Osteogenic Differentiation of Human Bone Marrow-derived Mesenchymal Cells Cultured on Alumina Ceramics
Abstract
Abstract: Alumina ceramics have excellent mechanical and biocompatible properties, but are bioinert and hence have no bone-bonding properties. We took a tissue-engineering approach in an attempt to modify the ceramic surface and so provide an osteogenic/osteoconductive milieu. We obtained human bone marrow mesenchymal cells from four donors and then cultured the cells for two weeks on alumina ceramic in the presence of β-glycerophosphate, ascorbic acid and dexamethasone. The cells showed extensive alkaline phosphatase staining and mineralization, as evidenced by Alizarin Red S staining and calcein uptake. Biochemical analyses revealed high levels of alkaline phosphatase activity, osteocalcin expression and calcium content. This data indicates the appearance of active osteoblasts that are concomitant with bone matrix formation, i.e., in vitro cultured bone. The cultured bone/alumina composites should prevent the aseptic loosening of all-alumina ceramic joints or the detachment of implanted alumina ceramics, and thus could have clinical significance in orthopedic reconstructive surgery.
Currently, many types of artificial materials are used for reconstructive bone treatments. For example, calcium phosphate ceramics such as hydroxyapatite (HA) are widely utilized as bone graft substitutes (1). They have bone-bonding properties that allow them to establish chemical bonds between the regenerated bone tissue and the surfaces of implants when they are implanted into bone defects. This bone/material interface is very firm and stable, and these materials are called “bioactive materials” (2,3). However, these materials are not suitable for implants that must bear large weight loads because they are relatively fragile.
On the other hand, alumina (Al2O3) ceramics are widely used for joint replacements, the fixing of fractures (as screws) and dental implants because of their excellent biocompatibility and high mechanical strength (4–7). Unfortunately, alumina ceramics are inert and have no chemical bone-bonding properties, and thus are not classed as “bioactive”. Instead, they are categorized as “bioinert materials” (8). Due to the nonbioactive nature of alumina ceramics, many failures, such as aseptic loosening in total joint replacements (5) and the detachment of dental implants from the host bone (7), have been reported. Therefore, a stable interface between the implants and bone is required to ensure success in clinical cases.
Previously, fibroblastic or osteoblastic cells were reported as being able to proliferate on an alumina ceramic surface (9,10). However, quantitative analyses of bone formation by cultured cells, especially through the cascade of osteogenic differentiation, have not been performed for alumina ceramic surfaces. Mesenchymal stem cells have been found in bone marrow. These can differentiate into osteoblasts, chondrocytes, adipocytes and muscle cells (11,12). We have succeeded in forcing bone marrow-derived mesenchymal cells (BMMCs) into active osteoblasts, which form a bone matrix on an HA ceramic surface by using a cell culture technology (13,14). The osteoblast/matrix construct formed on HA immediately exhibits a new bone-forming capability after in vivo implantation (14). Thus, we can fabricate in vitro cultured bone (osteoblasts/matrix construct) by taking a tissue-engineering approach (13–15).
To date, there have been no reports of cultured bone using human BMMCs (hBMMCs) on alumina ceramics. If we can effectively fabricate bone in vitro by culturing hBMMCs on an alumina ceramic surface, then the in vivo osteogenic capability of the cultured bone should give us early bone fixation between the host bone tissue and the in vitro bone formed on the alumina ceramic. Tissue culture polystyrene (TCPS) dishes are fabricated from crystal-grade polystyrene by a vacuum-gas plasma process. The TCPS surfaces exhibit a slightly hydrophilic nature since the treatment introduces carboxyl and hydroxyl groups into their surfaces. Under typical culture conditions using TCPS dishes, different types of cultured cells can adhere and proliferate on the surfaces, although the cells exhibit little attachment to the bare polystyrene surfaces because of their high hydrophobicity. For this reason, TCPS dishes are commonly utilized for maintaining cultured cells. The purpose of this study is to confirm, both qualitatively and quantitatively, the in vitro osteogenic differentiation capacity of hBMMCs cultured on alumina ceramics as well as on the TCPS dishes, the latter being recognized as the gold standard substratum for cell culture.
MATERIALS AND METHODS
Materials
Alumina ceramic disks (30 mm diameter, 1.5 mm thick) were kindly provided by Kyocera Co. (Kyoto, Japan). The disks, whose composition was 99.5% pure polycrystalline-alumina, were produced by sintering pressed-alumina powder at 1500°C. To enable the use of an optical microscope to observe the cultured cells on the ceramic, we also used transparent single-crystal alumina disks that were manufactured using the Czochralski crystal growth technique. All of the disks were as pure as possible, with the only source of contamination being the machine tools used in the forming process. Their surfaces were not treated with any coatings. We subjected the polycrystalline-alumina (P-alumina) and single-crystal alumina (S-alumina) disks to autoclave sterilization (120°C, 20 min) before using them as cell culture substrates.
