OPTIMIZATION OF DNA EXTRACTION FROM BROWN ALGAE (PHAEOPHYCEAE) BASED ON A COMMERCIAL KIT1
Received 5 March 2009. Accepted 7 December 2009.
Abstract
Large-scale DNA molecular studies require reliable and efficient tools for DNA extractions. However, for some plant species and brown algae, isolation of high-quality DNA is difficult. We developed a novel method for isolating high-quality DNA from the polysaccharide-rich and polyphenol-rich brown algae based on a commercial kit and protocol (Qiagen) by optimizing the lysis step and including a chloroform/isoamyl alcohol supplementary purification step. DNAs from 24 brown algal species extracted using the original and the modified Qiagen protocol were compared for yield, quality, and effectiveness in PCR amplification. There was no significant difference in the yields between protocols. However, a statistically significant increase in DNA purity was obtained with the modified protocol, for which the A260/A280 and A260/A230 absorbance ratios averaged 1.66 ± 0.05 and 1.31 ± 0.01, respectively, compared to 1.37 ± 0.04 and 0.52 ± 0.04 with the original protocol. DNAs extracted by the modified procedure were more successfully amplified by PCR (nuclear, mitochondrial, and chloroplastic regions) than DNAs extracted using the original commercial kit and protocol. Importantly, the modified protocol can be applied in a high-throughput (e.g., 96-well plate) format, allowing a higher efficiency for downstream molecular analysis. In addition, improved DNA quality could increase its stability for long-term storage.
Abbrevations:
-
- CIA
-
- chloroform/isoamyl alcohol
-
- PVP
-
- polyvinylpyrolidone
-
- TBE
-
- Tris Borate EDTA
Having sufficient amounts of high-quality DNA is a prerequisite for successful population genetic and molecular phylogenetic studies. However, for some taxa, isolation of high-quality DNA is difficult due to various secondary compounds that might be co-isolated with genomic DNA. Furthermore, these contaminants can cause further difficulties in downstream DNA applications, such as DNA amplification or restriction enzyme digestion (Kreader 1996, Huang et al. 2000). This issue is of a particular relevance to seaweeds and plants (Varma et al. 2007). The brown algae (class Phaeophyceae) are characterized by cell walls containing large amounts of complex, negatively charged polysaccharides (alginates) (Quatrano and Stevens 1976, Vreelan and Laetsch 1989) and phenolic compounds (Geiselman and McConnell 1981, Schoenwaelder and Clayton 1998). Phenolic compounds are believed to play a role in defense processes in intertidal benthic algal communities against UV radiation (Kim et al. 2000). Both polysaccharides and phenolic compounds are likely to interfere with the isolation of nucleic acid and the activity of enzymes (Pandey et al. 1996, Koonjul et al. 1999).
The commercial manufacturers’ protocols for DNA extraction are typically optimized for a variety of well-known plant species (e.g., tomato, rice, maize). However, their efficiency is clearly questionable for difficult taxonomic groups, such as brown algae. Thus, to improve the extraction efficiency and DNA quality, modifications of current extraction protocols are often required.
The development of advanced molecular technologies and methods has allowed large-scale molecular studies, such as the DNA-barcoding approaches (Saunders 2005) or large DNA banking, a new solution for the genomic conservation of biodiversity (Corthals and Desalle 2005, Thornton et al. 2005, Hodkinson et al. 2007). Thus, the need to achieve high throughput and meet high-quality standards is evident (Hajibabaei et al. 2005). However, high-throughput technologies rely on robotic procedures, which require standardized format, such as the widely accepted 96-well plate format.
Here, we report a study that aimed to develop an efficient DNA extraction procedure for brown algae. The novel DNA extraction protocol we developed is based on a commercial extraction kit and a modified protocol that minimizes polyphenol and polysaccharide co-isolations. We evaluated the procedure in terms of yield, purity, and integrity of isolated DNA, as well as effectiveness of downstream molecular applications, such as PCR amplification of various loci.
