Alcohol, Signaling, and ECM Turnover
Abstract
Alcohol is recognized as a direct hepatotoxin, but the precise molecular pathways that are important for the initiation and progression of alcohol-induced tissue injury are not completely understood. The current understanding of alcohol toxicity to organs suggests that alcohol initiates injury by generation of oxidative and nonoxidative ethanol metabolites and via translocation of gut-derived endotoxin. These processes lead to cellular injury and stimulation of the inflammatory responses mediated through a variety of molecules. With continuing alcohol abuse, the injury progresses through impairment of tissue regeneration and extracellular matrix (ECM) turnover, leading to fibrogenesis and cirrhosis. Several cell types are involved in this process, the predominant being stellate cells, macrophages, and parenchymal cells. In response to alcohol, growth factors and cytokines activate many signaling cascades that regulate fibrogenesis. This mini-review brings together research focusing on the underlying mechanisms of alcohol-mediated injury in a number of organs. It highlights the various processes and molecules that are likely involved in inflammation, immune modulation, susceptibility to infection, ECM turnover and fibrogenesis in the liver, pancreas, and lung triggered by alcohol abuse.
Excessive alcohol consumption has been reported to have causal relationship with more than 60 types of diseases (WHO, 2006). Chronic alcohol abuse damages nearly every organ in the body, especially the liver and pancreas. Investigations have shown that chronic alcohol invokes complex but common pathophysiological changes through altered metabolic, immunologic, and inflammatory processes in many organs. Chronic alcohol consumption is also responsible for approximately 3.6% of cancers worldwide (Seitz and Stickel, 2007). Alcohol as a direct hepatotoxin can induce cell damage through increased generation of reactive oxygen species (ROS) (Breitkopf et al., 2005; Parola and Robino, 2001) and by altering gut permeability to release bacterial endotoxin lipopolysaccharide (LPS) (Fukui et al., 1991; Nanji et al., 1994). Other mechanisms that have emerged from studies in several organs and experimental models of alcohol include changes in pro-inflammatory and pro-fibrogenic cytokines such as interleukins (IL-1α, IL-6, and IL-8) (Neuman et al., 1998), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β) (Tilg and Diehl, 2000). ROS produced during alcohol metabolism leads to inflammation mainly through TNF-α and TGF-β and cause cell death in the liver, lung, pancreas, heart and the brain. In addition, alcohol modulates local innate and adaptive immune responses (Neuman et al., 1993, 1998) that can alter resistance to a number of bacterial (Gamble et al., 2006; Szabo and Mandrekar, 2009) and viral (Graham, 2006; Neuman et al., 2008; Samet et al., 2003) infectious agents. Factors such as bacterial and viral infections enhance the inflammation and the deleterious effects of alcohol. Findings also suggest that acute and chronic alcohol exposure incur different mechanisms which respectively, either suppress or activate immune response on bacterial infection (D’Souza El-Guindy et al., 2007). Several cell–cell signaling pathways have been implicated that regulate the immune response(s), trigger fibrogenesis and wound healing through activation of transcription factors, such as, nuclear factor (NF)κB and activation protein-1 (AP-1). With persistent alcohol abuse, the injury progresses through impairment of tissue regeneration, cytokine production, leukocyte infiltration and extracellular matrix (ECM) turnover, and fibrogenesis leading to cirrhosis. As fibrosis develops there is a shift in the subendothelial ECM from low-density matrix to interstitial fibrillar collagen (Gressner and Bachem, 1990) and a significant increase in total collagen content (Rojkind et al., 1979). Several cell types are involved in this process, predominantly stellate cells and macrophages. Recent studies demonstrate that parenchymal cells, such as hepatocytes also play a major role in alcohol-induced pathogenesis (Dooley et al., 2008; Zeisberg et al., 2007). Advances in delineating the molecular mechanisms in alcohol-mediated tissue injury have been made mostly with alcoholic liver disease (ALD) which has also served as a model for alcohol damage in other organs.
Novel mechanisms of alcohol injury in diverse organs and tissues
Recent progress in alcohol research has revealed novel mechanisms, such as the discovery of sphingomyelinases playing a role in the TNF-α induced heptaocyte cell death and fibrogenesis (Marí and Fernández-Checa, 2007; Marí et al., 2004); TFG-β-mediated regulation of Cyp21A1 and ADH1 contributing toward liver fat accumulation and oxidative stress, and the antagonistic action of Smad 7 at different stages and locations of TGF-β-mediated signaling in ALD (Dooley et al., 2001, 2008). Regulation of plasmin homeostasis through PAI-1 (Arteel, 2008; Seth et al., 2008) and other fibrinolysis regulating molecules, such as osteopontin (Opn) (Apte et al., 2005b; Seth et al., 2006a,b) are also some of the recent discoveries that provide insights in the common mechanisms involved in alcohol-induced organ injury. More recently, Opn is also suspected to have a role in alcohol-mediated lipid accumulation in the hepatocytes by modulating peroxisome-proliferator activated receptor alpha (PPAR-α) (Nath and Szabo, 2009). PPAR-α, a transcription factor regulating genes involved in fatty acid oxidation, is known to suppress lipid accumulation and is downregulated with chronic alcohol consumption. However, chronic alcohol fed Opn knockout mice display increased PPAR-α, lipid accumulation and liver injury (Lee et al., 2008). In other forms of alcohol-mediated injury, alcohol as a co-factor modulates immune responses and can alter resistance to bacterial and viral infections. Mechanisms include increased cell toxicity and immune modulation via TNF-α led signaling events during viral infection. Various strains of Friend virus can induce erythroleukemias through activation of erythropoietin (Epo) (Shaked et al., 2005), and Epo is also associated with enhanced tumorigenicity of erythroleukemias (Cocco et al., 1996). A link between the severity of leukemia and alcohol consumption has also been reported (Ido et al., 1996), and mice treated with alcohol had enhanced Epo-R-mediated signaling and acceleration of preleukemic stage, suggesting that Epo pathway is important in enhancing preleukemic stage, and that alcohol plays a role in this process. These systems are highlighted in Fig. 1 and described below in more detail.
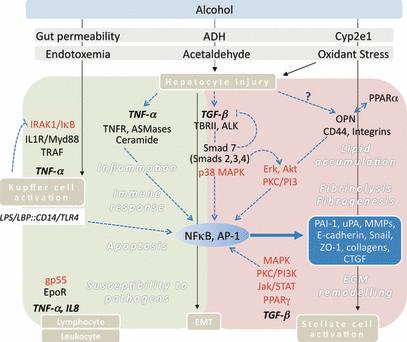
Overview of novel pathways in alcohol induced tissue injury in the liver. Alcohol mediates cellular injury in liver cells through endotoxin, oxidative stress, and its metabolites, such as acetaldehyde, mainly via TNF-α (gray box) and TGF-β (pink box). This invokes downstream signaling in a series of molecules (red) leading to activation of transcription factors NFκB and AP-1. The activated transcription factors regulate expression of various molecules (blue box) involved in immune response, inflammation, apoptosis, susceptibility to pathogens mainly via TNF-α, and lipid accumulation, fibrinolysis, fibrogenesis, and ECM remodeling via TGF-β. These processes occur in many cell types including Kupffer cells, stellate cells, lymphocytes, and hepatocytes (brown boxes). AP-1, activation protein 1; ALK, serine/threonine kinase receptor; ASMases, acidic sphingomyelinases; CTGF, connective tissue growth factor; Cyp2e1, cytochrome P450 2e1; EMT, epithelial mesenchymal transition; EpoR, erythropoietin receptor; Erk, extracellular signal-regulated kinases; IRAK1, Interleukin 1 receptor associated kinase; IκB: Inhibitor of κB; Jak/STAT: Janus kinases/Signal Transducers and Activators of Transcription; MAPK, mitogen associated protein kinases; MMPs, matrix metalloproteinases; MyD88, myeloid differentiated factor 88; NFκB, nuclear factor κB; OPN, osteopontin; PAI-1, plasminogen activator inhibitor-1; PI3K, phosphatidylinositol kinase; PKC, protein kinase C; PPARα, peroxisome proliferator activated receptor alpha; PPARγ, peroxisome proliferator activated receptor gamma; Smad, homolog of drosophila mothers against decapentaplegic; TNFR, TNF-α receptor; TRAF, TNFR associated factor; TBRII, TGF-β receptor II; uPA, urokinase plasminogen activator; ZO-1, zonula occludens.