Surface characterization
The surface roughness of the P- and S-alumina disks, as well as that of the tissue culture polystyrene (TCPS) dishes was measured by using a profilometer (Surfcorder SE-30D, Kosaka Laboratory, Tokyo, Japan) with a diamond stylus having a 5 µm tip. The results were used to obtain a roughness average (Ra). The sessile contact angles (SCA) of the P- and S-alumina disks, and that of the TCPS dishes, were determined by using Milli-Q water and a goniometer (Face Contact-Angle Meter, Kyowa Kaimenkagaku Co., Tokyo, Japan). The Ra and SCA values were represented as the mean value of six samples with standard deviation (SD). The significant difference in the Ra or SCA values was statistically analyzed by using a Student's t-test. A probability (P) of less than 0.05 was considered to be significant. To evaluate the surface structure of the materials, each material's surface was analyzed by using a scanning electron microscope (SM-300, Topcon Co., Tokyo, Japan). Prior to placing the samples in the chamber, the specimens were coated with a 200 Å layer of platinum to prevent charging.
Marrow cell preparation and culture
Human bone marrow cells were obtained from the iliac crest of four donors (the characteristics of the donors are listed in Table 1) by needle aspiration after obtaining informed consent. Then, 3 mL of the marrow aspirates were immediately combined with 3 mL of phosphate-buffered saline (PBS) containing 30 IU of heparin. After centrifuging, the plasma and fat layers were removed from the supernatant, and the remaining nucleated cells, together with the red blood cells, were poured into two T-75 flasks (Coster Co., MA, U.S.A.) containing 15 mL of medium to give us our primary culture. The medium (maintenance medium) consisted of Eagle's minimum essential medium alpha (α-MEM, Invitrogen Co., CA, U.S.A.) containing 15% fetal bovine serum (JRH Biosciences Inc., KS, U.S.A.) and antibiotics (100 units/mL penicillin, 0.1 mg/mL streptomycin and 0.25 µg/mL amphotericin B; Sigma-Aldrich Co., St. Louis, MO, U.S.A.). The cultures were maintained under a 5% CO2 atmosphere at 37°C. The maintenance culture medium was renewed three times each week. As part of the medium change, floating cells (red blood cells and hematopoietic cells) were removed so that the adherent cells became almost confluent about ten days after the creation of the primary culture. The adherent cells are known to contain cells of mesenchymal types (15). The nearly confluent cells were released from the substrates using 0.05% trypsin 0.53 mM EDTA and were seeded into control six-well Falcon tissue culture polystyrene (TCPS) dishes, and onto P- and S-alumina disks placed in six-well TCPS dishes at a cell density of 5 × 103 cells/cm2. The samples were then subcultured for two weeks with 2 mL of the maintenance medium supplemented with 10 mM β-glycerophosphate (affiliate of Merck KGaA, Darmstadt, Germany). To induce osteogenic differentiation, 20.5 µg/mL L-ascorbic acid 2-phosphate magnesium salt n-hydrate and 100 nM dexamethasone (Dex) (Sigma-Aldrich Co.) were also added to the medium.
| Cell | Age | Sex |
|---|---|---|
| 1 | 62 | Male |
| 2 | 58 | Male |
| 3 | 25 | Male |
| 4 | 75 | Female |
Alkaline phosphatase and Alizarin Red S staining of the fourteen-day subcultured cells
For the alkaline phosphatase (ALP) staining, the subcultured cell layers were washed twice with PBS (–) (phosphate-buffered saline without Ca2+ and Mg2+) and fixed with 4% paraformaldehyde in PBS (4°C, 10 min). Then, 2 mL of the substrate solution, which consisted of 1 mg of Naphthol AS-MX phosphate sodium salt and Fast Red Violet B salt (Nacalai Tesque Inc., Kyoto, Japan) dissolved in 2 mL of 56 mM 2-amino-2-methyl propanol (AMP) buffer (pH 9.9), was added to the culture well, incubated at room temperature for 10 min, and then washed several times with 10 mM Tris-buffer (pH 7.6, 1 mM EDTA).
For the Alizarin Red S staining, the subcultured cell layers were washed twice with PBS (–). After fixing with 95% ethanol (4°C, 15 min), they were washed with deionized water and then 0.4 mL of Alizarin Red S (Nacalai Tesque Inc.) solution dissolved in PBS (5 mg/mL) was added to the culture well. After 1 min, the wells were washed several times with deionized water to remove the remaining stain.
