinki and were approved by the Ethics Committee for Animals Experiments (Göteborg, Sweden) with the diary number 5/04.
Drugs and Chemicals
Acamprosate (kindly provided by Merck, Lyon, France) was diluted in saline. Strychnine, a competitive GlyR antagonist (Sigma-Aldrich, Stockholm, Sweden) was dissolved in Ringer (artificial cerebrospinal fluid) solution and perfused into the nAc or the VTA. The content of the Ringer solution was (in mmol/l): 140 NaCl, 1.2 CaCl2, 3.0 KCl, and 1.0 MgCl2. Ethanol (Kemetyl AB, Haninge, Sweden) was dissolved (2 to 4 to 6% v/v) with regular tap water.
Screening for Ethanol Preference
The EtOH concentration was gradually increased (2 to 4 to 6% v/v) over a 2-week period. After 1 week of access to 6% EtOH in addition to the tap water, the rats were housed individually in plastic cages (40 × 24 × 15 cm). They had continuous access to 2 bottles containing either tap water or 6% EtOH solution. Weight of the animals, water, and EtOH intake were measured over a 6-week period. The amount (g) of EtOH solution consumed, in percent of total fluid intake (g), was used as an index of EtOH preference. Animals were classified as EtOH low (drinking <20% of their daily fluid intake from the EtOH bottle), medium (20 to 60%), or high (>60%) preferring based on the average EtOH preference during the last 3 of the 6 weeks. Only medium- and high-preferring animals were used in this study. Animals included in the study were then equally, based on EtOH preference, divided into 4 treatment groups; (A) systemic acamprosate/local injections of strychnine, (B) systemic acamprosate/local injections of Ringer, (C) systemic NaCl/local injections of strychnine, and (D) systemic NaCl/local injections of Ringer.
Surgery and Limited Access Period
Bilateral guide cannulae (15 mm width [center to center] and 7 mm long, Plastics One, Roanoke, VA) were implanted in the EtOH medium- and high-preferring animals. The animals were anesthetized with isoflurane (Baxter; Apoteket AB, Stockholm, Sweden) and placed in a Kopf stereotaxic instrument where the guide cannulae were aimed at the bilateral nAc, with the coordinates +1.85 mm anterior and ±1.5 mm lateral to bregma, and −6.2 mm ventral to skull surface, according to the atlas of Paxinos and Watson (2007). The guides were fixed to the scull with 2 anchoring screws and Harvard cement (DabDental, Gothenburg, Sweden). A local anesthetic, Marcain (Apoteket AB), was applied to the wound and the animals were allowed to recover in their home cages for 2 days prior the start of the limited access (LA) paradigm. An obturator (Plastics One) was inserted into the guide cannulae to prevent contamination. For the remainder of the experiment, the animals had access to the bottles for 2.5 h per day during which they were able to choose between the bottle of water and the bottle of 6% EtOH solution. After 10 days on the LA paradigm, the animals demonstrated a new stable intake of fluids. Daily systemic injections of either acamprosate (200 mg/kg i.p) or NaCl 30 minutes before the animals had access to the bottles of fluids were then initiated. This procedure lasted throughout the remaining experiment.