Algal material. Twenty-four species of brown algae, covering seven orders (Dictyotales, Ectocarpales, Fucales, Laminariales, Scytothamnales, Sphacelariales, and Sporochnales) and 10 taxonomic families, were collected in Tasmania (Australia) and New Caledonia (Table S1 in the supplementary material). Seaweed samples were mostly preserved as herbarium plates or were silica dried and stored at room temperature until DNA extraction.
DNA extraction protocols. Two 20 mg samples of dried tissue were cut out from each brown algal specimen, resulting in 48 samples (i.e., two identical sets of 24 samples). Each sample was ground into a fine powder using a Mixer Mill MM300 (Retsch, Haan, Germany) for 2 min at 30 Hz. For one set of 24 samples, total genomic DNA was extracted using the DNeasy Plant Mini kit (Qiagen, Courtaboeuf, France) and protocol following manufacturer’s instructions. For the second set, the following modifications of a Qiagen protocol were carried out: (i) To each sample, 400 μL of a modified lysis buffer was added. The modified lysis buffer consisted of 2 M NaCl, 50 mM Na2EDTA pH 8.0, 2% polyvinylpyrolidone (PVP)-40, 0.1% BSA, 0.4 M sucrose, and 50 mM CaCl2. Antioxidant compounds, such as PVP and BSA, are known to also bind polyphenols (Couch and Fritz 1990, John 1992), and high salt concentration in the lysis buffer may decrease the levels of coextracted polysaccharides (Fang et al. 1992). (ii) To each sample, 40 μL of 10% Tween-20, 4 μL of Proteinase K (20 mg · mL−1), and 10 μL of RNase A (100 mg · mL−1) were added. (iii) Homogenates were then incubated for 30 min at 37°C (Hong et al. 1995) to minimize the release of polysaccharides. (iv) After lysis, a supplementary step of centrifugation (5415R, Eppendorf, Le Pecq, France) was carried out at 15,700g for 15 min at room temperature to eliminate high-molecular-weight polysaccharides (Patwary and Van Der Meer 1994). The supernatant was transferred into a new tube. (v) Then 130 μL of AP2 buffer (Qiagen) was added to each sample. The samples were kept on ice for 20 min and then centrifuged at 15,700g and at 4°C for 10 min. (vi) A supplementary purification step by CIA (chloroform/isoamylic alcohol, 96/4, v/v) was conducted as follows: one volume of CIA was added to each sample and mixed by gentle inversion for 10 min. The samples were then centrifuged at 15,700g and at 4°C for 10 min to separate the phases. The aqueous phase was transferred to the QiaShredder Mini spin column (Qiagen).
All subsequent steps of DNeasy Plant protocol were performed according to the manufacturer’s instructions.
For both protocols, precipitated DNA was eluted into 200 μL of buffer AE (10 mM Tris-Cl, 0.5 mM EDTA, pH 9.0).
DNA quantification and quality estimation. After extraction, the quantification of DNA was performed using three methods.
1. Measurements of UV absorbance: The spectrum of absorbance of the extracts for wavelengths ranging between 220 and 320 nm was obtained using a Nanodrop™ ND-1000 spectrophotometer (Labtech, Palaiseau, France). The absorbance value at 260 nm (A260) gave an estimation of the concentration of double-stranded DNA in the sample, assuming that [DNA] = 50 × A260 (in μg · mL−1) and the values of the A260/A280 and A260/A230 ratios provided information about the purity of DNA in the sample.
2. Fluorometry measurements: The measures were conducted using the Quant-iTTM dsDNA Broad Range kit (Invitrogen, Abington, UK) adapted for the Qubit fluorometer according to the manufacturer’s protocol.
3. Agarose gel electrophoresis: The integrity and the size of DNA were checked by electrophoresis (20 min at 135 V) against the molecular marker HindIII-digested Lambda DNA (Roche Applied Science, Meylan, France) in a 1% agarose gel stained with ethidium bromide and visualized under ultraviolet light.
