In Vivo Osteogenic Capability of Human Mesenchymal Cells Cultured on Hydroxyapatite and on β-Tricalcium Phosphate
Abstract
The aim of the current study was to examine in vitro osteogenic capability and in vivo bone formation of mesenchymal stromal cells (MSCs) on two kinds of calcium phosphate ceramics. MSCs derived from human bone marrow were seeded on either hydroxyapatite (HA) ceramic or β-tricalcium phosphate (β-TCP) ceramic and then cultured in a medium supplemented with a donor's serum, vitamin C, β-glycerophosphate, and dexamethasone. The culture revealed the expression of alkaline phosphatase activity, indicating the osteogenic differentiation of the MSCs on the ceramics (fabrication of tissue-engineered construct). The constructs were then implanted subcutaneously into nude rats for 8 weeks. New bone formation was observed in both types of ceramics, and human-specific Alu sequence was detected by in situ hybridization analysis. Quantitative microcomputed tomography showed that the volume of the new bone in the HA ceramic was greater than that in the β-TCP ceramic in six of seven cases. These results suggest that human MSCs cultured on ceramics could retain their osteogenic capability even after ectopic implantation and provide a rationale for the use of tissue-engineered constructs derived from a patient's MSCs and calcium phosphate ceramics in bone tissue regeneration.
Since 2001, we have treated patients with osteoarthritis, bone tumors, and osteonecrosis by the implantation of tissue-engineered constructs. The constructs can be fabricated by in vitro osteogenic differentiation of the patient's mesenchymal stromal cells (MSCs) on a variety of calcium phosphate ceramics (1–4). Fabrication of the constructs requires ceramic materials and many types of ceramics were developed and compared for their capability of supporting in vitro and in vivo osteogenesis (5–9). Additionally, it is also known that osteogenic capability is influenced by the physical parameters of the ceramics such as density and pore shape, size, and interconnection pathway (10–13). However, it is still not clear how these parameters influence the efficiency of osteogenesis. The present study focused on this issue using human MSCs cultured on two kinds of commercially available ceramics (hydroxyapatite [HA] and β-tricalcium phosphate [β-TCP]). We first tried to detect in vitro osteogenic differentiation of the MSCs on the ceramics to demonstrate the fabrication of tissue-engineered constructs and then implanted the constructs into immunocompromised athymic nude rats at subcutaneous sites to investigate their in vivo bone formation.
MATERIALS AND METHODS
In vitro cultivation and analysis
After we obtained informed consent from seven donors, bone marrow cells were harvested from their iliac crest by needle aspiration (Table 1). The details of cultivation were described previously (4). In brief, 3 mL of bone marrow was mixed with an equal volume of heparinized phosphate-buffered saline (PBS; Invitrogen Corp., Carlsbad, CA, USA) and centrifuged at 140 × g for 5 min. The supernatant was discarded, and the remaining cell layer was seeded in T-75 flasks (Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA) with basal medium that consisted of Eagle's minimum essential medium alpha (Invitrogen Corp.) supplemented with 15% of each donor's own serum and antibiotics. We used each donor's serum instead of fetal bovine serum (FBS) for our current clinical applications of the tissue-engineered constructs (1–4). Therefore, we decided to use each donor's serum in the present study. At subconfluence, the adherent cells (MSCs) were detached (passage 1) from the flasks with a trypsin-like enzyme (TrypLE Select; Invitrogen Corp.) and further cultured for several days to expand the number of the MSCs. The cells were detached from the flasks and resuspended with basal medium (passage 2; P-2). The P-2 cells were diluted into 1.5-mL centrifuge tubes and incubated with antibodies on ice for 15 min. The cells were pelleted, washed twice in PBS, and analyzed by a FACSCalibur flow cytometer (BD). The antibodies used were CD13-FITC (fluorescein isothiocyanate), CD34-FITC, CD44-FITC, CD45-FITC (Invitrogen Corp.), CD90-FITC (BD), and human leukocyte antigen region DR (HLA-DR; Serotec, Oxford, UK). Mouse immunoglobulin G (Beckman Coulter, Fullerton, CA, USA) was used as an isotype control. The fluorescence intensity of each antibody was compared with that of the isotype control and represented as a histogram.