Visualization of mineralized matrix by fluorescence emission during subculture
During the subculture periods, to enable the detection of the mineralized extracellular matrix of the cultured cells, 1 µg/mL of calcein (Dojindo Laboratory, Kumamoto, Japan) was added to the culture wells whenever the culture medium was renewed. Prior to the assay, the subcultured cell layers were washed twice with PBS (–), after which 2 mL of maintenance medium was added. The fluorescence of the calcein in the mineralized extracellular matrix of the cultured cells was visualized and observed by using a fluorescence microscope (IX 70, Olympus Co., Tokyo, Japan) (16). This assay was done for the same culture wells at each time point during the subsequent culture periods.
Measurement of DNA content and ALP activity of the fourteen-day subcultured cells
The subcultured cell layers were washed twice with PBS (–) and collected by scraping with 1 mL of 10 mM Tris-buffer (pH 7.4, 1 mM EDTA, 100 mM NaCl). The disks were then transferred into new dishes prior to the addition of Tris-buffer. The cell suspension was then sonicated, after which 20 µL of the sonicated cell suspension was used to quantify the DNA content. The DNA content was quantified using Hoechst 33258 (Molecular Probes Inc., OR, U.S.A.).
To measure the ALP activity, the same sonicated cell suspension was centrifuged at 13 000 × g for 1 min at 4°C. An aliquot (20 µL) of the supernatant was assayed for ALP activity using a p-nitrophenyl phosphate substrate (Zymed Laboratories Inc., CA, U.S.A.). The activity was represented by p-nitrophenol, which was released after incubation for 30 min at 37°C.
Measurement of calcium content of the fourteen-day subcultured cells
The subcultured cell layers were washed twice with PBS (–) and collected by scraping with 1 mL of 20% formic acid. The disks were then transferred into new dishes prior to the addition of 20% formic acid. The cell suspension was sonicated and stored at 4°C for 24 h. After centrifuging at 20 000 × g for 10 min at 4°C, the acid-hydrolyzed samples were then diluted with deionized water. The calcium content of the diluted samples was then measured by using an inductively-coupled plasma atomic emission spectrometer (SPS7800 plasma spectrometer, Seiko Instruments Inc., Chiba, Japan).
Quantification of osteocalcin content of the fourteen-day subcultured cells
After the measurement of the calcium content, the remnants of the samples in 20% formic acid were centrifuged at 20 000 × g for 10 min and the supernatant was then subjected to gel filtration to eliminate inorganic ions. The gel-filtered samples were then evaporated to increase the concentration. The resulting concentrated samples were added to an EIA plate immobilized with antihuman osteocalcin antibody to measure the concentration of osteocalcin with an intact human osteocalcin EIA kit (Biomedical Technologies Inc., MA, U.S.A.).
RESULTS
Surface characterization of the substrates
The roughness average (Ra) and sessile contact angles (SCA) of the surfaces of the P- and S-alumina disks, as well as those of the TCPS dishes, are shown in Table 2. The Ra values of the P- and S-alumina disks were very similar, and there were no statistical differences between them. The TCPS dish statistically exhibited larger Ra values than those of the others (P < 0.05). The SCA values of the P- and S-alumina disks were similar and not statistically different, while the TCPS dishes showed smaller SCA values than those of the others (P < 0.01). The results of scanning electron microscopy (SEM) analysis are shown in Fig. 1. The surface structure of the P-alumina disk (Fig. 1a), S-alumina disk (Fig. 1b) and TCPS dish (Fig. 1c) all proved to be very fine, while the grain of the P-alumina disk could barely be observed (Fig. 1a).
| Ra (nm) ± SD | SCA (°) ± SD | |
|---|---|---|
| P-alumina disk | 5.4 ± 1.1 | 73.5 ± 3.2 |
| S-alumina disk | 5.0 ± 2.0 | 72.9 ± 3.7 |
| TCPS dish | 11.4 ± 4.0 | 67.1 ± 2.4 |
- Data represent the mean value of six samples with standard deviation (SD).
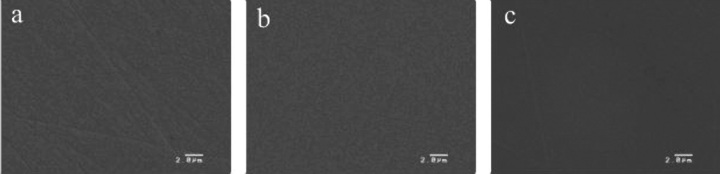
SEM micrographs of the surface of P- and S-alumina disks and a TCPS dish. The surface structures of a P-alumina disk (a), an S-alumina disk (b), and a TCPS dish (c) all exhibit a very fine surface, with little grain structure being visible on the surface of the P-alumina disk (a). (Original magnification: ×5000. Bar: 2 µm).