PCR analysis. PCR assays were carried out on both sets of the 24 brown algal DNA extracts. Three loci, each specific to one of the three genomes, were amplified: the chloroplast rbcL, the mitochondrial cox1, and a portion of the nuclear LSU rDNA regions. The rbcL and cox1 loci were amplified in two PCR reactions to cover the entire region (Table S2 in the supplementary material). All amplification reactions were performed in 25 μL volume containing 1 μL (e.g., 1–30 ng) of the extracted DNA, 0.3 μM forward and reverse primers (Operon, Köln, Germany; sequences are provided in Table S2), 0.26 mM dNTPs, 1X PCR buffer with 1.5 mM of MgCl2, 5% of DMSO, and 0.9 U Taq DNA Pol polymerase (MP Biomedicals, Illkirch, France). However, this premix was modified for the cox1a region using the Qbio Taq polymerase (MP Biomedicals) and adding 0.8 mg · mL−1 of BSA due to amplification issues with the initial BSA-free premix. The PCR conditions were as follows: initial denaturation step at 94°C for 3 min, followed by 35 cycles of 1 min at 94°C, 1 min at annealing temperature specific to the target region (Table S2), 1.5 min at 72°C, and a final extension step at 72°C for 3 min, using a Biometra thermocycler (Biometra, Goettingen, Germany).
Statistical analysis. To elucidate the effect of the extraction protocol on the yield of DNA, the A260/A280 and A260/A230 ratios, the nonparametric test of Tukey’s honest significant difference (HSD) was used. The tests were performed using the statistical software R (R Development Core Team 2009).
The yield and purity parameters of 24 brown algal DNAs extracted by the original and the modified Qiagen protocols are summarized in Figure 1. The estimates of the amount of isolated DNA depended on the quantification method used (Fig. 1a). Absorbance at 260 nm may provide a quick quantity estimate. However, any contaminant absorbing between 230 and 320 nm [e.g., RNAs, polysaccharides/alginates (Østgaard 1993), polyphenols (Mozetič et al. 2006), proteins, peptide compounds, carbohydrates (Ahmad et al. 2004), and organic salts] can cause an overestimation of DNA concentration. All these substances also tend to alter the shape of the DNA spectrum, and this can be used to estimate DNA purity by measuring the A260/A280 and A260/A230 ratios. No DNA sample can be qualified as satisfactorily pure if A260/A280 and A260/A230 values are <1.7 and 2.0, respectively (Sambrook and Russell 2001). More specifically, the A260/A280 ratio allows a rather lower sensitive estimation of protein contamination than the A260/A230 ratio. This ratio is considered more informative because most contaminants, including proteins, have generally much stronger absorbance at 230 nm than at 280 nm (Glasel 1995, Laws and Adams 1996).
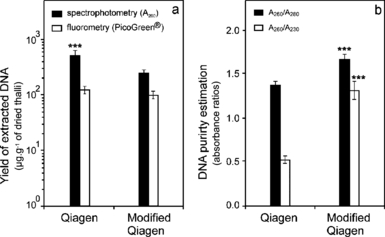
Effect of extraction protocol on (a) mean amount of recovered DNA in μg · g−1 of dried material (n = 24) estimated by spectrophotometry and fluorometry with PicoGreen® dye, and (b) purity of DNA evaluated by A260 nm/A280 nm and A260 nm/A230 nm ratios. Error bars represent SE. Significance level: ***P < 0.001.
Our fluorometric assay was much less sensitive to UV-absorbing contaminants due to the highly specific binding of the fluorescent dye PicoGreen® (Quant-iTTM kit) to the double-stranded DNA even in the presence of RNA, single-stranded DNA, protein, or phenol (Ahn et al. 1996).