Microinjection Procedure
During the 2 weeks between the implantation of guide cannulae and the start of microinjections each rat was handled 5 to 7 times to habituate the animal to the microinjection procedure. The animal was first held and then gently restrained in a towel and the guide cannulae obturators were removed and replaced. After the habituation procedure, the rat was returned to the home cage. Two days after the initiation of the systemic treatment, the injections were preceded with a local intracerebral microinjection. Extended syringes, with injection cannulae (Plastics One), were connected to a microperfusion pump (U-864 Syringe pump; AgnTho's, Lidingö, Sweden) and placed immediately above the nAc, approximately 1 mm below the tip of the guide cannulae. The pump was set to 0.5 μl/min and a total volume of 1 μl of either Ringer or strychnine (5 μg/μl) was injected bilaterally. After the injections the extended syringes were left in place for an additional minute, allowing the solution to stabilize in the tissue. The animals then received the systemic injection (2 ml/kg NaCl or 200 mg/kg acamprosate) followed by 30 minutes of rest before regaining access to the bottles (the time schedule for the entire experiment is depicted in Fig. 1A, and for the microinjection procedure Fig. 1B). After 3 consecutive days of microinjections, the animals received a microinjection of dye (Chicago Sky Blue diluted in Ringer) for verification of the injection area. The animals were then sacrificed and the brains were removed, fixed in Accustain (Sigma-Aldrich) and stored cold until verification of the injection site. Only animals with correctly placed guide cannulae and no visual errors (bleeding etc.) were included in the statistical analysis. Figure 2 demonstrates the approximate distribution of the microinjected drug in the brain 1 hour after dye injection.
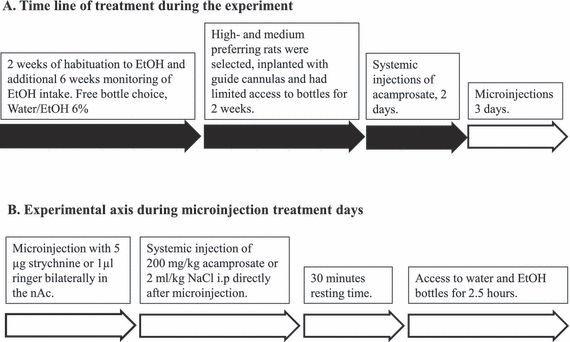
(A) Experimental procedure; 6 weeks of monitoring of ethanol intake during continual access to 6% ethanol and water was followed by implantation of guide cannulae and limited access to bottles for 2 weeks. Systemic treatment with either 200 mg/kg acamprosate or 0.9% NaCl was initiated 2 days before the microinjection procedures. (B) During microinjection days, all animals received bilateral microinjection with 1 μl of strychnine (5 μg) or Ringer in the nucleus accumbens (nAc) directly followed by a systemic injection. Thirty minutes later, the animals were allowed access to the bottles for 2.5 hours.
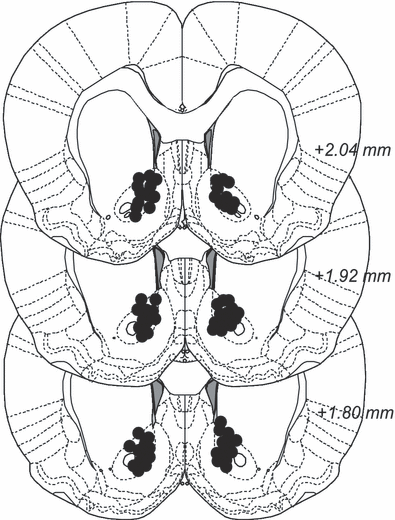
Histology. After experiments, a microinjection with diluted Chicago Sky Blue was performed in an attempt to see the dispersion of the injection in the brain. Black dots indicate dispersion of color. Animals with observable bleeding or leakage of stain were excluded from the statistical analysis.
Statistical Analysis
The data were statistically analyzed using an independent t-test or ANOVA (analysis of variance) with repeated measures followed by LSD (least significant difference) post hoc test. When appropriate, dependent variables were analyzed using paired t-test. A probability value (p) less than 0.05 was considered statistically significant. All values are expressed as mean ± SEM.