Proliferation and differentiation
As described in the Materials and Methods section, hBMMCs were subcultured in both the presence and absence of Dex. Under these conditions, the Dex induces the undifferentiated mesenchymal cells into osteoblasts that produce mineralized matrices about fourteen days after starting the subculture on a variety of materials, as we previously reported (17,18). During the two-week subculture period, the proliferation and differentiation of the hBMMCs on the transparent S-alumina disks, as well as the control TCPS dishes, were compared by phase contrast microscopy. The Dex-treated (Dex (+)) cells showed good cell adhesion accompanied by cell spreading, not only on the TCPS dishes (Fig. 2f), but also on the S-alumina disks (Fig. 2a). Subsequently, the cells cultured on both substrates showed a similar proliferating pattern (Fig. 2b,g), and reached confluence at day seven (Fig. 2c,h). The cells cultured without Dex (Dex (–)) also showed good cell adhesion and proliferated to reach confluence at day seven on both substrates. At day fourteen of the subculture, the Dex (+) cells cultured on both the S-alumina disks and TCPS dishes formed nodular aggregates, indicating mineralization (Fig. 2d,i). In contrast, the Dex (–) cells did not exhibit these aggregates (Fig. 2e,j). Thus, cell attachment to the substrates, proliferation and the mineralization of hBMMCs occurred in much the same way on all of the alumina surfaces, as well on as the TCPS dish surface.
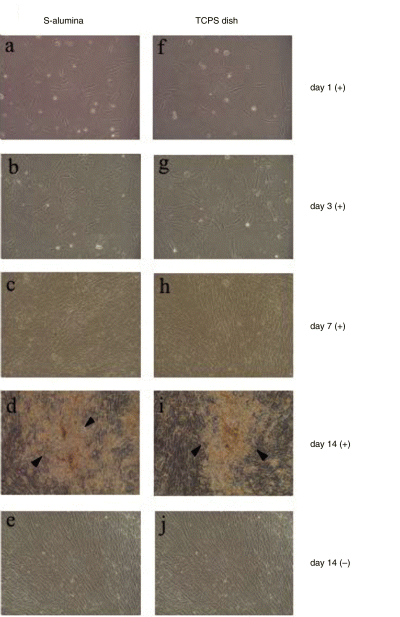
Phase contrast micrographs of hBMMCs subcultured on S-alumina disks and control TCPS dishes. After the primary culture of human bone marrow cells, adherent cells containing hBMMCs were released and subcultured on S-alumina disks and control TCPS dishes for two weeks in the presence (+) or absence (–) of Dex. The cells cultured on the transparent S-alumina disks and TCPS dishes were compared using phase contrast microscopy. The cells cultured with Dex on the S-alumina disks started to adhere and spread at day one (a), appeared to proliferate at day three (b), and reached confluence at day seven (c), as did those cultured on the TCPS dishes (f–h). At day fourteen of the subculture, the Dex-treated cells on both substrates formed nodular aggregates (arrows) indicating mineralization (d,i). In contrast, no mineralization was observed in the cells cultured without Dex, even after fourteen days of subculture (e,j). (Original magnification: ×100).
Qualitative evaluation of ALP activity and mineralized matrix
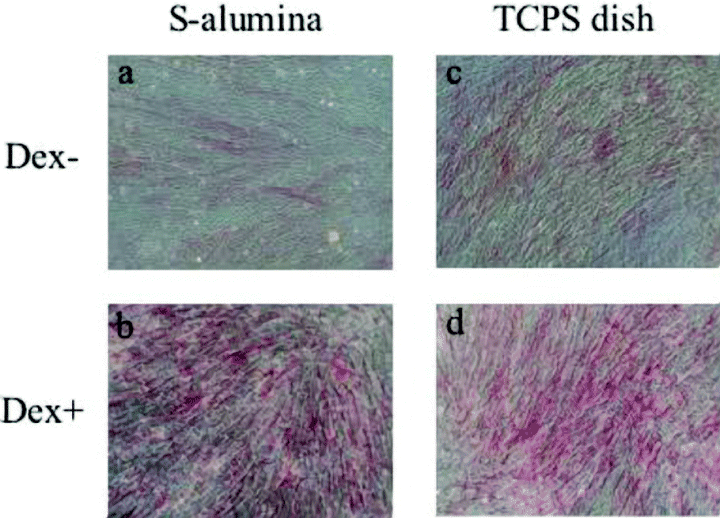
ALP and Alizarin Red S staining of the hBMMCs cultured on the P- and S-alumina disks, as well as those on the control TCPS dishes, were performed at day fourteen of the subculture. ALP staining on all the substrata could be detected but was more intense for the Dex (+) condition than the Dex (–) condition (Fig. 3). Under microscopic observation, the cells cultured on the S-alumina disks and TCPS dishes showed similar staining patterns (Fig. 4). Although some of the cells cultured without Dex showed positive activities (Fig. 4a,c), there was much less than that observed in the presence of Dex (Fig. 4b,d). As shown in Fig. 5, the Dex-treated cells on each substrate were strongly stained with Alizarin Red S, indicating calcium deposition, while the nontreated cells exhibited minimal staining. Microscopically, the nodular aggregates that appeared in the Dex-treated cells (Fig. 2d,i) were strongly stained with Alizarin Red S (Fig. 6b,d), but there were no stained areas in the Dex (–) cells (Fig. 6a,c). The microscopic findings showed that the ALP-positive areas tended to localize in the cellular regions, whereas the Alizarin Red S-positive areas were mostly found in the extracellular matrix regions. Strong signals of ALP and Alizarin Red S were detected in the cells cultured with Dex.
