A bridge between original and novel states: ontogeny and function of “suction discs” in the Branchiura (Crustacea)
Abstract
SUMMARY The emergence of novel structures in the course of evolution faces an explanatory problem, leaving the gap from the ancestral structures difficult to bridge. This difficulty is caused by the lack of intermediate stages. Branchiurans are ectoparasitic crustaceans which use a pair of “suction discs” to attach to their host. These structures are modified first maxillae. During ontogeny, the first maxillae transform from a normal cephalic appendage to the specialized suction disc. However, supposedly ancestral branchiurans lack the suction discs in the adults and the first maxilla remains a normal appendage throughout. We describe the muscular arrangements in the developing first maxillae in Argulus coregoni. The suction discs originate as a fusion of the first and second podomeres. The sucker muscles of the suction discs are homologous to the muscles that insert in the second podomere at the early larval stages. The developmental process of the suction disc can be seen as a “recapitulation” of the evolutionary process. We thus show how the first maxilla can maintain not just the biological role but also a functional continuity during the evolution of the novel structure. From this example it is obvious that the intermediate stages of the emerging novelty, if present in the ontogeny, can help solve at least some of the enigmatic appearances of novel structures.
INTRODUCTION
The small gradual changes of morphology in the animal kingdom stretching over long reaches of time beautifully fit the traditional “microevolutionary”-based explanatory frameworks for evolution (Futuyma 1998; Müller 2010). However, the question of how completely “new” traits are formed and their evolutionary background has vexed biologists for a very long time. If diversity is caused by variation of existing traits, how do these new traits then appear? (Frazzetta 1975; Futuyma 1998; Moczek 2008).
One of several problems of “novel” characters lies in the problem of interdependency of and correlations between structures. The organism's fitness and functionality risks being reduced if one of the interacting parts is altered (Futuyma 1998; Schwenk 2001). On the other hand, new body parts are often associated with new functions not present in the ancestral lineage (Müller and Wagner 2003). Accordingly, the problem is how to explain a novel feature's appearing and how it is accommodated into the preexisting, tightly correlated parts without a related loss of overall fitness (Mayr 1960; Riedl 1975; Wake et al. 1983; Roth and Wake 1989; Raff 1996; Müller and Wagner 2003). This problem is compounded by a general lack of “intermediate stages” in the evolution of novelties (Wagner 2001; Futuyma 2005). Conversely, in cases where “intermediates” can be convincingly argued for (typically then only in groups with well-resolved phylogenies where the plesiomorphic character states of the relatively basal groups are known), it is possible to theorize about the origins and explanations of novelties (Müller and Wagner 2003).
Here we provide new data from ontogenetic investigations on the specialized first maxilla “suction discs” in the Branchiura, which we consider a textbook example of an evolutionary novelty. The Branchiura is a group of ectoparasitic Crustacea found on fish hosts primarily in freshwater (Wilson 1902; Fryer 1968; Boxshall 2005; Møller 2009). The biological role (sensu Bock and von Wahlert 1965) of the first maxilla stays the same throughout the life of the parasite; it serves as the main attachment structure with which the parasites attach to the fish host (Leydig 1850, 1889; Claus 1875; Wilson 1904). However, the specific function of the appendage in the adult stage is different among the Branchiuran genera. Thus, species of the genus Dolops attach by hooks on the distal part of the first maxilla, while species of Argulus, Chonopeltis, and Dipteropeltis attach by the highly modified first maxilla suction disc (Møller et al. 2008; Møller 2009; Møller and Olesen 2009) (see Fig. 1). The functional morphology of the adult first maxilla is completely different from any normal arthropod appendage and is completely remodeled for the sucker function with muscles operating the central piston-like part in a suction cup like fashion (see Gresty et al. 1993; Møller et al. 2008 for details).
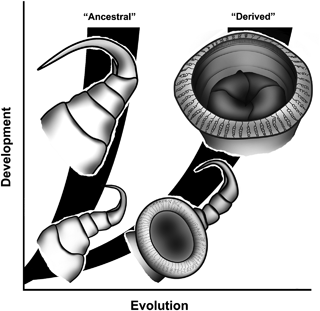
Proposed evolutionary and developmental pathway of the first maxilla.