| Donor | Age | Gender | Differentiation period (days) | After differentiation period (in vitro) | After 8 weeks of implantation (in vivo) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ALP (mmol/well) | New bone volume (%) | Histological results | |||||||
| Dex (+) | Dex (−) | HA | β-TCP | HA | β-TCP | ||||
| #1 | 31 | M | 11 | 2.03 | 3.7 | 10.8* | 4.8 | + | + |
| #2 | 25 | F | 11 | 7.14 | 1.24 | 34.7* | 10.1 | + | + |
| #3 | 48 | M | 15 | 1.9 | 0.427 | 14.1* | 3.1 | + | + |
| #4 | 39 | F | 15 | 1.24 | 0.925 | 13.8* | 5.6 | − | − |
| #5 | 46 | M | 16 | 0.589 | 0.231 | 9.5 | 12 | + | − |
| #6 | 32 | F | 18 | 1.79 | 1.11 | 5.1* | 0.1 | − | − |
| #7 | 49 | M | 20 | 3.28 | 1.36 | 5.5* | 0.4 | + | − |
| Average | 38.6 | 15.1 ± 3.34 | 2.57 ± 2.18 | 1.28 ± 1.14 | 13.4 ± 10.06* | 5.16 ± 4.55 | |||
- Histological detection of the bone is represented as + and nondetection as −.
- * P < 0.05 compared with β-TCP.
To fabricate tissue-engineered constructs, 500 µL of the P-2 cell suspension at 5 × 105 cells/mL was seeded on each type of HA and β-TCP ceramic and incubated overnight at 37°C. The average seeding density per scaffold was 1 × 105 cells/scaffold. An additional 2 mL of the medium was supplied the next day. To induce in vitro osteogenic differentiation of the MSCs on the culture plate and the ceramics, the cells were cultured in basal medium supplemented with 10 mM β-glycerophosphate (Merck, Darmstadt, Germany), 0.07 mM L-ascorbic acid phosphate magnesium salt n-hydrate (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and 0.1 µM dexamethasone (Dex, Sigma-Aldrich Corp., St. Louis, MO, USA) (4). The culture periods were from 11 to 20 days (Table 1). In addition, to check the differentiation capability of the MSCs, the P-2 cell suspension was seeded on a 12-well culture plate (BD) at 2 × 104cells/well. The cells on the culture plate were cultured with or without Dex for the same periods as the MSCs on the ceramics.
Granular types of HA and β-TCP ceramics were used as scaffolds of the constructs. The size of the HA ceramic granules (100% HA, Pentax Corporation, Tokyo, Japan) was from 2.0 to 4.0 mm. The granules had pores with diameters from 2 to 500 µm, according to information from the manufacturer. The size of the β-TCP ceramic granules (100% β-TCP; Olympus Corporation, Tokyo, Japan) was from 2.8 to 5.0 mm; the granules had pores with diameters from 100 to 400 µm, according to information from the manufacturer.
To confirm the in vitro osteogenic differentiation of MSCs on the culture plate, calcium (Ca) deposition and alkaline phosphatase (ALP) activity of the cells were assayed. The Ca deposition was detected by using Ca-binding fluorescent dye, calcein (14). Four hours before the assay, 1 µg/mL of calcein (Dojindo Laboratories, Kumamoto, Japan) was added. After the differentiation period, the cells were rinsed with PBS, and fluorescence from the deposited calcein was visualized by an image analyzer (Typhoon 8600; GE Healthcare UK Ltd., Buckinghamshire, UK). Then, the cells were harvested with 0.5 mL of 10 mM Tris-buffer (pH 7.4, 1 mM ethylenediaminetetraacetic acid [EDTA], 100 mM NaCl). The harvested cells were sonicated and centrifuged. The supernatant was assayed for ALP activity by using p-nitrophenyl phosphate (pNPP) substrate (Zymed Laboratories Inc., San Francisco, CA, USA). The samples were incubated at 37°C for 30 min and measured on a microplate reader (1420 ARVOsx Multilabel Counter; PerkinElmer, Inc., Waltham, MA, USA). ALP activity was examined by the specific conversion of pNPP into p-nitrophenol (pNP) and was represented as µmol of pNP produced per 30 min.