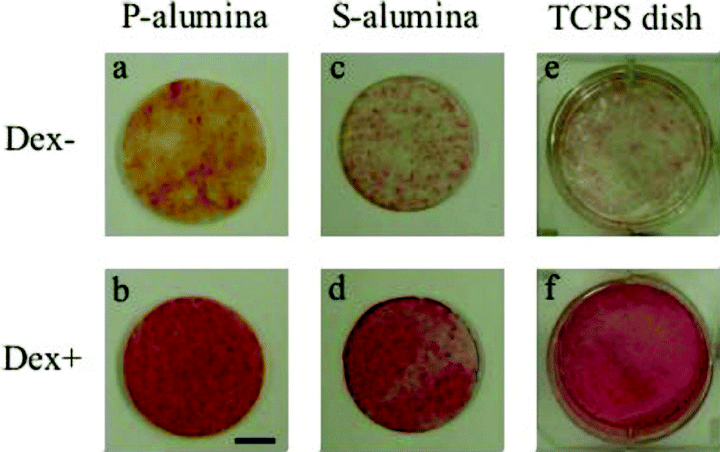
ALP stain of hBMMCs subcultured for two weeks. The hBMMCs were subcultured on P-alumina disks, S-alumina disks and control TCPS dishes in the presence (Dex +) or absence (Dex –) of dexamethasone. After two weeks, ALP staining was performed. The cells cultured with Dex (b,d,f) were found to be more intensely stained with ALP than those cultured without Dex (a,c,e) on all culture substrates. (Bar: 10 mm).
Microscopic views of the hBMMCs subcultured on S-alumina disks and control TCPS dishes after ALP staining. (a) and (b) show microscopic views of the cultured cells in (c) and (d) of Fig. 3, respectively. (c) and (d) show microscopic views of the cultured cells in (e) and (f) of Fig. 3, respectively. The cells cultured on both substrates showed similar ALP staining patterns. Numerous cellular regions were found to be positive for ALP in the presence of Dex (b,d), although some positive cells were also observed in the absence of Dex (a,c). (Original magnification: ×100).

Alizarin Red S staining of hBMMCs subcultured for two weeks on P- and S-alumina disks, and control TCPS dishes. The Dex-treated cells cultured on each substrate were strongly stained with Alizarin Red S (b,d,f), while the nontreated cells were barely stained (a,c,e). (Bar: 10 mm).
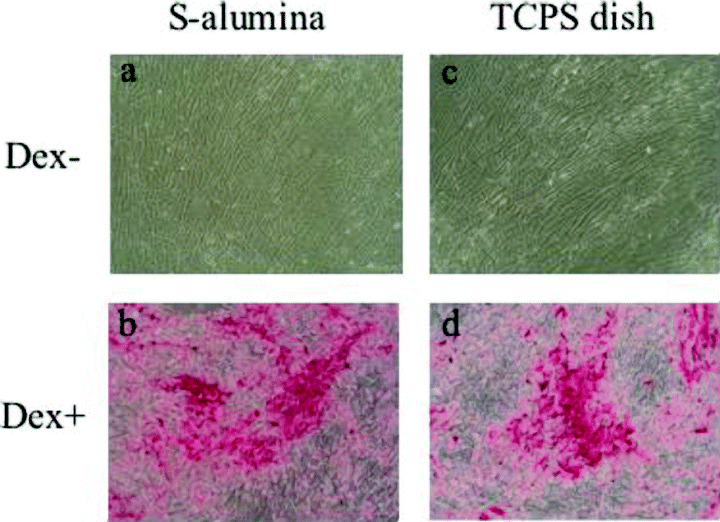
Microscopic views of hBMMCs subcultured on S-alumina disks and control TCPS dishes after Alizarin Red S staining. (a) and (b) show microscopic views of the cultured cells in (c) and (d) of Fig. 3, respectively. (c) and (d) show microscopic views of the cultured cells in (e) and (f) of Fig. 3, respectively. The cells cultured on both substrates were found to stain similarly with Alizarin Red S. The mineralized extracellular matrix that appeared in the Dex-treated cells was heavily stained with Alizarin Red S (b,d), but there was no obvious stainable mineralization in the untreated cells (a,c). (Original magnification: ×100).