Toxicity of Alcohol Metabolite Acetaldehyde
Alcohol, directly or via its metabolites, acetaldehyde and acetate, can evoke mechanisms that promote cellular and tissue injury. The metabolism of alcohol to acetaldehyde and acetate by way of alcohol and acetaldehyde dehydrogenases (ADH and ALDH, respectively), and microsomal cytochrome P450 2E1 (Cyp2E1), leads to persistent redox stress associated with lipid peroxidation, increased collagen production (Tsukamoto, 1993) and fibrogenesis. Acetaldehyde forms neo-antigenic protein- and DNA-adducts and also generates IgAs that are deposited in the tissues and are responsible for inflammatory and immune responses in the cells (Seitz and Homann, 2007; Tuma and Casey, 2003). DNA adducts may subsequently lead to replication errors and point mutations. Acetaldehyde may directly bind to proteins related with DNA repair and methylation and thus interfere with processes controlling gene activity and integrity of DNA. In addition, acetaldehyde-induced chromosome aberrations are likely to eventually lead to cancer development. There is now convincing evidence that the carcinogenic effect of alcohol is due to the DNA mutagenic properties of acetaldehyde operating at multiple levels. These diverse mutagenic mechanisms are summarized in detail in a recent review by Seitz and Stickel (2007). However, as acetaldehyde is rapidly metabolized in the liver, it only plays a minor role in hepatocellular carcinoma development, whereas these mechanisms are pivotal in other alcohol-related cancers, like that of the upper aerodigestive tracts, breast and colorectal cancer (Seitz and Becker, 2007). Acetaldehyde also disrupts the barrier function provided by tight junctions in epithelial cells. In the hepatocytes, tight junctions are disrupted by acetaldehyde thereby increasing paracellular permeability. In vitro studies using intestinal epithelial Caco-2 cells have shown that the effect of acetaldehyde on tight junctions is mediated via tyrosine kinase dependent inhibition of protein tyrosine phosphatase activity (Atkinson and Rao, 2001). Acetaldehyde-induced disruption of tight junctions is also associated with increased tyrosine phosphorylation of several related proteins, such as zonula occludens (ZO)-1 and β-catenin, indicating that acetaldehyde can modify intracellular signaling pathways to destabilize the tight junction protein complex leading to increased permeability to endotoxin (Atkinson and Rao, 2001) and fibrogenesis. Acetaldehyde also induces collagen 1 synthesis in the hepatic stellate cells (HSCs) (Greenwel, 1999) and promotes ECM remodeling by altering matrix metalloproteinases (MMPs) (Casini et al., 1994). Acetaldehyde-induced downstream signaling occurs through several transcription factors and complex pathways (Tommaso et al., 2008). For example, collagen α1 and α2 promoters have acetaldehyde-responsive elements within binding sites for transcription factors, as well as overlapping with TGF-β and TNF-α response elements (Greenwel, 1999) evident of complexities and multilayered controls. Acetaldehyde triggers phosphorylation of protein kinase C (PKC) involving extracellular receptor kinase (Erk)1/2 and phosphatidylinositol kinase (PI3K) signaling and activation of transcription factor AP-1, leading to collagen induction (Svegliati-Baroni et al., 2001, 2005).
LPS-Mediated Alcohol-Induced Tissue Injury
One of the main features of alcohol-induced tissue injury is via LPS. The onset of ALD is steatohepatitis characterized by progressive tissue damage initiated by lipid accumulation in the liver. This is accompanied by infiltration of inflammation promoting cells that migrate toward the liver in response to activation of Kupffer cells (KC), the liver resident macrophages. Alcohol augments the translocation of gut-derived LPS which is central to the activation of KCs in ALD (Wheeler et al., 2000).When activated, KCs produce signaling molecules, such as cytokines, that promote inflammation and increase ROS. Inactivating KCs prevents alcohol-induced liver injury underscoring the significance of these cells in ALD pathogenesis (Adachi et al., 1994). The LPS and LPS binding protein (LBP) complexes with CD14 and toll like receptor-4 (TLR-4) on activated KC surface (Su, 2002), triggering the pro-inflammatory signaling cascades that are important for the development of ALD. TLR-4 also mediates inflammatory signaling by LPS in HSCs (Paik et al., 2003). In activated stellate cells, TLR-4 is upregulated, further sensitizing this cell type leading to fibrogenic responses in ALD (Paik et al., 2003). The importance of this mechanism in ALD has been impressively documented by Uesugi and colleagues (2001) and is further supported by the finding of TLR-4 gene polymorphisms connected to liver fibrosis (Guo et al., 2009; Huang et al., 2007). Similarly, development of chronic pancreatitis was enhanced in the alcohol-fed rats with repeated injections of LPS as evidenced by acinar atrophy and pancreatic fibrosis (Vonlaufen et al., 2007) (Fig. 2A).
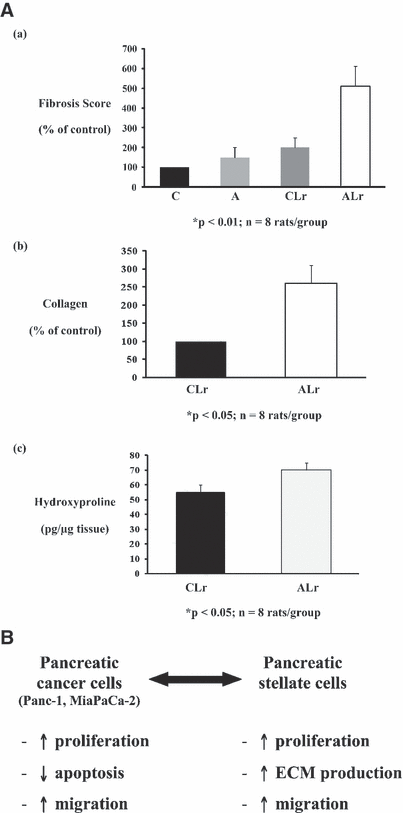
(A) Effect of alcohol±LPS on histological and biochemical indices of pancreatic fibrosis. Pancreatic sections and tissue homogenates from 4 rat groups were analyzed: non-alcohol-fed no LPS (Control, C), alcohol-fed no LPS (A), nonalcohol fed, LPS (CLr) and alcohol-fed LPS (ALr). Panels (a) and (b) represent data for morphometric analysis of sections stained with Masson’s trichrome and Sirius Red (for collagen) respectively. Panel (c) depicts data for hydroxyproline content of the pancreas. Alcohol-fed rats subjected to repeated LPS injections showed significant increases in Masson’s trichrome and Sirius Red staining as well as in pancreatic hydroxyproline levels, compared to the C, A, or CLr animals. (B) Interactions between pancreatic cancer cells and pancreatic stellate cells. Pancreatic cancer cells increase PSC proliferation, extracellular matrix synthesis, and migration. In turn, PSCs increase cancer cell proliferation and migration but decrease cancer cell apoptosis.