Results
Acamprosate Decreases Ethanol Intake
The first phase of the study involved systemic saline or acamprosate (200 mg/kg) administration while monitoring EtOH consumption. Groups C and D, receiving saline injections, maintained their EtOH consumption. Groups A and B, consuming moderate amounts of EtOH (0.89 g/kg/d [ranging from 0.39 to 1.65 g/kg/d] in a LA paradigm) decreased their EtOH intake after 2 days of systemic acamprosate injections (Fig. 3; paired sampled t-tests between treatment days LA (average value of the last 5 LA days) and systemic injection days 1 (SI1) and 2 (SI2)—acamprosate; p = 0.390 (LA vs. SI1) and p < 0.001 (LA vs. SI2), NaCl; p = 0.589 (LA vs. SI1) and p = 0.519 (LA vs. SI2). Also a 2-way ANOVA with repeated measures indicated an effect of acamprosate over time (time effect [LA-SI2; F2,58 = 7.233, p = 0.002], a significant interaction term [F2,58 = 4.486, p = 0.015], but no group effect [F1,29 = 0.414, p = 0.525]). After the initial phase, where acamprosate decreased EtOH intake, a second phase of the study was commenced to investigate the role of accumbal GlyRs in the acamprosate-induced decrease in EtOH intake. All groups of animals received microinjections of either strychnine (5 μg) or Ringer into the nAc prior to the systemic injection (acamprosate or saline) and access to the fluid bottles.
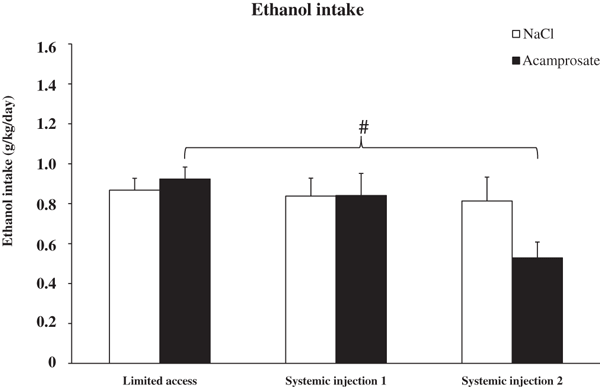
Ethanol intake during limited access (mean value of the last 5 limited access days) and the 2 systemic injection days. The ethanol intake of the 2 treatment groups, acamprosate (n = 13) and NaCl (n = 18) were indistinguishable during limited access and systemic injection day 1. During the second day of systemic treatment, acamprosate administration significantly decreased ethanol intake (2-way anova with repeated measures and t-test #represents p value <0.01). Bars represent group mean ± SEM.
Accumbal Strychnine Reverses the Acamprosate-Induced Reduction in Ethanol Intake But Does Not Influence Total Fluid Intake
The acamprosate-treated group of rats receiving Ringer locally in the nAc maintained their decreased EtOH intake for the remainder of the experiment, indicating that the microinjection per se did not influence the consumption of fluids during the 2.5 hours that the animals were allowed access to the bottles (Fig. 4A; paired sampled t-test acamprosate (SI2) vs. microinjection treatment days 2 (MI2; p = 0.874) and 3 (MI3; p = 0.407). The acamprosate-treated animals receiving strychnine locally in the nAc elevated their EtOH intake to the same level or higher, than that observed prior to acamprosate treatment (Fig. 4A, SI2 vs. MI2 p = 0.002; SI2 vs. MI3 p = 0.009). When comparing the 2 acamprosate-treated groups during the second microinjection day (MI2) a significant difference in EtOH intake was observed (acamprosate/Ringer vs. acamprosate/strychnine p = 0.03, unpaired t-test). Microinjections of either Ringer or strychnine to rats receiving systemic saline did not influence the choice of fluids (Fig. 4B). Insets in Fig. 4A and 4B illustrate the EtOH intake in acamprosate- and NaCl-treated animals for the last 5 LA days (LA1–LA5), the systemic injection days (SI1–SI2), and the microinjection days (MI1–MI3). A 2-way ANOVA for repeated measures performed between SI2 and MI3 revealed significant time effect (F3,81 = 3.870, p = 0.012) and interaction term (F9,81 = 2.668, p = 0.009) but no group effect (F3,27 = 0.564, p = 0.644).