Mineralized matrix formation detected by fluorescent emission
Recently, we reported a novel quantitative method for detecting mineralization by cultured cells utilizing a calcium-binding fluorescent calcein dye. Calcein has been found to be specifically incorporated and deposited into extracellular bone matrix, as evidenced by costaining with Alizarin Red S (16). The advanced characteristics of the method included the monitoring of the mineralization of the same specimens of cultured cells in a time-dependent manner due to continuous cultivation without the fixation processes of the cell layers. To demonstrate the calcium deposition of the cultured hBMMCs over time during the subsequent subculture period, we added calcein to the culture medium whenever the medium was renewed. The fluorescence of the calcein incorporated into the mineralized extracellular matrix of the S-alumina disks and control TCPS dishes was clearly visualized by using a fluorescence microscope (16). The calcein uptake of the cells cultured on the S-alumina disks appeared to be comparable to that of cells cultured on TCPS dishes (Fig. 7). At day seven of subculture, many fluorescent spots could be seen in the cells cultured with Dex (Fig. 7a,d). Subsequently, the fluorescent areas enlarged with time such that, at day fourteen, fluorescent aggregates were observed in those locations where mineralization (dark/dim areas) was shown by phase contrast microscopy (Fig. 7b,e). In contrast, we could barely detect any fluorescence in those cells cultured without Dex (Fig. 7c,f).
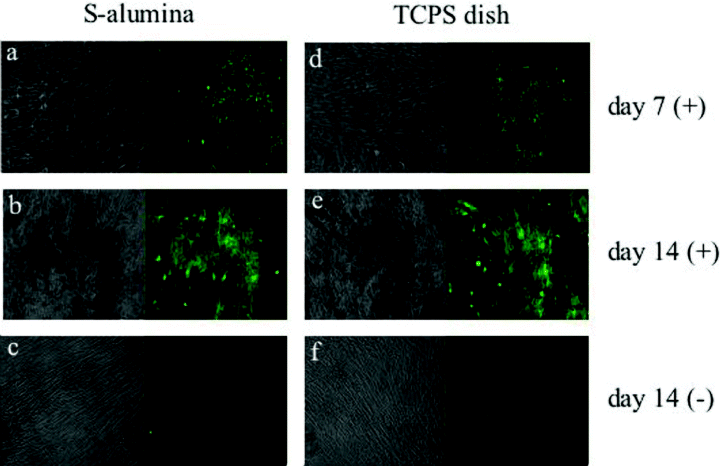
Fluorescent micrographs of the calcein incorporated into the hBMMCs subcultured on the S-alumina disks and control TCPS dishes. As described in Fig. 2, the hBMMCs were subcultured on S-alumina disks and control TCPS dishes with (+) or without (–) Dex. To demonstrate calcium deposition with time during the subsequent subculture periods, calcein was added to the culture medium whenever the medium was renewed. The fluorescence of the calcein incorporated into the mineralized matrix of the hBMMCs cultured on the S-alumina disks and TCPS dishes was clearly visualized by fluorescent microscopy. Each micrograph (a–f) represents both the phase contrast view (left-hand side) and fluorescent view (right-hand side). The calcein uptake of the cell layers on the S-alumina disks appeared to be comparable to those on the TCPS dishes. At day seven, many fluorescent spots were clearly observed in the cells cultured with Dex (a,d). After fourteen days of subculture, the location of the fluorescent uptake appeared to coincide with the dark dim areas seen in the phase contrast view (b,e). The dark areas in the phase contrast micrographs are the mineralized matrix of the cultured cells. Fluorescence (mineralization) was barely detected in those cells cultured without Dex (c,f), even after fourteen days of subculture. (Original magnification: ×100).
Biochemical findings
After fourteen days of the subculture, biochemical analyses were comparatively demonstrated for the cell layers on the P-alumina disks and control TCPS dishes. The DNA content, which reflects the cell numbers, was measured and represented as DNA content/area. The DNA content of the cell layers on the P-alumina disks was similar to that on the TCPS dishes, regardless of the Dex treatment, for every case of hBMMC (Fig. 8a). ALP activity, which is recognized as an osteoblastic marker, was measured and represented as ALP activity/area. The ALP activity on all of the substrates was significantly higher in the Dex-treated cells than in the untreated cells, and the activity of the cell layers with Dex on the P-alumina disks showed slightly lower (in cells 1 and 3) or higher (in cells 2 and 4) levels relative to those on the TCPS dishes (Fig. 8b). The bone-specific osteocalcin and calcium contents were measured and calculated as content/area (Fig. 9). The contents of the cell layers on both the P-alumina disks and TCPS dishes were also significantly higher in the Dex (+) cells than in the Dex (–) cells in all cases. For the Dex (+) cells, both of the contents on the P-alumina disks were slightly lower (in cells 1, 2 and 3) or higher (in cell 4) relative to those on the TCPS dishes (Fig. 9a,b). Taken together, the results clearly show that the P-alumina disks as well as the TCPS dishes support Dex-dependent osteogenic differentiation of the hBMMCs.