Bacterial toxins are known to be injurious to the lung by impairing microcirculation and, similar to the liver and pancreas, by promoting chemical mediators release and inducing oxidative stress. However, unlike the liver, the lung is constantly exposed to particulate matter from the external environment in performing the function of gas exchange. Live microorganisms and LPS are common biological constituents of the particulate matter. These are usually cleared by the mechanical (e.g., mucociliary apparatus) and chemical processes in the upper respiratory tract. When these processes are impaired or fail the particulate matter enters into the alveoli, evoking a self resolving inflammatory response by the alveolar macrophage (AM). Alcohol abuse perturbs the mucociliary clearance of the pathogens by cilia, the AM recognition and phagocytosis of the stimuli, generation of chemical mediators, and the recruitment of auxiliary defenses, as well as the secretion of surfactant by lung epithelial cells (D’Souza El-Guindy et al., 2007; D’Souza et al., 1996; Gamble et al., 2006; Joshi and Guidot, 2007; Szabo and Mandrekar, 2009; Wyatt et al., 2004). These adverse effects of acute and chronic alcohol intoxication on the lung have been reviewed extensively elsewhere (Boé et al., 2009; Happel and Nelson, 2005). Like the liver KC, the AM is the primary defender of the lung. It recognizes the stimulus, that escapes expulsion by the upper respiratory tract, through pattern recognition receptors including the TLRs, and then releases chemical mediators (e.g., cytokines, chemokines, and ROS). These are bactericidal in nature and/or facilitate the recruitment of other leukocytes for effective removal of the stimulus and restoration of lung homeostasis. In addition to the AM, other innate immune cells including neutrophils, monocytes, dendritic, and natural killer cells are also equipped with the pattern recognition receptors and can aid in defense against a variety of lung infections.
Role of Stellate Cells in Alcohol-Induced Fibrogenesis and Cancer
Pancreatic injury in response to alcohol can occur via pancreatic stellate cells (PSCs) that are shown to be the key players in alcoholic pancreatitis (Apte et al., 1998; Bachem et al., 1998). In normal tissue, PSCs play an important role in maintaining a delicate balance between ECM synthesis and degradation. During alcoholic pancreatic injury, PSCs are activated in a paracrine fashion, by growth factors, pro-inflammatory cytokines, and oxidant stress (Apte et al., 2005a) and are the major source of collagen deposition in fibrotic pancreas (Haber et al., 1999). The likely activating factors include proliferative and pro-fibrogenic growth factors such as platelet derived growth factor (PDGF) and TGF-β, as well as oxidant stress generated within the tissues by oxidative metabolism of alcohol to acetaldehyde and subsequently to acetate (Apte et al., 1999; Masamune et al., 2002). Notably, PSCs can also synthesize endogenous cytokines resulting in an autocrine loop of cell activation (Shek et al., 2002; Sparmann et al., 2005). Due to the generally accepted fact that chronic alcohol administration is insufficient to cause overt pancreatic (and other tissue) injury in experimental animals, additional triggers such as LPS, may be needed to produce demonstrable tissue injury. In vivo models of pancreatic injury using alcohol and LPS exhibited increased collagen staining around ducts and near acinar cells, associated with increased hydroxyproline levels and increased expression of pro-collagen and alpha smooth muscle actin (α-SMA), an indication of PSC activation (Perides et al., 2005). Given that PSCs are now established as key players in the fibrosis of chronic pancreatitis, it is not surprising that the putative role of these cells in pancreatic cancer has attracted the attention of researchers. It is well known that one of the important risk factors for pancreatic cancer is chronic pancreatitis, and as noted earlier, the 2 diseases have a striking histopathologic feature in common i.e., extensive fibrosis (desmoplasia). Indeed, microarray analyses have demonstrated that the stromal compartments of chronic pancreatitis and pancreatic cancer have at least 107 genes in common (Binkley et al., 2004). Using in vitro and in vivo models, it has also been demonstrated that PSCs are responsible for producing the desmoplastic reaction in pancreatic cancer (Apte et al., 2004). A bidirectional interaction exists between PSCs and pancreatic cancer cells, indicating that cancer cells recruit PSCs which, in turn, facilitate local tumor progression and distant metastasis (Apte et al., 1998; Bachem et al., 1998). In vitro studies using PSCs either co-cultured with or exposed to conditioned media from cancer cells have established that (i) pancreatic cancer cells induce PSC activation (as indicated by increased proliferation, extracellular matrix synthesis and migration) and that (ii) PSCs induce cancer cell proliferation but inhibit apoptosis, thereby effectively enhancing the survival of cancer cells (Fig. 2B) (Apte et al., 2004; Bachem et al., 2005; Vonlaufen et al., 2008). Thus pancreatic cancer cells are able to recruit host PSCs to their immediate vicinity, and in turn, PSCs can establish a growth permissive environment for tumor cells. Activation of PSCs by cancer cells is mediated by fibroblast growth factor (FGF) and PDGF secreted by cancer cells (Bachem et al., 2005) and Apte’s group recently demonstrated that the proliferative effect of PSC secretions on pancreatic cancer cells is mediated, at least in part, by PDGF (Vonlaufen et al., 2008). An in vivo orthotopic tumor model shows accelerated pancreatic tumor growth in the presence of PSCs, fibrosis within tumor tissue, increased cancer cell proliferation, decreased apoptosis and significantly higher regional and distant metastasis (Vonlaufen et al., 2008). These novel observations show that PSC activation is a central feature of alcoholic pancreatic fibrosis as well as pancreatic cancer. The orthotopic tumor model of alcoholic pancreatitis presents an important tool for (i) the assessment of the chronological sequence of events in the pancreas during injury, (ii) the evaluation of efficacy of therapeutic strategies, and (iii) determination of the potential reversibility of pancreatic acinar injury and fibrosis upon withdrawal of alcohol.
Alcohol-induced factors such as ROS, LPS, cytokines, chemokines, and growth factors drive the liver response by activating hepatic stellate cells (HSCs), similar to PSCs in the pancreas. Role of HSCs in liver injury of any etiology is well established as these cells undergo activation through stages of initiation and perpetuation similar to PSCs (Friedman, 1993). Initiation results from paracrine stimulation from neighboring cells and the ECM disruption by metalloproteinases (Li and Friedman, 1999). Perpetuation involves the maintenance of the activated state through enhanced cytokine expression resulting from both autocrine and paracrine stimulation and continued ECM remodeling (Friedman, 2000).