To confirm the in vitro osteogenic differentiation of MSCs on the ceramics, ALP staining was performed after the MSC culture on the ceramics (15). After being washed with PBS, the ceramics were fixed with 10% neutral formalin. The ceramics were stained with 1.3 mM naphthol AS-MX phosphate (Nacalai Tesque, Kyoto, Japan) and 1.3 mM Fast Red Violet LB salt (Nacalai Tesque) in 56 mM 2-amino-2-methyl propanol (Nacalai Tesque) buffer for 5 min and washed with water to remove any remaining stain.
In vivo implantation and analysis
The tissue-engineered constructs were subcutaneously implanted into a total of nine nude rats. Ceramics without MSCs were also implanted as controls. After 8 weeks of implantation, the implants were harvested, fixed with 10% neutral formalin, and analyzed by microcomputed tomography (micro-CT, Hitachi Medical Corp., Tokyo, Japan) for evaluation of the new bone volume. Micro-CT was performed on fixed samples 10 µm thick at 50 kV and 150 µA. The porosity of the ceramics and the new bone formation areas in the micro-CT images were extracted, and their volumes were measured by using TRI3D-BON software (Ratoc System Engineering Co., Ltd., Tokyo, Japan). The micro-CT image showed areas having high, middle, and low intensities. As in our previous article, we could define the high (white), middle (gray), and low (black) areas as ceramics, new bone formation, and fibrovascular tissue with fat cells, respectively (16). We used the program to measure the total volume of these areas and the pore volume by the sum of the middle and low areas, respectively. New bone formation was expressed as a percentage of total bone volume per total ceramic pore volume.
After micro-CT analysis, the implants were decalcified in EDTA, embedded in paraffin, and serially sectioned. The serial sections were used for hematoxylin-eosin (HE) staining and in situ hybridization. The sections were washed by using xylene, rehydrated by serial ethanol, and treated with Proteinase K (DakoCytomation, Inc., Carpinteria, CA, USA) for 40 min at room temperature. The treated sections were then washed with PBS, rehydrated, and incubated with digoxigenin (DIG)-labeled human Alu probe (PanPath B.V., Amsterdam, The Netherlands) for 2 h at 37°C. Then the sections were rinsed with tris buffered saline and treated with anti-DIG antibody for 40 min.
Statistical analysis
Data were examined with JMP 5.0 software (SAS Institute, Cary, NC, USA). The Wilcoxon rank-sum test was used for comparison of the new bone volume on the HA ceramics and the β-TCP ceramics. Fundamental significance for the analyses was set at P < 0.05.
RESULTS