Møller et al. (2008) presented a preliminary phylogeny of the Branchiura based on molecular data, showing Dolops as the possible sister group to the remaining Branchiuran genera. Based on the fact that all known branchiuran larvae have a distal bi-partite hook on the first maxilla in the early stages obviously homologous to the distal hook in adult Dolops, the authors proposed to apply the ontogenetic criterion (sensu Patterson 1982) in the following scenario of evolution: the distal hooks are to be considered the ancestral mode of attachment in the Branchiura and hence, the suction disc is the derived, and possibly later evolved mode (Møller et al. 2008) (Fig. 1).
In general, however, knowledge of larval development in the Branchiura is still very patchy, and limited to ca. 15 species, most of them from the genus Argulus. The development of the external morphology has been described using light and scanning electron microscopy (SEM) (Wilson 1902; Fryer 1961; Thomas 1961, Shimura 1981; Rushton-Mellor and Boxshall 1994; Møller et al. 2007). The muscular system of the first maxillae in the adult A. japonicus was described by Gresty et al. (1993) and in the hatching larvae of A. foliaceus by Boxshall (1998). Until now, it has been the general consensus, based on external morphology, that the suction discs originate from the proximal most limb portion of the first maxilla (forthwith termed podomere 1) (Claus 1875; Tokioka 1936; Shimura 1981; Rushton-Mellor and Boxshall 1994; Møller et al. 2008). Only a few authors claim that the suction discs originate from podomere 2 (Thomas 1961; Van Niekerk and Kok 1989). Both interpretations state that the suction disc arises from only one podomere of the first maxilla. If this is correct, it must be assumed that the sucker muscles operating the central piston-like part would have to be developed de novo for this function. Clearly, no muscles attach in the middle of the podomere 1, which means that there are no possible muscles to recruit for a new function. So how does the suction disc become equipped with a central muscle? What is the precise nature of this highly elaborated suction disc? How is the problem of functional constraints managed? In this study, we endeavor to answer these questions by looking into the ontogeny of the muscular system of the first maxilla during its development into the suction disc using various microscopic techniques (confocal laser scanning microscopy, SEM, and histological sections). We hope to provide a well-documented example of a highly elaborated evolutionary novelty and its ontogenetic and possible evolutionary origin.
MATERIALS AND METHODS
The examined species in this study is Argulus coregoni, collected from Amago Salmon Oncorhynchus masou ishikawai in the Fisheries Laboratory (Kinki University, Shingu City, Japan) (33°72.98′N/135°90.43′E). Specimens for cLSM observation (Leica TCS-SL, Mannheim, Germany), were fixed for approximately 5 h in icecold 4%p-formaldehyde in phosphate-buffered saline (PBS; 130 mm NaCl; 7 mm Na2HPO4·2H2O; 3 mm NaH2PO4·2H2O; pH 7.0), and washed in 0.3% Triton-X 100 in PBS (PBT). For F-actin immunoreactivity, specimens were stained with rhodamine phalloidin (Molecular Probes, Carlsbad, CA, USA) at 100 nM in PBT, and then washed five times with PBT. Appendages were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA). The cuticle signal is an endogenous autofluorescence. Three-dimensional reconstructions were performed using the ImageJ software.
For SEM (JEOL JSM-5600LV, JEOL, Tokyo, Japan), specimens were fixed in 5% formaldehyde. Specimens were dehydrated in an ethanol series, freeze-dried, and then coated in an osmium plasma coater (Nippon Laser and Electronics Lab OPC 40, Aichi, Japan). For histological sections, specimens were fixed in 2% glutaraldehyde and 2%p-formaldehyde in 0.1 m sodium-cacodylate buffer (pH 7.4) supplemented with 5% sucrose for 2 h at 4°C and postfixed in 1% osmium tetroxide in the same buffer with 5% sucrose for 0.5 h at 4°C. Specimens were then dehydrated in an ethanol series, embedded in Spurr's resin and polymerized, and finally sectioned at 1 μm on a ULTRACUT-N (Reichert Nissei, Depew, NY, USA). Sections were stained with toluidine blue (Kiernan 2008) and observed in a light microscope.