Similar mechanisms, to those in the pancreas, may also be operating in the liver during alcohol-mediated carcinogenesis, where HSCs are known to mediate fibrosis and interact with epithelial cells on alcohol activation. Strikingly again like pancreatitis, cirrhotic livers have a higher incidence of developing cancer (Kojiro and Roskams, 2005). The inflammatory and fibrotic milieu, driven through several cytokines including TGF-β, TNF-α, PDGF, and connective tissue growth factor (CTGF) triggering several signaling pathways, seem to be similar in both organs. The profibrotic TGF-β plays an important role during pathogenesis of liver disease (premalignant stages) and malignant transformation by a switch to a tumor progression (Dooley et al., 2009). In addition, TNF-α-mediated NFκB/IκB kinase 2 (IKK2) activation elicits cell survival and anti-apoptotic signals favoring tumorigenesis. Maeda et al. showed that IKK2 knockout mice were protected from chemical-induced carcinogenesis (Maeda et al., 2005) and by extension, suggests a role for this pathway even for alcohol-mediated carcinogenesis. In the liver, other alcohol metabolic products also seem to be involved in carcinogenesis. ROS produced by a number of enzymatic reactions during alcohol metabolism by ADH1 and Cyp2E1, is the driving force in malignant transformation, together with ongoing cirrhosis. Elevated ROS levels increase lipid peroxidation and generate reactive molecules such as malondialdehyde, which then react with DNA bases. This results in highly mutagenic exocyclic DNA adducts (Hu et al., 2002), which increasingly occur during ALD progression (Frank et al., 2004). Cyp2E1 activity is also elevated up to 20-fold in patients chronically consuming alcohol (Oneta et al., 2002). This amplifies ROS formation upon alcohol challenge and accelerates DNA damage. Elevated Cyp2E1 activity further contributes to transformation by activation of pro-carciongens like nitrosamines (Seitz and Stickel, 2007). Additionally, reactive nitrogen species is also known to precede the development of hepatocellular carcinoma. Cyp2E1 is also implicated in retinoic acid receptor-mediated c-jun kinase (JNK) signaling pathway and AP-1 transcription factor expression in experimental models of alcohol, favoring hepatic cell proliferation and survival in malignant transformation (Wang et al., 1998b). Moreover, genetic predispositions, such as polymorphisms in genes coding for ADH, ALDH, Cyp2E1, CD14, TNF-α, and others can considerably contribute to the risk of alcohol abuse and addiction, and thus indirectly to development of alcohol-induced cancer (Edenberg, 2007; Stickel and Osterreicher, 2006).
Both HSCs and PSCs, when activated have a myofibroblast phenotype, increased cytokine production and secrete excessive amounts of ECM proteins encouraging development of fibrosis (Friedman, 1999b, 2000). Histological, immunohistochemical, and in situ hybridisation techniques have established that PSCs and HSCs are the predominant source of the collagen deposited in fibrotic pancreas (Haber et al., 1999) and liver, respectively. Other mesenchymal cell types have also been identified that can differentiate into active pro-fibrogenic fibroblast and contribute to tissue injury via TGF-β signaling (Beaussier et al., 2007) likely toward carcinogenesis.
Role of Hepatocytes: Epithelial to Mesenchymal Transition
Emerging evidence implicates hepatocytes as a source of pro-fibrogenic fibroblastoid cells by virtue of undergoing epithelial to mesenchymal transition (EMT) in chronic liver injury (Dooley et al., 2008; Kaimori et al., 2007; Zeisberg et al., 2007). This change is driven by active TGF-β signaling. TGF-β synergizes with alcohol in inducing oxidative stress (Zhuge and Cederbaum, 2006) and increases the inflammatory response of endotoxins (Seki et al., 2007). Noteworthy is the TGF-β-mediated anti-proliferative and pro-apoptotic change in hepatocyte characteristics under certain conditions. For example, in cancer, TGF-β growth inhibitory capacity is often lost (Inagaki et al., 1993) leading to promotion of proliferation and migration, making the cells more aggressive in infiltrating surrounding tissue and enhancing metastasis. A recent report provides evidence for laminin-5 and TGF-β acting together for transition of noninvasive hepatocellular carcinoma cells to an invasive hepatocellular carcinoma type. This change in phenotype is driven by TGF-β and is sustained by increase in expression of PDGF (Giannelli et al., 2005; Gotzmann et al., 2006). The TGF-β-mediated switch of phenotype is not only valid in hepatocellular carcinoma cells, but also in adult, nontransformed hepatocytes. A recent study demonstrated that TGF-β-induced apoptosis occurred only in a minor fraction of cultured hepatocytes (Dooley et al., 2008). The majority of hepatocytes lost epithelial characteristics and acquired a mesenchymal phenotype, called EMT, regulated by a switch in the cell’s expression pattern. This illustrates the potentiality of diverse responses of hepatocytes to TGF-β, as well the importance and influence of surrounding cellular matrix in a 3-dimensional context.
In intact liver, hepatocytes show a strong apical-basal polar phenotype enabling them to fulfill their multiple and spatially directed physiological functions. These functions, among others, include uptake from blood, carbohydrate homeostasis, catabolism, and detoxification, as well as bile secretion. This is achieved by an impermeable layer of hepatocytes connected by cell–cell junctions, mainly tight junctions. Upon TGF-β-induced cell transition this epithelial phenotype is lost, tight junctions are dissolved and several factors like zonula occludens-1 and E-cadherin are downregulated. In parallel, mesenchymal marker proteins are induced, such as, vimentin and type I collagen. Another property of the cells undergoing EMT is a gain in migratory capacity accompanied with a change in morphology toward a fibroblastoid shape (Fig. 3). Similar characteristics of EMT are also shown by Zeisberg and colleagues in hepatocytes treated with TGF-β (Zeisberg et al., 2007). In their very elegant study using double transgenic mice in a carbon tetrachloride (CCL4)-induced liver fibrosis model they demonstrated that a considerable fraction of fibroblast specific protein 1 (FSP-1) positive fibroblasts was derived from hepatocytes. Gene expression profiling in hepatocyte cells treated with TGF-β revealed a precise and detailed picture of TGF-β-induced pro-fibrotic and EMT target genes (Breitkopf et al., 2005). Using this approach, known targets involved in growth control, like p21 and apoptosis, were identified. Further they found differentially regulated genes related to EMT (vimentin, Snail, E-cadherin, ZO-1, β-catenin, and others), fibrosis (CTGF, collagen type I, TIMP-1, and others), and also to alcohol metabolism (ADH1 and CYPs). Studies from Dooley’s group and others reveal that upon induction with TGF-β, Snail-a TGF-β target gene and a critical regulator of E-cadherin expression, facilitates the downregulation of cell junction protein E-cadherin. These investigations also demonstrate TGF-β dependent upregulation of Snail in hepatocytes around inflamed and fibrotic tissue, further supporting the idea of hepatocyte transition and profibrogenic action in liver diseases.