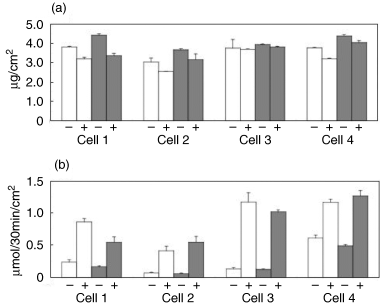
DNA content and ALP activity of hBMMCs subcultured for two weeks on P-alumina disks and control TCPS dishes. The hBMMCs obtained from four donors (cells 1–4) were used for the subculture. The subculture was performed on the P-alumina disks (▪) and control TCPS dishes (□) in the presence (+) or absence (–) of Dex. After two weeks, the DNA content and ALP activity of the cultured cell layers were measured using the method described in the Materials and Methods section. The DNA content (a) and ALP activity (b) of each cell layer was divided by the cultured area (the area of the P-alumina disk or the TCPS dish). The data represents the mean ± SD of the four samples.
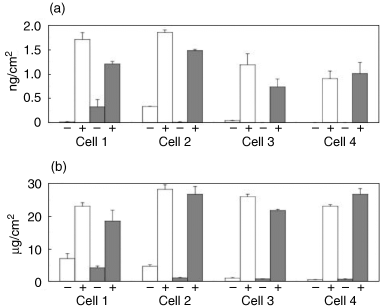
Osteocalcin and calcium content of hBMMCs subcultured for two weeks on P-alumina disks and control TCPS dishes. The hBMMCs obtained from four donors (cells 1–4) were subcultured on P-alumina disks (▪) and control TCPS dishes (□) in the presence (+) or absence (–) of Dex. After two weeks, the osteocalcin and calcium contents of the cultured cell layers were measured. The osteocalcin (a) and calcium (b) content of each cell layer was divided by the cultured area (the area of the P-alumina disk or the TCPS dish). The data represents the mean ± SD of the four samples.
DISCUSSION
For this study, we added Dex to the culture medium because Dex is known to induce osteogenic differentiation in bone marrow cells. Maniatopoulos et al.reported that cultured rat bone marrow cells could form in vitro mineralization in the presence of Dex (19). We then reported that Fourier transform infra-red spectra and X-ray analysis had revealed that the physical properties of the mineralization that appears in this culture condition were comparable to those of natural bone (17). Moreover, the Dex-treated cells (Dex (+) cells) showed high levels of ALP activity. We also demonstrated osteocalcin messenger RNA (mRNA) expression by Northern blot analysis, and the location of the gene expression was identified in the cells surrounding the mineralized area as a result of in situ hybridization (17). These previous results confirm that mesenchymal cells (rBMMCs) derived from rat bone marrow can differentiate into active osteoblasts, which fabricate genuine bone in a culture. Furthermore, the osteogenic differentiation of cultured rBMMCs could occur on a glass (18) and hydroxyapatite ceramic surface (13).
Recently, human mesenchymal stem cells derived from bone marrow have been found to have an osteogenic ability in vitro (20). We have also demonstrated the osteogenic differentiation of human marrow-derived mesenchymal cells (hBMMCs) under the Dex-contained culture condition (21). To utilize the osteogenic capability of hBMMCs for bone treatment in clinical cases, several studies have investigated the in vitro osteogenic capacity of hBMMCs cultured on certain types of bioceramics (21,22). The aim of these studies is to fabricate hybrid materials (i.e., in vitro cultured bone on the bioceramics), which show the formation of new bone in vivo, and thus the methods can be categorized as a “tissue-engineering” approach. In the present study, we tried to induce hBMMCs to express osteogenic differentiation on alumina ceramics by employing “tissue engineering” that could be used in clinical situations.