RESULTS
Ontogeny of exoskeletal structure of the first maxilla
The ontogenetic sequence was studied using confocal laser scanning microscopy. In the stage 2 larva (Fig. 2A), the first maxilla has four podomeres and a large bi-partite hook present at the tip. Each podomere is distinct and the boundary between the first and second podomeres is clearly observed. Weak/indefinite autofluorescence is observed in the first and second podomeres. In the stage 3 larva (Fig. 2B), the first maxilla still has a distal hook and four podomeres. A disc-like structure is created by a combination between the first and second podomeres. The second podomere makes out approximately 90° of the disc. The podomeres are still separate and show weak/hazy autofluorescence. The anlagen of the “circular sucker muscle” (Gresty et al. 1993) is observed, but hard to distinguish. In the stage 5 larva (Fig. 2C), the first and second podomeres are enlarged and distinctly disc shaped. The border between the first and second podomere is still visible. The second podomere makes up approximately 90° of the disc. The support rods in the disc margin are visible, but not fully differentiated. The distal two podomeres and the hook are still present, but already semivestigial. A circular sucker muscle is observed in the basal part of the first podomere. The anlagen of disc rim muscle are observed in the first and second podomeres. In the stage 6 larva (Fig. 2D), the border between the first and second podomeres is not visible, and has fully developed into a (functional?) suction disc. The vestige of the distal two podomeres is observed but is reduced. A circular sucker muscle is observed around the disc rim and the disc rim muscles are observed.
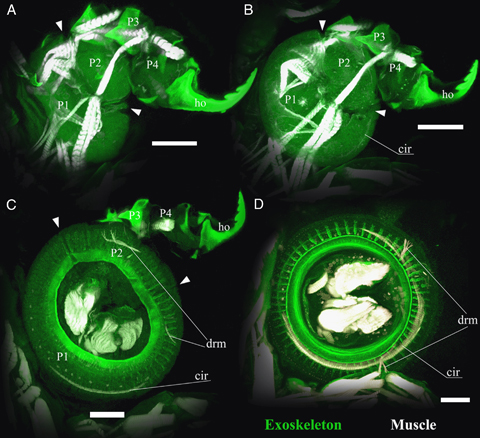
Ontogeny of exoskeletal structure of the left first maxilla from ventral aspect observed by confocal microscopy. (A) Stage 2 larva. (B) Stage 3 larva. (C) Stage 5 larva. (D) Stage 6 larva. P1, P2, P3, and P4 indicate the first, second, third, and fourth podomeres, respectively. Arrowheads indicate the boundary between first and second podomeres. ho, distal hook; cir, circular sucker muscle; drm, disc rim muscle. Scale bars, 50 μm.
The detailed microstructures of the first and second podomeres were observed by scanning electron microscope and histological sections and are presented in Fig. 3. Stage 3 larvae were used for these observations. The weakly sclerotized first podomere and the second podomere with a ventral plate are combined to form a disk-like shape (Fig. 3A). The first and second podomeres both have a corresponding dorsal plate (Fig. 3B). The weakly sclerotized area covers not only the first podomere but also the lateral side of the second podomere (Fig. 3B). These identifications of podomeres by external observation were compared and confirmed also by the histological section data (Fig. 3C). The dorsal plates are useful landmarks for identifying the specific podomere configuration. Dorsal plate 1 is associated with the ventral swelling (weakly sclerotized area) as a first podomere; dorsal plate 2 is associated with the ventral plate as a second podomere. The fate of each podomere's ventral region cells remains to be investigated.