For the Qiagen protocol, the mean yield measured by spectrophotometry appeared to be significantly higher than the one obtained by fluorometry (P < 0.001). The same trend was also observed for the modified Qiagen protocol, albeit not statistically significant. Nevertheless, the fluorometric quantification gave similar yield values for both protocols: mean ± SE of 97 ± 17 μg of genomic DNA per gram of dried material extracted by the modified Qiagen protocol and 122 ± 18 μg · g−1 extracted by the Qiagen protocol. Furthermore, the purity of DNA estimated by A260/A280 and A260/A230 ratios averaged 1.66 ± 0.05 and 1.31 ± 0.10, respectively, in the modified Qiagen protocol, and only 1.37 ± 0.04 and 0.52 ± 0.04 in the Qiagen protocol (Fig. 1b). The modified procedure allowed significant increase in the quality of the extracted DNA (P < 0.001). Indeed, for the Qiagen protocol, the estimates obtained by UV quantification were 4-fold higher than those obtained by fluorometry, whereas the difference was reduced to 2.5-fold for the modified Qiagen protocol (Fig. 1a). The low purity of DNA extracted by the Qiagen protocol (Fig. 1b) was in accordance with the inaccuracy of yield values evaluated by the spectrophotometric method.
The improvement of the quality of extracted DNA when the modified Qiagen protocol was used is demonstrated on one of the samples [Caulocystis cephalornithos (Labill.) Aresch., Fig. 2]. The A260/A230 ratio of this sample was 2.6-fold higher with the new procedure, whereas the A260/A280 ratio increased 1.4-fold. Thus, the modified protocol clearly allowed a decrease in the amount of UV-absorbing contaminants at 230 nm (Fig. 2b). Reduction of contaminants in the DNA solution is also believed to enhance the stability of DNA for long-term storage (Blunden et al. 1994).
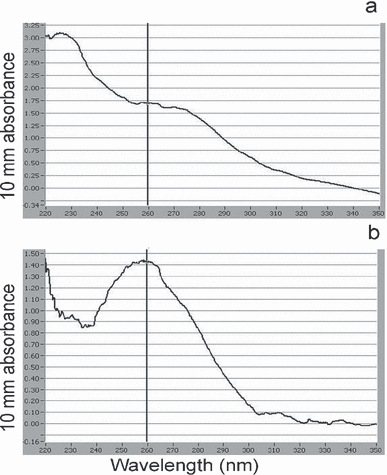
Spectrum of absorbance between 220 and 350 nm obtained by the Nanodrop® ND-1000 using 1 μL of DNA from Caulocystis cephalornithos extracted by (a) the Qiagen protocol and (b) the modified Qiagen protocol.
Using both extraction protocols, high-molecular-weight DNA (∼23 kbp) was obtained for all samples (Fig. 3), although some degree of DNA degradation was observed for some samples. This degradation was independent of the extraction protocol and probably resulted from tissue handling and/or storage of the material before extraction. All samples were air-dried, apart from four of them that were dried and stored in silica gel (Fig. 3, lanes 3 to 6), and it is possible that DNA was damaged during the handling. It is known that the DNA extracted from herbarium specimens is often degraded (Ribeiro and Lovato 2007, Telle and Thine 2008). It appears that both methods of preservation might not be ideal procedures, because in either case, drying was rather slow and could have led to stresses triggering DNA degradation. Generally, for animal samples, once the tissues are dried, DNA is fairly stable; however, in plants and algae, DNA degradation frequently occurs, even in dried tissues (Johnson et al. 2003, Ribeiro and Lovato 2007). Hence, obtaining good-quality DNA may require special procedures, such as extraction from freeze-dried material stored at very low temperature (Saha et al. 1997).
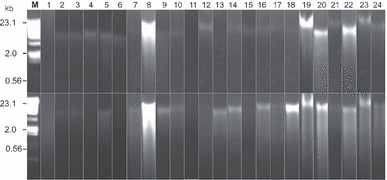
Total genomic DNA isolated from brown algal taxa eletrophoresed on 1% agarose TBE gel. Three microliters of DNA extract mixed with 2 μL of blue bromophenol was loaded on agarose gel. Lane M1 = molecular weight ladder (HindIII-digested Lambda DNA); lanes 1–24 = brown algal samples (lanes 1–2 = Dictyotales, lanes 3–6 = Ectocarpales, lanes 7–17 = Fucales, lanes 18–20 = Laminariales, lane 21 = Scytothamnales, lane 22 = Sphacelariales, lanes 23–24 = Sporochnales; see Table S1 in the supplementary material for species’ names). Top image corresponds to the Qiagen protocol, and the bottom image to the modified Qiagen protocol.