P-2 cells were analyzed with flow cytometry for the expressions of CD13, CD34, CD44, CD45, CD90, and HLA-DR surface antigens (Fig. 1). The cells were negative for hematopoietic cell markers CD34, CD45, and HLA-DR. However, the cells were strongly positive for CD13, CD44, and CD90, which are known to be present in MSCs (17). Therefore, the cells were mesenchymal types, and we refer to these cells as MSCs.
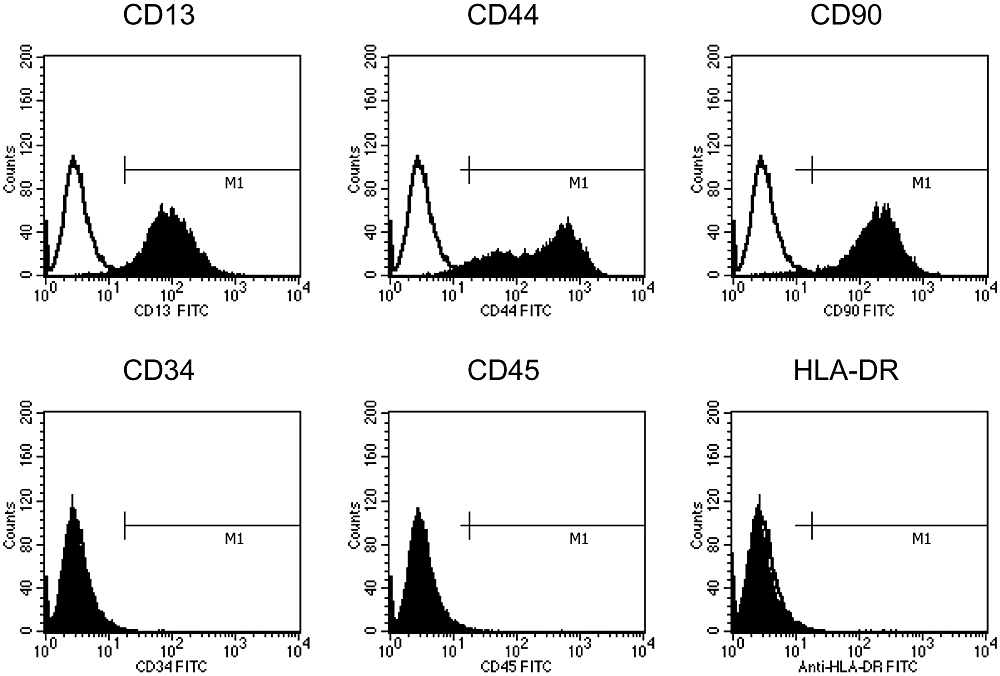
Flow cytometry analyses of the MSCs from donor #7. The MSCs from donor #7 were reacted with antibodies against CD13, CD34, CD44, CD45, CD90, HLA-DR, or immunoglobulin G isotype control antibodies. The cells were then loaded into a flow cytometer. The open and solid histograms indicate the results using isotype control immunoglobulin G and specific antibodies, respectively.
To confirm whether the MSCs have osteogenic capability, we cultured them on a 12-well plate in the presence or absence of Dex. As we previously reported, mineralization could be detected after 10–14 days of culture and more obviously after 21 days (18). As shown in Fig. 2, the culture with Dex showed mineralization (amorphous areas in Fig. 2a), which was confirmed by calcein uptake (black areas in rectangles of Fig. 2). In contrast, the culture without Dex showed no mineralization (Fig. 2b). To demonstrate the results quantitatively, we measured the ALP activity of the MSCs on the culture plate (Table 1). In six of seven cases, the expression of ALP activity was higher for the cultures in the presence of Dex than it was for those in the absence of Dex. Although the data varied among donors, when cultured on the dish, the MSCs showed osteogenic capability after differentiation periods.
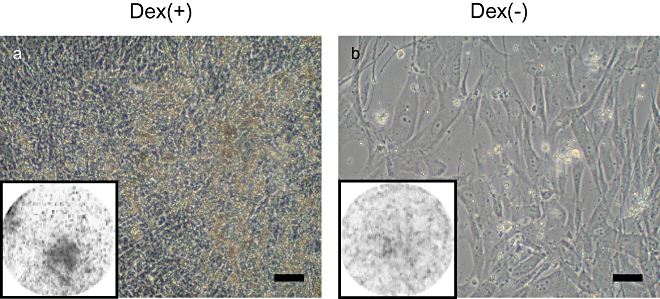
Cell morphology and mineralization capacity of MSCs from donor #7. The MSCs were cultured on 12-well culture plates for 20 days with (a) or without (b) dexamethasone. Calcium deposition detected by calcein uptake was visualized with an image analyzer. The round area indicates the entire area of the well in a 12-well culture plate. Scale bar = 200 µm.
ALP staining of MSCs cultured on HA and β-TCP ceramics was performed to assess the in vitro osteogenic differentiation of MSCs on the ceramics (Fig. 3). A red color indicates high ALP activity of the MSCs on the ceramics. Although stainability varied among the specimens, all the ceramics stained red. The stainability on HA correlated well with that on β-TCP. For example, strong staining was seen on both HA and β-TCP using MSCs from donor #1, whereas weak staining was seen on both types of ceramics from donor #4. These findings indicate that the MSCs on both the HA and β-TCP ceramics differentiated into osteoblasts in vitro. Therefore, we could confirm the fabrication of tissue-engineered constructs.