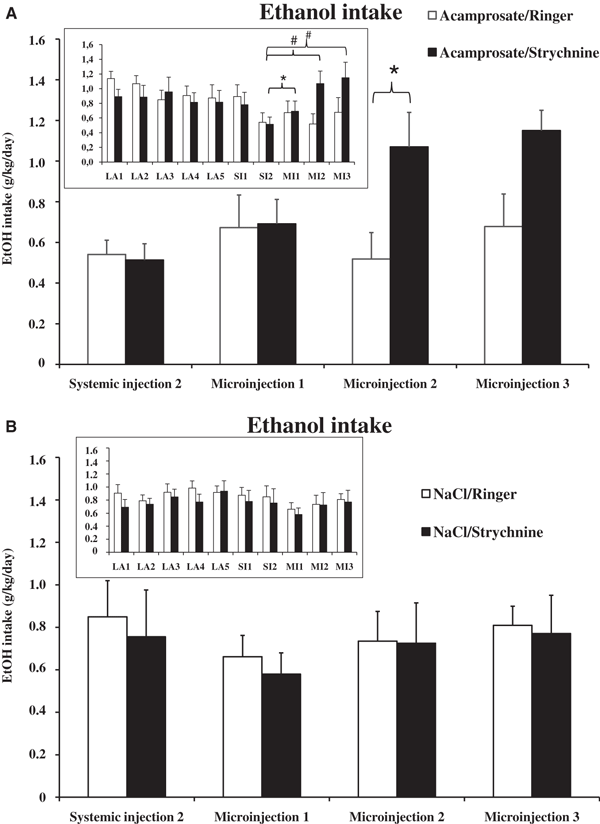
(A) Ethanol intake in acamprosate-treated groups during the systemic injection day 2 (SI2) and the 3 microinjection days (MI1-3). Data are presented as mean ± SEM. The 2 groups (acamprosate/Ringer, n = 7; acamprosate/strychnine, n = 6) were indistinguishable during systemic injection day 2 and microinjection day 1. On the second and third microinjection day, the acamprosate/Ringer group remained at the same level of ethanol intake, while the acamprosate/strychnine group increased their ethanol intake, indicating the absence of acamprosate’s ethanol intake-reducing effects. For statistical details, see Results. Inset; An overview of ethanol intake during the last 5 limited access days (LA1-5), systemic injection days (SI1-2), and microinjection days (MI1-3) in the 2 acamprosate-treated groups. (B) Ethanol intake in NaCl-treated groups during microinjection days compared with the last systemic injection day revealed no differences. Inset; An overview of ethanol intake during the last 5 limited access days (LA1-5), systemic injection days (SI1-2), and microinjection days (MI1-3) in the 2 saline-treated groups. *p < 0.05, #p < 0.01.
The total fluid intake in all 4 groups during the LA, systemic injections (SI1 and SI2), and microinjection days (MI1, MI2 and MI3) is shown in Fig. 5A. The total fluid intake in both the acamprosate/Ringer and the acamprosate/strychnine group was significantly reduced on systemic treatment day 2 (SI2), compared with the respective LA baseline levels (paired sampled t-test p = 0.015 and 0.042, respectively). These effects are probably explained by an acamprosate-induced decrease in EtOH intake without a simultaneous increase in water intake. Figure 5B illustrates water intake in all of the groups, within groups comparisons (paired sample t-test) revealed no significant differences when compared to the pretreatment baseline water intake.