As a result of microscopic observation, in the Dex (+) culture condition the cultured hBMMCs showed good proliferation and obvious mineralization on the S-alumina disks as well as on the control TCPS dishes (Fig. 2). Moreover, the cells with Dex on both substrates exhibited extensive ALP staining (Fig. 4) in addition to the mineralized formation evidenced by the Alizarin Red S staining (Fig. 6) and calcein uptake (Fig. 7). Although the cells cultured on the opaque P-alumina disks could not be used for microscopic observation, macroscopically, the cells cultured with Dex on the P-alumina disks were strongly stained with ALP and Alizarin Red S, in much the same way as those cultured on the S-alumina disks (3, 5). Regarding this, we should pay careful attention to the differences in the surface characterization that may influence the adhesion and function of the cells in tissue culture experiments. Neupert et al. reported no differences in the adhesion and proliferation of the fibroblast-like cells cultured on the surfaces of alumina ceramics (9). In contrast, Schwartz and Boyan demonstrated the importance of the material's surface roughness and topography on mesenchymal cell response (23). In this study, the P- and S-alumina disks showed very similar characteristics in their surface roughness and topography (Table 2 and Fig. 1). Thus, we can assume that the cultured cells will achieve a level of adhesion, proliferation and mineralization on the P-alumina disks that is similar to those actually observed on the S-alumina disks.
According to the results of biochemical analysis, the P-alumina disks and control TCPS dishes showed comparable levels of DNA content/area, regardless of the Dex treatment (Fig. 8). This indicates that hBMMCs could proliferate, even on the P-alumina disks. ALP is a protein that localizes on the cell membranes of the osteoblasts. Osteocalcin is a protein that is secreted by active osteoblasts and is thus generally recognized as an osteoblast-specific maker. Bone matrix consists of hydroxyapatite crystals made of calcium and phosphorus. Therefore, these three factors (ALP, osteocalcin and calcium) provide important clues for detecting osteogenic differentiation of the cells. In our study, although the similarity of the relative levels of the factors in the cultured cells on the P-alumina disk to those on the control TCPS dishes seemed to depend on cellular individuality, all of the cases showed significantly higher levels of these factors in the Dex (+) cells than in the Dex (–) cells (8, 9). This data indicates the Dex-dependent osteogenic differentiation capability of hBMMCs cultured on P-alumina disks as well as on TCPS dishes. Interestingly, the hBMMCs from elderly persons (62 and 75 years old: Table 1), a middle-aged person (58 years old) and a young person (25 years old) showed similar high levels of ALP activity (Fig. 8b), osteocalcin content (Fig. 9a) and calcium content (Fig. 9b). These results proved that the hBMMCs obtained from an aged person could proliferate and differentiate into active osteoblasts that are concomitant with bone matrix formation on the alumina ceramic surface.
We have reported the in vivo bone-forming capability of fresh bone marrow cells combined with porous ceramics (24,25). When we used porous HA ceramics, the bone formation appeared to start on the HA surface and proceeded toward the center of the pore, and there was no intervening connective tissue at the bone/HA interface (24). In contrast, when we used porous alumina ceramics, the bone formation started at a point distant from the material surface, and intervening connective tissue was observed at the bone/alumina interface (25). The in vivo cell/alumina interaction (osteogenesis with intervening connective tissue) can be attributed to the nonbioactive nature of the alumina (8), and the intervening connective tissue is predicted to weaken the stability of the interface between the de novo bone and the implant surface. In this study, we succeeded in differentiating the hBMMCs into active osteoblasts that are concomitant with bone matrix formation on the alumina ceramic surface. Our recent study demonstrated that cultured osteoblasts from bone marrow can fabricate bone matrix consisting of hydroxyapatite directly onto the surface of the culture substratum and, moreover, the cultured bone on the ceramic exhibits the ability to form new bone immediately after in vivo implantation (14,15). The in vitro cultured bone, however, cannot bond to the alumina ceramic surface. So, we would not be able to achieve mechanical stability if we used flat-surfaced alumina ceramic. Therefore, if we can fabricate the in vitro bone in the pores of porous alumina ceramic, then the prefabricated cultured bone in the porous regions would remain after in vivo implantation, and should then actively form de novo bone. The de novo bone in the pores, together with the regenerated bone derived from the host, can have a significant effect on the stable mechanical interface between the host bone tissue and the porous implants.
In conclusion, our current results confirm the osteogenic differentiation capability of human mesenchymal cells cultured on alumina ceramics. Importantly, the cells were derived from bone marrow cells obtained with minimum invasion using needle aspiration. Cultured bone/alumina composites that are prefabricated by tissue-engineering approaches may prevent the aseptic loosening of total joint prostheses having rough/porous alumina surfaces, as well as other failures of alumina ceramic implants. Therefore, current approaches with the least morbidity may have clinical significance in reconstructive surgery in the orthopedic field.
Acknowledgments: This work was undertaken by the Three-Dimensional Tissue Module Project, METI (A Millennium Project). The author (M. Hirose) is grateful for a fellowship from the New Energy and Industrial Technology Development Organization of Japan. We wish to express our gratitude to Kyocera Co., Ltd. (Kyoto, Japan) for supplying the alumina ceramic disks.