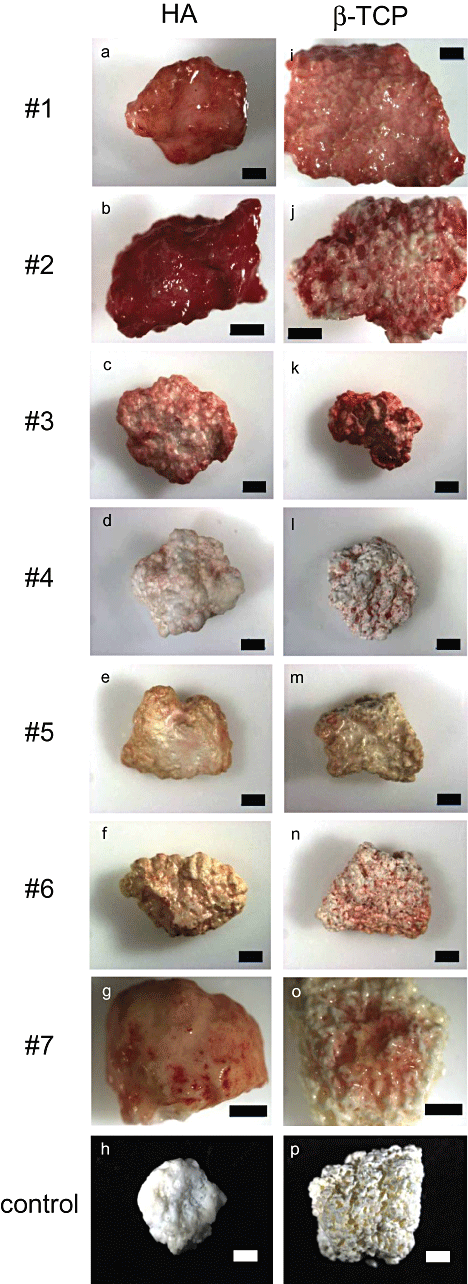
ALP staining of tissue-engineered constructs after in vitro culture. The MSCs from donors #1–7 were cultured on either HA (a–g) or β-TCP (i–o) ceramics under osteogenic conditions. After differentiation periods, ALP staining was performed to confirm osteogenic differentiation of the MSCs. Negative controls of HA (h) and β-TCP (p) without culturing MSCs are also presented. Red color indicates the location of ALP activity. Scale bar = 1 mm.
A total of 35 tissue-engineered constructs (17 constructs using HA and 18 constructs using β-TCP ceramics) were implanted into subcutaneous sites of nude rats to assess the in vivo osteogenic capability of the constructs. After 8 weeks of implantation, histological sections after HE staining showed new bone formation with osteoblasts and osteocytes in the pore regions of both the HA and β-TCP ceramics (Fig. 4). The new bone formation was detected in 7 of 17 (41%) constructs using HA and in 4 of 18 (22%) constructs using β-TCP ceramics. As expected, ceramics not seeded with MSCs showed no bone formation (Fig. 4d,h). An analysis of the in situ hybridization showed human Alu sequence in the cells around the newly formed bone (Fig. 5). The cells were located in or on the bone matrix, indicating that the cells were osteocytes or osteoblasts. Thus, we confirmed that the human donor cells initiated the new bone formation.
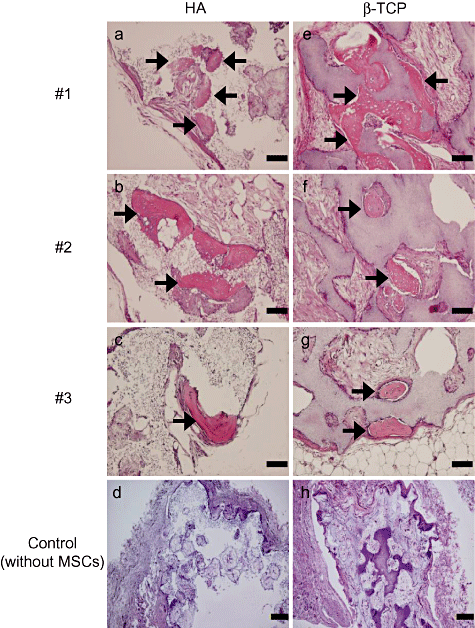
Histological findings of the constructs from three donors (#1–3) after 8 weeks in vivo implantation. The harvested constructs using HA (a–c) and β-TCP (e–g) ceramics were stained by HE. HA (d) and β-TCP (h) ceramics without MSCs were implanted as negative controls. The constructs using both HA and β-TCP ceramics showed new bone formation in the pore areas. Arrows denote newly formed bone. Scale bar: (a–c, e–g) = 100 µm and (d, h) = 200 µm.