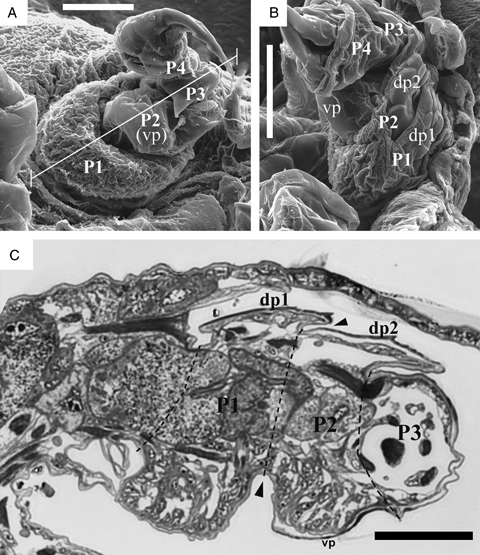
Microstructures of the first maxilla in the stage 3 larvae. (A) Ventral aspect of the right first maxilla. The white line corresponds to the section of Fig. 2C. (B) Lateral aspect of left first maxilla. (C) Transverse section of the first maxilla. P1, P2, P3, and P4 indicate the first, second, third, and fourth podomeres, respectively. Arrowheads indicate the boundary between first and second podomeres. vp, ventral plate; dp1, dorsal plate of first podomere; dp2, dorsal plate of second podomere. Scale bars, 50 μm.
Three-dimensional reconstruction of the musculoskeletal ontogeny of the first maxilla (Fig. 4)
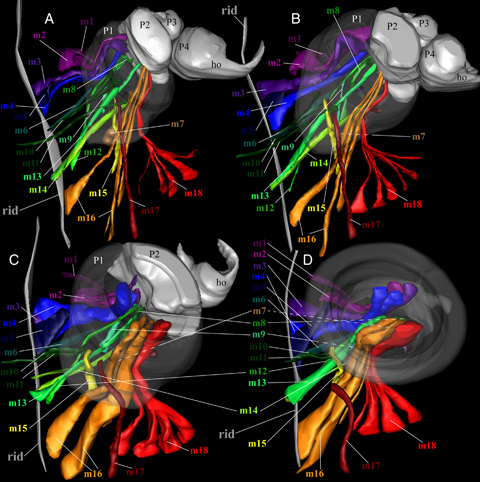
Three-dimensional reconstruction of musculoskeletal ontogeny of the left first maxilla (ventral aspect). (A) Stage 2 larva. (B) Stage 3 larva. (C) Stage 5 larva. (D) Stage 6 larva. m1–18 indicate the first to eighteenth muscles, respectively. P1, P2, P3, and P4 indicate the first, second, third, and fourth podomeres, respectively. The “rid” indicates dorsal ridge.
The muscles of the suction disc were reconstructed and are shown in Fig. 4. Muscles are numbered 1–18, ad referendum. Every muscle in the suction disc (stage 6 larva; Fig. 4D) (except for the circular sucker and disc rim muscles) was already present in the larval hooked first maxilla (stage 2 larva; Fig. 4A). Also, the muscular arrangement itself is not changed through ontogeny. The details of muscular arrangement and their functions are described below.
Muscles 2–4, 8, 13, 16, and 18 insert on the ventral side of the boundary between the first and second podomeres in the stage 2 larva (Fig. 4A). These muscles retain their arrangement throughout ontogeny (Fig. 4, B–D). The exoskeletal area at the muscle attachments (the apodeme) is transformed into the “sucker plate” (central movable part of the disc, inside the rim), and the muscles subsequently to a new function as “sucker muscles” in the stage 6 larva. Muscles 5, 10, 11, 15, and 17 insert in and around the ventral side of the boundary between the cephalon and first podomere in the stage 2 larva (Fig. 4A). These muscles retain their arrangement through ontogeny (Fig. 4, B–D). The ventral side of the boundary between the trunk and first podomere is transformed into the proximal inner side of the suction disc in the stage 6 larva. The muscles are recruited to a new function as the “cup adductor muscle.”Muscle 1 inserts in the dorsal side of the boundary between the trunk and first podomere in the stage 2 larva (Fig. 4A). This muscle also retains its specific position through ontogeny (Fig. 4, B–D). The dorsal side of the boundary between the trunk and first podomere is transformed into the proximal outer side of the suction disc in the stage 6 larva. Muscle 1 is recruited for the novel function as the “cup abductor muscle.”Muscles 6, 7, 9, 12, and 14 insert on the dorsal side of the first podomere in the stage 2 larva (Fig. 4A). These muscles retain their arrangement throughout ontogeny (Fig. 4, B–D). The dorsal side of the first podomere corresponds to the dorsal side of the suction disc in the stage 6 larva. These muscles probably function as cup abductors. For the sake of comparison, Table 1 gives an overview of the terms used in this article and the corresponding muscles as described in Gresty et al. (1993).