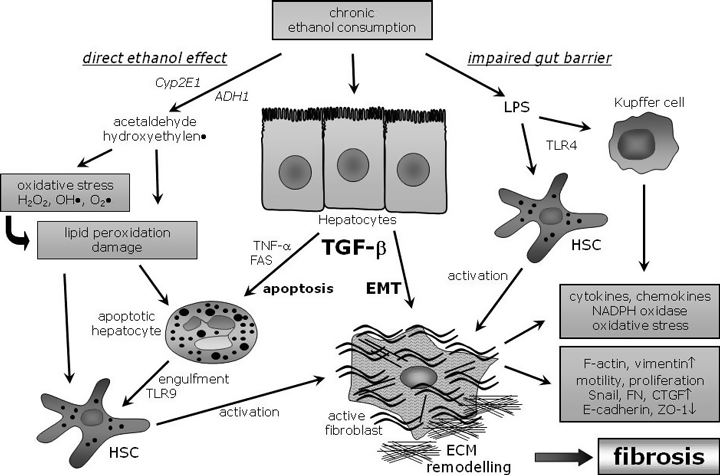
Effects of ethanol uptake in liver cells. Ethanol metabolites induce lipid peroxidation and ROS with subsequent damage of hepatocytes and HSC activation. Alcohol challenge is accompanied with an impaired gut barrier function that leads to elevated endotoxin levels in the liver. LPS then triggers HSC and Kupffer cell activation by TLR-4 and CD14 signaling. TGF-β stimulates hepatocytes to undergo apoptosis or epithelial mesenchymal transition (EMT). Apoptotic hepatocytes can be phagocytosed by hepatic stellate cells and trigger their activation (in part by TLR-9 signaling), thereby contributing to inflammation and fibrosis. The recently described mechanism of hepatocyte EMT upon TGF-β signaling actively drives inflammatory and fibrotic processes. A considerable part of the active, profibrogenic fibroblasts derive from hepatocytes (see text for details). FN, fibronectin.
High CTGF levels have been associated with severity of fibrotic diseases and might reflect a therapeutic node for treating chronic liver fibrosis (Grotendorst, 1997; Lasky et al., 1998). CTGF is a strong pro-fibrotic mitogen and an early target gene of TGF-β signaling. Until recently CTGF was accepted to be expressed by activated stellate cells in liver diseases (Paradis et al., 2002). The involvement of CTGF in hepatocytes of hepatitis B virus infected livers as well as in CCL4 treated mice (Weng et al., 2007) is a novel finding. It is noteworthy that expression of CTGF was mainly mediated by TGF-β/serine/threonine kinase receptor (ALK)5/Smad3 signaling, although additional pathways have been described to contribute to TGF-β-mediated CTGF expression in other cells types (Arnott et al., 2008; Gressner and Gressner, 2008; Gressner et al., 2008; Woods et al., 2008).
Signaling pathways important in alcohol-mediated tissue injury
Alcohol toxicity invokes several signaling pathways/molecules. These include mitogen associated protein kinases (MAPK), PI3K, TGF-β signaling cascade, PKC, PPARγ and the JAK-STAT pathway regulating expression of a number of inflammatory and ECM molecules through transcription factors NFκB and AP-1 in both liver (Dooley et al., 2000; Furukawa et al., 2003; Mandrekar and Szabo, 2009) and pancreas (Apte et al., 2006). Recent research has revealed novel molecules and their roles in mediating these signaling pathways leading to progression of tissue damage, including activation of KCs and transformation of quiescent to activated stellate cells. Some of these novel mechanisms are explained with special reference to hepatic cells.
LPS-TLR-4-Mediated Signaling
Endotoxin-mediated activation of KCs via LPS-LPB-CD14-TLR-4 complex initiates a variety of signaling cascades in the cell. One such signaling pathway involves IL-1 receptor associated kinase (IRAK), myeloid differentiated factor 88 (MyD88), and TNFR associated factor (TRAF) to modulate NFκB transcription factor activity. NFκB is maintained in an inactive state by inhibitory molecule IκB. Signals generated on endotoxin binding to CD14/TLR-4 receptor complex releases IκB, resulting in activated form of NFκB. The consequence is generation of superoxide anions and production of cytokines which further damage the tissue. IRAK1 is also rapidly inactivated in response to macrophage activation decreasing signaling, suggesting a negative feedback mechanism (Li et al., 2000). Consistent with this, alcohol administration in mice rapidly suppressed the production and activity of IRAK1 in the liver and IRAK1 expression correlated with the degree of liver cell response (decreased TNF-α) to endotoxin levels after acute alcohol exposure inducing tolerance to LPS (Yamashina et al., 2000). In contrast, long term exposure to alcohol increased IRAK and TNF-α, thereby inducing sensitization to LPS (Yamashina et al., 2000). It is hypothesized that the role of IRAK1 may be central to the dual effects of alcohol observed with acute alcohol typically inducing tolerance and chronic alcohol sensitizing the liver to the effects of endotoxin (Mathurin et al., 2000). Evidence strongly indicates that these effects are brought upon by changes in levels and activity of IRAK1 and CD14 (Wheeler and Thurman, 2003; Yamashina et al., 2000). The critical role of TLR-4 in this pathway is also demonstrated in mice strain C3H/HeJ that has a mutated form of TLR-4 gene and cannot initiate these signals. These mice are resistant to endotoxin effects and show no signs of chronic alcohol induced liver injury compared to wild type mice that develop severe alcoholic hepatitis (Uesugi et al., 2001). Other experimental models that lacked CD14 or LBP also demonstrate that LPS-mediated signaling pathways are critical for alcoholic liver injury (Yin et al., 2001). On LPS induction, early growth response-1 (Egr-1), another transcription factor, signals through MAPK (Erk1/2) via TNF-α in hepatic macrophages on chronic alcohol exposure (Kishore et al., 2002). Egr-1 is known to contribute toward LPS-induced increased sensitivity of macrophages in chronic alcohol models (Pritchard and Nagy, 2005). The lack of steatosis or increased ALT and TNF-α in Egr-1 knockout mice after chronic alcohol (McMullen et al., 2005) exposure also supports this proposition.
TGF-β-Mediated Signaling
The TGF-β signaling pathway has recently been studied in great detail by Dooley’s group and a comprehensive picture of the TGF-β pathway interactome is provided by Taylor and Wrana (Taylor and Wrana, 2008). TGF-β can induce apoptosis via TNF-α related pathway through activation of transcription factor AP-1 and via Smad signaling. Smads (homolog of drosophila mothers against decapentaplegic) are a class of proteins that modulate the activity of TGF-β ligands and act as nuclear transcription factors on complexing with other Smads. TGF-β forms a complex with TGF-β receptor II (TBRII) and ALK5, phosphorylating Smad 2 and Smad 3. This complex in association with Smad 4 translocates to the nucleus, regulating gene transcription (Shi and Massague, 2003; Taylor and Wrana, 2008). Among others, Smad target genes include ECM-related molecules such as (plasminogen activator inhibitor-1) PAI-1, urokinase plasminogen activator (uPA), and collagen. TGF-β signaling activates several positive and negative feedback mechanisms, particularly through Smad7, a key negative feedback participant that modulates TGF-β signaling in a site- and stage-specific manner (Dooley et al., 2008; Nakao et al., 1997; Shi and Massague, 2003; Shi et al., 2004; Zhang et al., 2007). In hepatocytes, these feedback mechanisms tightly regulate TGF-β signaling in order to check excessive apoptosis and inhibition of proliferation. This mechanism presents an extremely sophisticated way to prevent hepatic failure that would otherwise result due to substantial cell loss (Schrum et al., 2001). Importantly, only a small proportion of hepatocytes go through apoptosis but the majority have been shown to undergo EMT induced by TGF-β signaling (Dooley et al., 2008). The involvement of hepatocytes in modulating fibrosis in this manner is novel and intriguing and has important consequences in ALD pathogenesis. ALK5 dependent phosphorylation of Smad 2 translocates it to the nucleus where it binds to p38-MAPK-dependent phosphorylated Smad 3 and Smad 4. This hetero-complex can then bind with high affinity and specificity to the Smad binding element (SBE) in the promoter region of PAI-1 and stimulates PAI-1 transcription (Furukawa et al., 2003). Recent reports increasingly suggest a role for PAI-1 in liver injury (also see plasminogen system below). Besides the canonical Smad signaling, TGF-β can transduce signaling via other pathways (Moustakas and Heldin, 2008). As TGF-β plays a prominent role in/during development and tissue homeostasis, its signaling is tightly controlled. The complexity of TGF-β signaling manifests in cofactors’ binding to and associating with TGF-β signaling cascade components as well as in cross talks with other signaling molecules, such as through Akt and Erk pathways.