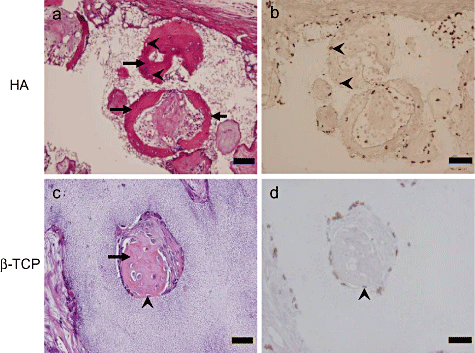
In situ hybridization of constructs from donor #2 after 8 weeks of in vivo implantation. Histological sections of the construct using HA (a and b) and β-TCP (c and d) ceramics were stained by HE. Osteocytes (arrowheads) appear to be embedded in the newly formed bone matrix. The sections adjacent to the HE staining of (a) and (b) were used for the in situ hybridization seen in (c) and (d), respectively. Brown cells in the sections of (b) and (d) indicate cells having the human Alu sequence. Arrows indicate newly formed bone. Arrowheads indicate osteocytes. Scale bar: (a, b) = 100 µm and (c, d) = 50 µm.
The implants were also analyzed by micro-CT to quantitate the volume of newly formed bone and pore areas. Typical micro-CT images are shown in Fig. 6. Using a software, the gray areas of the newly formed bone (Fig. 6, a1,d1) are depicted in yellow (Fig. 6, a2,b2), and the pore areas are depicted in blue (Fig. 6, a3,b3). The porosities of HA and β-TCP ceramics were 20.8 ± 5.01 standard deviation (SD) and 38.9 ± 5.41 SD, respectively. We calculated the bone volumes and compared those in the HA and β-TCP ceramics (Table 1). In six of seven cases, the bone volumes in the HA ceramics were greater than those in the β-TCP ceramics, and the difference was significant (P = 0.041). This result suggests that both types of ceramics supported new bone formation in vivo, but the HA ceramics tended to promote more of it than the β-TCP ceramics.
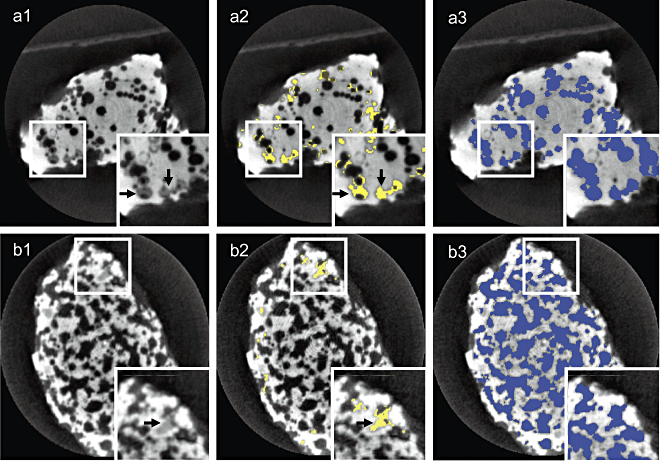
Typical micro-CT image of newly formed bone in pore areas of the constructs. Micro-CT images of the constructs using HA and β-TCP ceramics after 8 weeks of implantation are seen in (a1) and (b1), respectively. The micro-CT images depict the areas having a high (white), middle (gray), and low (black) intensity. We defined the white, gray, and black areas as ceramics, newly formed bone (arrows), and soft fibrous tissue, respectively. The bone areas are converted to yellow areas as seen in (a2) and (b2). The pore areas (newly formed bone and fibrous tissue) are converted to blue areas (a3 and b3). The rectangular areas in the corners show close-up views of the small rectangular areas.
DISCUSSION
The adherent cells obtained from the marrow of individual donors were negative for hematopoietic markers (CD34, CD45, and HLA-DR) but positive for markers present in mesenchymal cells (CD13, CD44 and CD90; Fig. 1). These cells had a fibroblastic shape and showed in vitro mineralization on a culture dish when cultured in the presence of Dex (Fig. 2). These findings indicate that the adherent fibroblastic cells were mesenchymal types. The culture dish surface supported the osteogenic differentiation of the cells (MSCs) as previously reported (4). In the present study, HA and β-TCP ceramics were evaluated for their capability to support in vitro as well as in vivo osteogenic differentiation of human MSCs.