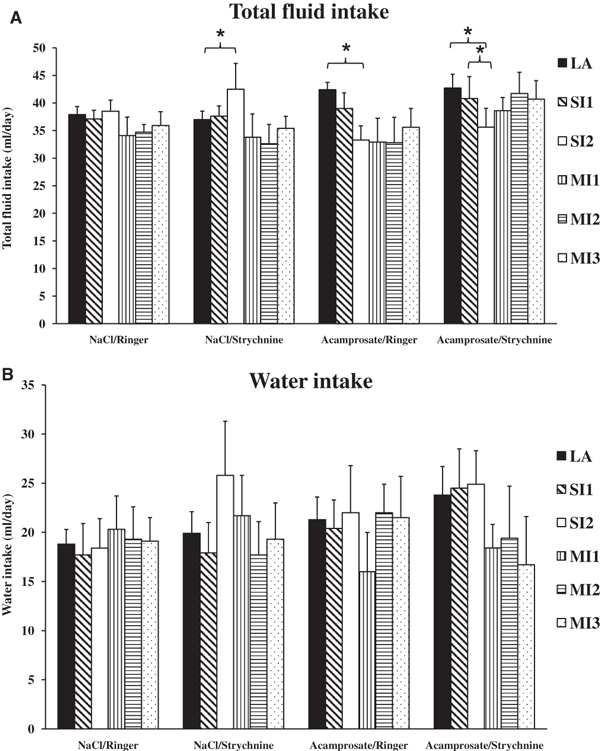
(A) Illustration of total fluid intake in all groups during limited access (LA; mean value of the last 5 days of limited access), systemic injection days (SI1-2), and microinjection days (MI1-3). Fluctuations are most pronounced in the 2 acamprosate-treated groups due to their alterations in ethanol intake, while the fluctuations observed in the NaCl-treated groups are, except for 1 day (SI2) in NaCl/strychnine, not significantly different during the final 10 experimental days. (B) Overview of the water intake in all groups during the same experimental days as in Fig. 5A (LA, SI1-2, MI1-3). Within group comparisons (paired sample t-test) revealed no significant differences when compared with the pretreatment baseline water intake (LA).
Discussion
To our knowledge this is the first time that an acamprosate-induced reduction in EtOH intake has been shown to be reversed by another pharmacological substance, in this case the competitive GlyR antagonist strychnine. These results, in conjunction with the neurochemical results presented in an accompanying paper, strongly indicate that acamprosate produces its EtOH intake-reducing effect via direct or indirect activation of GlyRs in the nAc and further highlights the importance of these receptors in the regulation of EtOH intake in the rat.
In the accompanying article, we demonstrate that both local and systemic administration of acamprosate increase extracellular dopamine levels in the nAc, and, in consonance with findings with EtOH (Molander and Söderpalm, 2005b) and taurine (Ericson et al., 2006), nAc administration of the competitive GlyR antagonist strychnine blocks both these effects. As augmented accumbal dopamine levels may be involved in the reinforcing effects of EtOH, the acamprosate-induced dopamine modulation might be one of the mechanisms mediating acamprosate′s EtOH intake-reducing effect. Due to technical limitations, extracellular dopamine levels were not measured in the present study, but based on the results obtained in the accompanying article and on studies performed by Molander and colleagues (2005), it is highly likely that extracellular dopamine levels are concomitantly modulated by EtOH consumption and acamprosate. In the study by Molander and colleagues (2005), EtOH consumption was associated with elevation of nAc dopamine levels in animals perfused with Ringer through the dialysis probe. Rats perfused with glycine also increased their dopamine levels in the nAc, but simultaneously decreased their EtOH consumption and no further elevation of dopamine was observed. In analogy, it is reasonable to assume that acamprosate in this study, elevates dopamine via GlyR activation in the same brain region and that this increase is related to the observation of a reduced EtOH intake. After administration of strychnine, the dopamine activation by acamprosate most likely was antagonized (as in the accompanying paper) and, hence, the EtOH intake-reducing effect was reversed.
Molander and colleagues (2005) demonstrated that local bilateral perfusion of strychnine and glycine reciprocally altered accumbal dopamine levels and strychnine slightly increased EtOH intake, although water intake tended to also increase. In the present study, microinjections of strychnine did not alter EtOH intake in saline-treated animals. The reason for this discrepancy may be the difference in experimental designs. In this study, both medium- and high-preferring animals were tested in contrast to strongly high-preferring rats in the former study. Moreover, in this study repeated microinjections were given, whereas in the former study the animals were exposed only once to strychnine by reversed microdialysis. Further, during a microinjection the substance is applied directly in the nAc, which leads to diffusion of the drug to areas surrounding the injection site. The concentration of strychnine in the volume applied will, during the period between injection and acamprosate administration, be further diluted by an unknown factor. During microdialysis administration, the tissue concentration of the substance will be 5 to 10% of the perfusate concentration and the diffusion kinetics might differ from that after microinjection.