| In this paper | From Gresty et al. (1993) |
|---|---|
| Muscle 1 | N/A |
| Muscle 2 | Cup promotor muscle |
| Muscle 3 | Cup abductor muscle 2 |
| Muscle 4 | Sucker muscle 1 |
| Muscle 5 | Cup adductor muscle 1 |
| Muscle 6 | Cup adductor muscle 2 |
| Muscle 7 | Cup abductor muscle 5 |
| Muscle 8 | Cup abductor muscle 4 |
| Muscle 9 | Cup abductor muscle 1 |
| Muscle 10 | Cup abductor muscle 6 |
| Muscle 11 | Cup abductor muscle 7 |
| Muscle 12 | Cup abductor muscle 3 |
| Muscle 13 | Sucker muscle 5 |
| Muscle 14 | Cup remotor muscle 3 |
| Muscle 15 | Cup adductor muscle 3 |
| Muscle 16 | Sucker muscle 3 |
| Muscle 17 | Sucker muscle 4 |
| Muscle 18 | Sucker muscle 2 |
DISCUSSION
Maintained functional continuity
The results clearly show that the suction discs originate from the cross-boundary area of the first and second podomeres of the first maxilla (see Fig. 5, A–C). This cross-boundary nature is critical for the functional continuity of the first maxilla during ontogeny, as the sucker function (specifically: the piston action of the central part) is carried out by the muscle inserted in the central part of the suction disc. In a hypothetical suction disc originating from only the first podomere, the central part of the disc would correspond to the central part of the podomere, and indeed there are no muscles attaching in this area. Thus, the sucker function would have to have muscles forming de novo during ontogeny. As mentioned above, the notion that such muscles develop de novo seems improbable and is difficult to explain in an evolutionary context. A dipodomere origin of the suction disc as we present strong evidence for here, is more easily explained as the muscles inserting on the boundary between the first and second podomere being “exaptated” for the sucker function; they are already in place and in use by the larval second podomere (see Fig. 5A). Thus the remodeling of the appendage during ontogeny is made possible. In the case of the branchiuran suction disc, the biological role of the limb does not change, but the principal function definitely does. Most of the muscles of the larval podomeres are identical (but have changed relative function) to those in the adult suction disc. Two curious exceptions from this are the disc rim and circular sucker muscles, both of which seemingly have no larval podomere origin (see 4, 5).
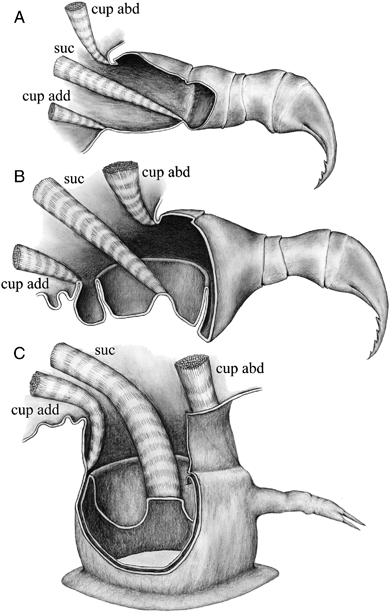
Schematic illustration of ontogeny of the first maxilla. (A) Normal appendage with hook in the early larvae. (B) Intermediate stage between normal appendages and suction disc. (C) Suction disc with distal podomere vestige. The “cup abd” indicates cup abductor muscles, corresponding to m1, 6, 7, 9, 12, and 14 in Fig. 3. The “cup add” indicates cup adductor muscles, corresponding to m5, 10, 11, 15, ad 17 in Fig. 3. The “suc” indicates sucker muscles, corresponding to m2, 3, 4, 8, 13, and 18 in Fig. 3.