We first made tissue-engineered constructs consisting of in vitro cultured human MSCs on ceramic scaffolds, and then the constructs were implanted in vivo. After the culture, a strong donor dependency was observed with regard to the ALP activity of the MSCs on the ceramics (Fig. 3). This is in agreement with previous studies that reported a large variability in ALP expression by cultures derived from human bone marrow cells of different donors (19–22). In spite of the variability, ALP activity was only observed in ceramics with MSCs and not in control ceramics without MSCs. Thus, both ceramics supported proliferation and osteogenic differentiation of donor MSCs on their surfaces. After implantation of the constructs, histological sections showed newly formed bone in the constructs but not in the control (Table 1 and Fig. 4). As seen in the data of in situ hybridization, human cells can be detected around the newly formed bone in the pore regions of the ceramics (Fig. 5). Therefore, the donor cells survived and formed new bone in the recipients. It is important to note that the cells were cultured in a medium containing donor serum and that the culture condition was the same as that used for clinical applications, which are ongoing in our facility (1–4). Although we could detect the donor cells in the newly formed bone at the ectopic implantation, recipient cells were also detected in and around the bone. Therefore, the initial bone from the donor induced surrounding undifferentiated recipient cells to show osteogenic differentiation within the ceramic pores. The induction might be attributed to the bone matrix of the initial bone because the matrix is known to contain various cytokines including bone morphogenetic proteins. Using composites of marrow cells and HA ceramics, Goshima et al. also reported similar findings (23). They found that the newly formed bones in the composites derived from donor cells were replaced by those derived from host cells 8–12 weeks after implantation of the composites.
As described in the histological findings, both HA and β-TCP had the capability to support osteogenic differentiation, which resulted in new bone formation. We also performed a quantitative analysis of the bone formation by micro-CT and found that more bone formation was detected in the HA ceramics than in the β-TCP ceramics after implantation (Table 1). However, it is well known that porosity, pore size, and pore interconnection influence the bone-forming capability of the ceramics, and two ceramics used in this study differed in these parameters. In fact, HA ceramics had smaller porosity and pore size than β-TCP ceramics. Many have proposed the hypothesis that scaffolds having higher porosity and pore size result in greater bone ingrowth in vivo (10). In contrast to the methods used in these reports, which utilized in vivo implantation of the ceramics, we first cultured the MSCs on the ceramics and then implanted the constructs at ectopic sites. In this regard, we already reported that rat MSCs well proliferated and differentiated into osteoblasts on HA ceramics having low porosity (30%) (24). Furthermore, Mastrogiacomo et al. reported that new bone formation had occurred more rapidly and efficiently in a scaffold with smaller pore size and smaller interconnection size (11).
As described, both HA and β-TCP ceramics supported in vitro as well as in vivo osteogenic differentiation of MSCs. We also reported that both ceramics showed excellent in vivo bone-forming capability using freshly isolated marrow cells (25). We also should consider the difference of composition that affects the in vivo resorbability of the ceramics. The HA ceramics are known to be nonresorbable, but the β-TCP ceramics are resorbable. The difference in resorbability influences bone formation because it is reported that an imbalance of β-TCP resorption and bone formation resulted in bone loss (13). Although our implantation periods of 8 weeks at rat subcutaneous sites did not cause massive resorption of the β-TCP ceramics, it might cause microresorption, which could influence the bone-forming capability of the constructs using β-TCP.
CONCLUSIONS
Calcium phosphate ceramics clearly supported the osteogenic differentiation of human MSCs and showed ectopic bone formation that originated from donor cells. Importantly, the differentiation could occur by culturing MSCs on the porous ceramics and the in vitro differentiated cells continued to fabricate new bone even after the in vivo implantation. Therefore, combining in vitro manipulated MSCs with porous ceramics can be expected to effect sufficient new bone-forming capability, which can thereby provide tissue engineering approaches to patients with skeletal defects in order to regenerate skeletal tissues.
Acknowledgments
Acknowledgments: This study was financially supported by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, and the New Energy and Industrial Technology Development Organization (NEDO), Japan.