The specificity of strychnine was discussed in the accompanying article. High concentrations of strychnine are believed to antagonize also GABAA receptors and to interact with alpha 7 nAChRs (Hussy et al., 1997; Maksay, 1998; Palma et al., 1999; Shirasaki et al., 1991; Xu, 1999) and inhibition of GABAA (for review see Korpi, 1994) or accumbal nAChRs (Nadal et al., 1998) decreases EtOH intake. However, in this study strychnine per se did not affect EtOH intake, which would argue against interference with these receptor populations in the dose applied.
According to Ikemoto (2007), microinjected substances are only active a few minutes and will probably diffuse into other areas in the brain, whereas in this study, systemic injections were given 30 minutes after the nAc microinjection of strychnine. Based on the outcome of the study, i.e. that strychnine clearly antagonized the EtOH intake-reducing effect of acamprosate, it is obvious that strychnine maintained its pharmacological effect throughout the time period studied. However, given the diffusion of the microinjected substance, the localization of the effect to the nAc may be questioned. Microinjection with color via the guide cannulae after the final experimental day was done in order to confirm the positions of the guide cannulae and to estimate the diffusion of strychnine/vehicle in the nAc area. Although, no divergences/deviations were observed, the color can probably not fully represent the diffusion of strychnine and it cannot be excluded that the substance has reached also other brain areas.
The effect of acamprosate on EtOH self-administration and consumption is in general agreement with previous studies (Boismare et al., 1984; Cowen et al., 2005; Hölter et al., 1997; Le Magnen et al., 1987; Paille et al., 1995; Spanagel et al., 1996). Acamprosate’s EtOH intake-reducing effect has been reported to be associated with an increase in dopamine transporter binding and a significant decrease in dopamine D2-like binding (Cowen et al., 2005), as well as a reduction in extracellular glutamate overflow in the ventral striatum (Spanagel et al., 2005). The effects on the dopamine system could very well be secondary to the effects of acamprosate reported in the accompanying paper, i.e., the dopamine-activating effect mediated via GlyR activation. Also, the reduction in glutamate overflow in the nAc could theoretically derive from GlyR activation in the nAc, as GlyRs generally are inhibitory. Both the dopamine and the glutamate system have previously been heavily implicated in the context of alcohol addiction and here we implicate also GlyRs, the activation of which may be the primary mechanism of action underlying the EtOH intake-reducing effect of acamprosate. GlyRs were recently implicated also by the findings that a glycine transporter inhibitor, ORG 25935, which increases extracellular glycine levels, robustly and consistently reduces EtOH intake and preference in Wistar EtOH high- and medium-preferring rats (Lidö et al., 2009; Molander et al., 2007; for review see Söderpalm et al., 2009).
In conclusion, even though the EtOH intake-reducing effects of acamprosate need to be studied in greater detail to pinpoint the exact mechanism for its actions, we believe that this study demonstrates involvement of accumbal GlyRs. In addition, the results here reported provide further support for our general hypothesis that GlyRs are functionally important for regulating EtOH consumption in the rat.
Acknowledgments
The authors would like to acknowledge Dr. Chris Pickering for reading the manuscript and correcting linguistic errors. Financial support for this work was obtained from the Swedish Medical Research Council (Diary numbers 2006 to 4988 and 2006 to 6385), the Swedish Labor Market Insurance (AFA) support for biomedical alcohol research, governmental support under the LUA/ALF agreement, the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, Wilhelm and Martina Lundgrens Veternskapsfond, Magnus Bergvalls Stiftelse, Gunnar och Märta Bergendahls Stiftelse and Sigurd och Elsa Goljes minne.