TNF-α-Mediated Signaling
TNF-α is a pro-inflammatory cytokine induced primarily in macrophages and is considered to be a major player in ALD as a therapeutic target. It has been shown in experimental models of alcohol, that anti-TNF-α antibodies attenuate hepatic necrosis and inflammation. Also, in the intragastric infusion model, TNF receptor 1 (TNFR1) knockout mice were resistant to ALD. In human, anti-TNF-α antibody showed initial promise as a specific molecular treatment for ALD, however, a randomized controlled trial closed after cases of serious sepsis emerged in treated patients.
In addition to the signaling pathways described above, novel mechanisms involving several sphingolipids have been implicated in TNF-α-mediated hepatocellular death. For example, Sphingosine-1-phosphate is shown to have an anti-apoptotic role (Marí and Fernández-Checa, 2007). Glycosphingolipids which act as second messengers mediate stress and death ligand-induced cell death, differentiation, proliferation and gene regulation (Marí and Fernández-Checa, 2007; Morales et al., 2007). Sphingomyelinases (SMases), specifically acidic SMase (ASMase), is required for hydrolysis of sphingomyelin and is involved in the activation of the mitochondrial pathway of apoptosis (Hannun and Luberto, 2000; Kolesnick and Krönke, 1998). Ceramide is generated on activation of SMases and plays a crucial role in determining the fate of cells in response to stress such as induced by alcohol and death receptor-mediated signaling. In hepatocytes, TNF-α binding to TNFR activates ASMase dependent signaling and induces cell death (García-Ruiz et al., 2003; Marí et al., 2004). Cultured hepatocytes depleted of mitochondrial glutathione (mGSH) are sensitive to TNF-α, and undergo apoptotic cell death but are rescued by Cyclosporin A treatment. In contrast, hepatocytes from ASMase−/− mice are insensitive to TNF-α, but responsive to exogenous ASMase-induced down-regulation of methionine adenosyltransferases (MAT)1A. Moreover, in an in vivo model of lethal hepatitis by TNF-α, depletion of S-adenosyl-l-methionine (SAM) preceded activation of caspase-8 and -3 with massive liver damage and death of the mice. In contrast, minimal hepatic SAM depletion, caspase activation, and liver damage are seen in ASMase−/− mice. More importantly, therapeutic treatment with SAM abrogates caspase activation and liver injury, thus rescuing ASMase+/+ mice from TNF-α-induced lethality. This demonstrates a dual role for ASMase in TNF-α-induced liver failure by targeting glycosphingolipids to the mitochondria (García-Ruiz et al., 2003) and through downregulation of MAT1A and subsequent SAM depletion (Marí et al., 2004) (Fig. 4). In ASMase null mice there was minimal TNF-α as well as LPS-induced liver injury (García-Ruiz et al., 2003) suggesting that this mechanism may also be important in alcohol-mediated injury. TNF–TNFR1 interactions are responsible for generating survival and death signals through NFκB and caspase-8, respectively (Micheau and Tschopp, 2003; Muppidi et al., 2004). NFκB prevents apoptosis by inhibiting caspase-8 (Thorne and Tschopp, 2001), upregulating inhibitors of apoptosis (Wang et al., 1998a) and downregulating ROS-mediated JNK activation (Kamata et al., 2005; Sakon et al., 2003). Studies have shown that stress induced by selective depletion of mitochondrial glutathione (mGSH) sensitizes hepatocytes to TNF in an unconventional pathway that is independent of NFκB inactivation. These observations highlight the overall relevance of sphingolipids/ASMase pathway and imply that such mechanisms may also be important in alcohol-induced liver and other organ injury.
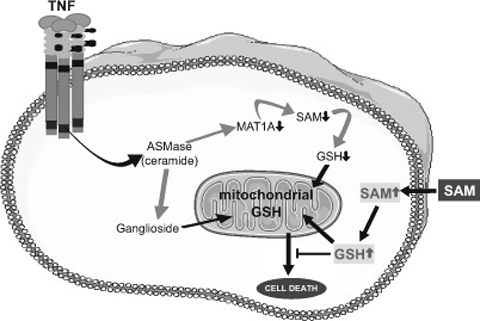
Mechanisms used by ASMase in mediating the cytotoxic effects of TNF-α. Upon binding to its receptor, TNF activates ASMase resulting on one hand in the downregulation of MAT1A, and consequently in SAM and GSH depletion; and on the other hand ASMase fuels the synthesis of complex glycosphingolipids, such as gangliosides, able to target mitochondria and inducing mitochondrial oxidative stress. Both processes promote the damaging effects induced by TNF. SAM treatment would replenish GSH stores, including those in mitochondria, producing favorable conditions in which hepatocytes withstand the harmful effects of TNF.