In the larval stages showing an intermediate morphology (2, 4, 5) the first maxillae fulfill their biological role as attachment structures at all times. Thus there is a smooth transition from hooks to suction discs, and Shimura (1981) described what he saw as a developed suction disc in stage 5 larvae of Argulus coregoni as well as “functional” distal hooks. Van As and Van As (1996) also described a supposed co-occurring functional proximal suction disc and distal hook in the first maxilla of Chonopeltis lisikili. The examined specimens here also display both distal hooks and a developed proximal suction disc, but it remains unclear if both “systems” are in use at the same time, as the force needed to sink the hook into the fish skin for anchoring needs to have a strong and fully functional proximal appendage in order to work (2, 4).
Based on the discussions in Møller et al. (2008), the recapitulated nature of the suction disc ontogeny is a further piece of evidence suggesting that the functional continuity was maintained through the evolutionary process leading to the suction disc. Probably, the muscular arrangement itself may differ among the taxa (e.g., in Dipteropeltis or Chonopeltis). Nevertheless, functional continuity should be preserved through the development and evolution across the three taxa that have them, given the morphological origin of the suction discs.
Is the “suction disc” a novelty?
We do not aim to explore all the proposed definitions of “novelty” in the literature (for recent reviews see, e.g., Moczek 2008; Brigandt and Love 2010; Müller 2010), but a few of these are worth exploring with our example in mind. Mayr (1960) defined a novelty as “any newly arisen character, structural or otherwise, that differs more than quantitatively from the character that gave rise to it,” and regarded novelty as a new functional character (Mayr 1963, 1982). According to Mayr's definition, the branchiuran suction disc is clearly a novelty as the suction disc has a highly elaborated morphology and contains newly arisen structures.
Müller and Wagner (1991) provided an alternative definition from a morphological point of view, “a morphological novelty is a structure that is neither homologous to any structure in the ancestral species nor homonomous to any other structure of the same organism.” From this perspective, novelty is different from adaptive modification (Müller and Wagner 2003). According to this definition, the interpretation of novelty in suction discs is difficult, because we have established that the adult suction disc consists of the modified elements of the first and second podomeres.
Müller (2010) provided a new classification of novelty using three classes or types. According to this classification, the suction disc could be a composite of two different types of novelty. The transformation descried here is corresponding to a Type III novelty (major change of an existing character). But the specialized circular muscles (circular sucker muscle and disc rim muscle) could represent examples of Type II novelties (discrete new elements). In this view, the suction disc should be interpreted as the novelty.
In summary, our results provide insight into the possible origin of an “evolutionary novelty.” The highly elaborated (and novel) structure, the first maxilla “suction disc,” originates from the cross-boundary area of the first and second podomeres in the larval first maxilla. During ontogeny, the elements are retained but remodeled and internal structures are recruited to serve new functions. While the biological role of the limb stays the same, the functional morphology completely changes. However, there is seemingly never a true loss of function, as the limb muscles are in use throughout this transition during ontogeny. Based on this, we find it reasonable to assume that the functional continuity was also maintained through the evolutionary process leading to the formation of the suction disc. This process is probably best explained by a mixture of major change of an existing morphology coupled with some discrete new elements.
Acknowledgments
The authors express deep gratitude to Dr. Yutaro Suzuki (Shizuoka University) for valuable discussions. We also appreciate Dr. Kyosuke Ikuta (Osaka Kyoiku University) for providing the abundant branchiuran literature. Our deep thanks are extended to Fisheries Laboratory, Kinki University for practical help in sample collection. O. S. M. was financed by the Carlsberg Foundation (Copenhagen, Denmark) grant no. 2009_01_0006.