The electrophoresis analysis shown in Figure 3 suggested much greater variation in the yield of DNA in favor of the modified Qiagen protocol than the fluorometric yield estimated in Figure 1. However, such discrepancy is likely resulting from the presence of polyphenolic compounds, coextracted in greater amount with the Qiagen protocol. It has indeed been demonstrated that phenolic acids decreased the intensity of fluorescence of ethidium bromide through a competition for DNA binding (Labieniec and Gabryelak 2006) and interfered in the gel analysis if used as a quantification method. This phenomenon is called the fluorescence quenching.
In summary, our results indicated that spectrophotometry and electrophoresis analysis might be a suitable method for estimation of algal DNA quality but should not be recommended for quantification of algal DNA. Fluorometry with PicoGreen® seemed to be the most suitable method for quantification of algal DNA.
The extracted DNAs were further used for routine downstream molecular application, such as PCR. A clear improvement of PCR success in all tested loci was observed on the samples extracted by the modified Qiagen protocol (Fig. 4). We used uniform PCR conditions except for the cox1a fragment, which was amplified using a different type of Taq polymerase and an addition of BSA (known to bind polyphenol). Even with optimized PCR conditions that could mask the differences in quality of DNA, the amplification success increased with our modified protocol for all loci. For instance, the sample C. cephalornithos characterized in Figure 2 failed to amplify for some loci when processed using the Qiagen protocol, whereas it succeeded when extracted with the modified protocol (Fig. 4a, lane 8). For the cox1, LSU, and rbcL regions, the rates of positive PCR, respectively, reached 77% versus 58%, 75% versus 63%, and 83% versus 79% for modified Qiagen protocol versus Qiagen protocol, respectively (Fig. 4b).
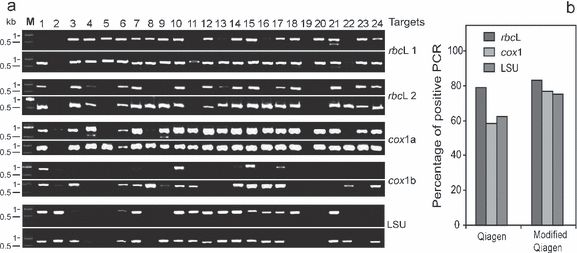
PCR amplifications of DNA isolated from 24 brown algal samples. (a) PCR products of the two parts of the rbcL chloroplast region, the two parts of the cox1 mitochondrial region, and the LSU nuclear region on 1.5% agarose TBE gel. Lanes 1 to 24 = samples (lanes 1–2 = Dictyotales, lanes 3–6 = Ectocarpales, lanes 7–17 = Fucales, lanes 18–20 = Laminariales, lane 21 = Scytothamnales, lane 22 = Sphacelariales, lanes 23–24 = Sporochnales; see Table S1 in the supplementary material for species’ names) and lane M = DNA molecular weight marker XIV (Roche Applied Science). For each locus, top image refers to the Qiagen protocol, and bottom image to the modified Qiagen protocol. (b) Percentage of successful amplification of the three genomic regions.
In conclusion, we provide here a novel DNA extraction protocol for brown algae based on a Qiagen commercial kit. The modifications included the use of an alternative lysis buffer and an additional step of purification by chloroform/isoamyl alcohol. This new DNA extraction procedure provided a higher quality of genomic DNA extracted from small tissue samples. The DNA extraction procedure as described here can easily be performed in the 96-well plate format and is thus adapted to automatic devices. Most importantly, the DNA isolated by the modified Qiagen procedure across number of brown algal species has proved to be suitable for downstream DNA molecular application, such as PCR amplification, which is of first importance when working with a wide range of species.
Acknowledgments
This work was supported by the BQR Genotheque and the MNHN’s “Direction des collections” directed by Michel Guiraud and Thierry Bourgoin. We thank Claude Payri who kindly provided us with specimens from New Caledonia. We thank Monika Zavodna, Philippe Gaubert, and anonymous reviewers for useful comments on the early draft of the manuscript, and Céline Bonillo for her technical help.