Alcohol-Induced Fibrogenesis Modulated via Plasmin-Plasminogen System
The mechanisms by which hepatocytes alter fibrosis have been shown in in vitro studies (Seki, 1996; Seki et al., 2007). One such mechanism is by dysregulating plasmin-plasminogen homeostasis and altering fibrinolysis. The expression and activities of pro- and anti-fibrinolytic molecules are meant to balance the formation and resolution of fibrotic scar thereby modulating fibrogenesis. The major players involved in this pathway are the pro-fibrinolytic uPA, tissue-type plasminogen activators (tPA) and anti-fibrinolytic PAI-1. The first hint of involvement of hepatocytes and TGF-β in fibrosis and matrix remodeling came from a study in AML12 cells, an immortalized nontransformed hepatocyte cell line, showing that TGF-β strongly induces PAI-1 in a rapid and transient manner (Seki, 1996). PAI-1 is an acute-phase protein and a major inhibitor of uPA and tPA that regulate liver matrix remodeling through the activation of plasminogen to plasmin. The activation of plasminogen to plasmin breaks down fibrin through fibrinolysis and both are increased in alcoholic cirrhosis (Leiper et al., 1994; Shanmukhappa et al., 2006). Recently, Seth et al. have shown increased PAI-1, uPA, tPA, plasminogen, plasmin and fibrinolysis in in vivo mouse and in vitro hepatic cell culture models of alcohol (Seth et al., 2008). The data from their study suggest a pro-fibrinolytic profile with exposure to low dose alcohol and a propensity for anti-fibrinolytic response with high-dose alcohol presumably, due to several log-fold increase in PAI-1 expression (Seth et al., 2008). Similarly, PAI-1 is strongly induced and is known to regulate ECM remodeling in experimental models of alcoholic as well as other forms of liver injury (Arteel, 2008; Bergheim et al., 2006; Seth et al., 2008). PAI-1 affects activity of MMPs further contributing to ECM remodeling (Bergheim et al., 2006; Ramos-DeSimone et al., 1999). In other experimental models of chronic liver injury, increased ECM accumulation was shown in plasminogen knockout mice (Pohl et al., 2001) suggesting the significance of this pathway in liver fibrogenesis. Plasmin activation is also known to be regulated through Osteopontin (Opn), a Th1 cytokine. As shown by comprehensive signaling studies in cancer cells, Opn interacts with several integrins (αvβ3, α4β1, α9β1) and CD44 receptors initiating a variety of signaling cascades that regulate gene transcription via NFκB and AP-1 (Das et al., 2004, 2005; Philip and Kundu, 2003; Rangaswami et al., 2005). Binding of Opn to its receptors induces PI3K/Akt-dependent NFκB activation and uPA secretion in cancer cells (Das et al., 2005). uPA/tPA, MMP9, and plasmin are also activated via MAPK and Erk pathways activating transcription factors AP-1 in breast and prostate cancer cells (Angelucci et al., 2002; Das et al., 2004; Philip and Kundu, 2003). Intriguingly, findings in human ALD show for the first time a significant increase in expression of Opn, Opn receptors (integrins, CD44), Opn modifiers (thrombin, MMPs, tissue inhibitors of MMP [TIMPs]) and downstream effectors (uPA, tPA, plasminogen) (Seth et al., 2003, 2006a,b). Increased Opn plasma levels are a marker for fibrosis (Szalay et al., 2009), matrix remodeling (Simoes et al., 2009), and metastatic progression in several cancers (Bellahcène et al., 2008; Patani et al., 2008), including hepatocellular carcinoma (Kim et al., 2006; Zhang et al., 2006). Hepatic Opn expression is also increased in CCL4 model of liver injury and the methionine choline deficient diet model of nonalcohol steatohepatitis (NASH) (Lee et al., 2004; Sahai et al., 2004). Opn upregulation was shown to directly mediate stellate cell activation in vitro in the CCL4 rat model (Lee et al., 2004). Alcohol also induced Opn in the lung and liver, along with a panel of other inflammatory mediators, such as TNF-α, IL-10, macrophage inflammatory protein (MIP)-2, and MMP-9 (Xu et al., 2007). There is compelling evidence that suggests these mechanisms may also be occurring in liver and other organs in response to alcohol-induced injury.
Alcohol Modulates Immune Response During Bacterial and Viral Infection
The recognition that alcoholics are at a greater risk for acquiring and dying from tuberculosis and bacterial pneumonias dates back to the clinical observations made by Benjamin Rush in 1784 (Rush, 1943). These observations have since then been confirmed by epidemiological and laboratory studies (MacGregor et al., 1978; Mason et al., 2004; Saitz et al., 1997; Schmidt and De Lint, 1972; Zaridze et al., 2009). More recently, alcohol abuse has also been associated with increased incidence and severity of acute respiratory distress syndrome (ARDS) (Esper et al., 2006; Moss et al., 1996), increased risk for viral and fungal infections and, subsequent susceptibility to secondary bacterial infections (Fong et al., 1994; Jerrells et al., 2007). The increased susceptibility of alcohol abusers to a variety of lung infections is attributed largely to alcohol-mediated alterations in innate and adaptive immunity (Happel and Nelson, 2005; Joshi and Guidot, 2007; Szabo and Mandrekar, 2009). As is the case with other organs, the effects of alcohol on the lung may be direct or mediated via its metabolites (Sisson 2007). Findings using in vivo animal models of acute alcohol intoxication and/or in vitro exposure of isolated AM to alcohol indicate that a single exposure to intoxicating levels (acute) of alcohol can suppress lung innate immune responses to acute immune stimulus. Studies performed using either lungs or immune cells isolated from human and rodents suggest that acute alcohol can suppress innate immune responses by altering the balance between pro- and anti-inflammatory mediators (D’Souza El-Guindy et al., 2007; Gamble et al., 2006; Szabo and Mandrekar, 2009). It is suspected that alcohol inhibits inflammatory cytokine secretion by affecting the NF-κB signaling pathway (Happel and Nelson, 2005). More recently, using a murine model of acute alcohol intoxication, alcohol is shown to impair LPS-induced pulmonary chemokine LIX production and alveolar neutrophil influx (Walker et al., 2009). On the other hand, excessive and prolonged alcohol consumption is suspected to induce a state of low grade inflammation, making the lungs hyporesponsive to bacterial insult and increasing susceptibility to the infection. In this context, chronic alcohol feeding in drinking water to mice is reported to increase basal levels of secreted TNF-α, osteopontin, MMP-9, Il-12p40, MIP-2, and blunt the inflammatory response to subsequent infection with live Streptococcus pneumoniae. This is accompanied by a high pathogen burden both in the lung and systemic circulation (Xu et al., 2007). Impaired phagocytosis of inactivated bacteria by AM, decreased cellular glutathione, decreased ROS generation, impaired lipid peroxidation, TNF-α secretion and increased apoptosis are also reported in AM isolated from rat and mouse models of chronic alcohol feeding (Brown et al., 2007a; D’Souza et al., 1996). Interestingly, supplementing alcohol diet with acetylcysteine, a precursor of glutathione, is reported to prevent the alcohol-induced defects in phagocytosis and loss of cell viability (Brown et al., 2007a), offering a potential mechanism to restore alcohol-induced defects in lung cell functions. Studies performed on bronchoalveolar lavage (BAL) and immune cells isolated from lungs of subjects (human and rodents) ingesting alcohol or with naïve lung immune cells exposed in vitro to alcohol suggest that alcohol modulates not only innate but also adaptive immune cell functions (Szabo and Mandrekar, 2009). Throughout bacterial invasion, the antigen presenting cells (dendritic cells, monocytes, and macrophages) assist the transition of innate to adaptive immune responses by T-lymphocyte activation (Bartlett et al., 2008; Tsai and Grayson, 2008). IL-23, a cytokine produced by activated AM and involved in the activation of memory T-cell proliferation and T-cell derived IL-17 production, is suppressed by acute alcohol intoxication in mice infected with Klebsiella pneumoniae (Happel and Nelson, 2005). Using murine model of chronic alcohol intake and K pneumonia infection, alcohol abuse is also reported to suppress IL-12, a critical cytokine driving T-cell IFN-γ response, and IFN-γ expression in the lung (Shellito et al., 2001). In this same model of infection, the expression of IL-17, a cytokine which serves as a link between innate and adaptive immunity, is shown to be inhibited by chronic alcohol intake (Ye et al., 2001). In mouse models of tuberculosis, excessive alcohol feeding impaired pathogen clearance, blunted CD4+ and CD+ T lymphocyte responses, decreased lymphocyte proliferation, and IFN-γ levels in CD4+ T (Mason et al., 2004). Similarly, decreased ability to clear respiratory syncytial virus (RSV) infection despite increased IFN-α and IFN-β induction, progressive loss of CD+ T cells, increased injury and lethality along with increased inflammation reported in animal models of chronic alcohol feeding (Jerrells et al., 2007).
Both acute and chronic alcohol exposure can also direct cytotoxicity via increased production of pro-inflammatory cytokines and chemokines and inhibiting T-cell activation and IL-12 production (Szabo and Mandrekar, 2009). In particular, alcohol-induced cell toxicity is mediated via TNF-α led signaling events that activate transcription factors that can alter resistance to viral infections (Neuman et al., 1999, 2008). Alcohol is a known co-factor promoting hepatitis C virus (HCV) infection. It accelerates disease progression of HCV-related cirrhosis and increases risk of hepatocellular carcinoma. Alcohol modifications of cytokine profiles can manipulate the transformation of cells from a preleukemic to leukemic state. In the Friend murine leukemia virus (F-MuLV), the role of alcohol was examined by Neuman’s group. They showed that alcohol could induce erythroleukemia in a BALBc mouse model. The alcohol fed mice showed an accelerated progression of the preleukemic stage with enhanced proliferation of erythroblasts and splenic infiltration (Shaked et al., 2005). In this model, the TNF-α and IL-8 were greatly increased in the liver and spleen of mice exposed to alcohol. In addition, erythropoietin-receptor (Epo-R) transduction pathway was also upregulated altering gp55/Epo-R-mediated signaling, early initiation of tumorigenesis and enhancing preleukemic stage. Activation of Epo is known to be associated with increased tumorigenicity of erythroleukemias (Cocco et al., 1996). Studies in ALD and other viral infections also reveal that exposure to alcohol alters the immune responses to infection and accelerate disease progression. Early studies provide clinical evidence in patients with chronic ALD showing reduced IL-2 indicating a deficient Thl activity (Saxena et al., 1986), and elevated serum levels of IgA and IgE, suggestive of Th2 activation. Similar observations are recorded in a murine retroviral model of acquired immune deficiency syndrome (MAIDS) (Fitzpatrick et al., 1995), and this model has been successfully used to evaluate the effects of alcohol on retrovirus-induced immunodeficiency. In this model, chronic alcohol altered the course of the disease and in the noninfected mice, chronic alcohol feeding led to immune enhancement rather than suppression consistent with the hypothesis that chronic alcohol abuse depresses Th1 function and activates Th2 responses. The study of Friend erythroleukemia has made a contribution to the understanding of tumor progression events associated with human cancer (Ben-David and Bernstein, 1991; Shaked et al., 2005). Neuman et al. have demonstrated that alcohol changes the splenic microenvironment promoting a hematologic malignancy via Epo/Epo-R pathway (Neuman et al., 2008), and paves the way to test possible pharmacological treatments for alcohol-induced tumor progression in human leukemia.
Alcohol, ECM Remodeling, and Fibrogenesis: Role for ASMase, MMPs, TIMPs
During liver fibrosis and cirrhosis, ECM remodeling is accompanied not only by scar formation and increased deposition of fibril forming collagens (like type I collagen and others), but also by degradation of normal liver ECM. These orchestrated processes are regulated by expression and activation of MMPs as well as by TIMPs (Arthur, 1995, 2000; Siegmund et al., 2005). Remarkably, among others, acetaldehyde and TGF-β directly contribute to this process, in part by downregulating MMPs and upregulating type I collagen (Siegmund et al., 2005).
As described above, ASMase-mediated ceramide generation modulates the fibrogenic potential of HSC during fibrogenesis, via regulation of cathepsins (Cts) (Moles et al., 2009). Cathepsins are known to be involved in tumorigenesis, cell death and are necessary for HSC transdifferentiation into myofibroblasts. During in vitro mouse HSC activation, CtsB and CtsD are increased in parallel to α-SMA and TGF-β levels (Moles et al., 2009). Inhibition of CtsB or transfection with CtsB siRNAs in vitro blunted Akt phosphorylation and HSC proliferation and decreased expression of α-SMA and TGF-β mRNA in activated HSC. Furthermore, in in vivo murine model of CCl4-induced fibrogenesis, CtsB expression increased in HSCs and its inactivation abrogated HSC activation, inflammation, and collagen deposition (Moles et al., 2009), suggesting that the antagonism of cathepsins in HSC may be of relevance for the treatment of liver fibrosis.
Little is known on the effects of alcohol abuse on lung ECM regulation. Limited published studies performed in rodent models of alcohol suggest that alcohol abuse can increase the expression of fibronectin by alveolar type II cells, and activate lung MMP-9 and MMP-2 without affecting their production. The studies also suggest these effects of alcohol may be mediated by alcohol-induced oxidative stress (Brown et al., 2007b; Lois et al., 1999). A recent clinical study reports increases in fibronectin gene expression by alveolar macrophages and epithelial lining fluid recovered from alcohol abusing individuals (Burnham et al., 2007). ECM turnover is regulated by endogenous neutral endopeptidases in the lung, amongst which are the Zinc (Zn) dependent MMPs and TIMPs: the balance between MMPs and TIMPs determines either tissue homeostasis or disease state (Chirco et al., 2006; Greenlee et al., 2007; Murphy and Nagase, 2008). Recent studies suggest that pathogens can bind to ECM molecules via adhesins and use the ECM for survival and dissemination. The pathogens also release endopeptidases that can disintegrate the ECM and release ECM protein fragments with pro-inflammatory properties (Morwood and Nicholson, 2006). In many pulmonary diseases, including ARDS and bacterial pneumonias, abnormal remodeling or destruction of ECM has been reported (Elkington and Friedland, 2006; O’Reilly et al., 2008; Okamoto et al., 2004). Not only do the pro-inflammatory and apoptotic mediators such as TNF-α, IL-1-β and FAS regulate the expression and activation of MMPs and TIMPs, but the MMPs and TIMPs can also regulate the expression of pro-inflammatory and apoptotic mediators as well as the recruitment of inflammatory cells to the inflammation site. The ability of the TIMPs to balance the MMPs activity may allow these molecules to regulate communication between immune cell signaling networks and the ECM (Elkington and Friedland, 2006; Greenlee et al., 2007). Elevated levels of MMPs (MMP-2, MMP-8, and MMP-9, specifically) are reported in BAL recovered from patients with acute lung injury and hospital acquired pneumonia (Fligiel et al., 2006; Schaaf et al., 2008). Also, increased activitation of lung MMP-9 and MMP-2 in response to acute endotoxemia have been reported in rodent model of chronic alcohol ingestion (Lois et al., 1999). Taken together, these studies suggest that activation of tissue remodeling may contribute to the increased susceptibility for ARDS in individuals consuming excessive alcohol.
Described above are several lines of investigation that reveal common molecular pathways that are important for the initiation and progression of alcohol-induced tissue injury. However, it is prudent to note that some pathways may only be important under certain conditions, in specific organs and arbitrated through a diverse range of molecules. These molecules and pathways are complex and context dependent, and collectively contribute to alcohol-induced abnormalities, impaired immune and wound repair responses. Comprehensive and diverse approaches are required for exploration and understanding of these mechanisms of alcohol-mediated pathogenesis.
Acknowledgments
The work described in this review was supported by National Health and Medical Research Council (NH&MRC) of Australia/Department of Veteran’s Affairs (DVA) (Seth); NIH, NIAAA grant R01AA013168 (D’Souza El-Guindy); NH&MRC and the Cancer Council of New South Wales, Australia (Apte); CIBEREHD and grant PI070193 (Instituto de Salud Carlos III, Spain), and by grant P50-AA-11999 (Research Center for Liver and Pancreatic Diseases, U.S. National Institute on Alcohol Abuse and Alcoholism) (Mari); BMBF (HepatoSys) and the European Alcohol Research Foundation (ERAB) (Dooley).




