Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns
Abstract
SUMMARY Root development is traditionally associated with the formation of Hertwig's epithelial root sheath (HERS), whose fragments give rise to the epithelial cell rests of Malassez (ERM). The HERS is formed by depletion of the core of stellate reticulum cells, the putative stem cells, in the cervical loop, leaving only a double layer of the basal epithelium with limited growth capacity. The continuously growing incisor of the rodent is subdivided into a crown analog half on the labial side, with a cervical loop containing a large core of stellate reticulum, and its progeny gives rise to enamel producing. The lingual side is known as the root analog and gives rise to ERM. We show that the lingual cervical loop contains a small core of stellate reticulum cells and suggest that it acts as a functional stem cell niche. Similarly we show that continuously growing roots represented by the sloth molar and K14-Eda transgenic incisor maintain a cervical loop with a small core of stellate reticulum cells around the entire circumference of the tooth and do not form a HERS, and still give rise to ERM. We propose that HERS is not a necessary structure to initiate root formation. Moreover, we conclude that crown vs. root formation, i.e. the production of enamel vs. cementum, and the differentiation of the epithelial cells into ameloblasts vs. ERM, can be regulated independently from the regulation of stem cell maintenance. This developmental flexibility may underlie the developmental and evolutionary diversity in tooth patterning.
INTRODUCTION
The teeth can be roughly subdivided into three groups. The first group consists of brachydont or low-crowned teeth where the root is relatively long compared with the crown. This is the tooth type usually described in textbooks when describing root formation (Nanci 2003). The second group consists of hypsodont, or high–crowned, teeth, where the crown is high compared with the root. The third group consists of the hypselodont teeth, ever-growing or open-rooted teeth that grow continuously during the lifetime of the animal. Open-rooted refers solely to the large apical opening present in all continuously growing teeth and does not imply that the tooth actually needs to have a root in a classical sense as described in the textbooks.
It is thought that brachydonty is the ancestral state of mammalian teeth. During evolution the shift from brachydont to hypsodont teeth is a common phenomenon (Macfadden 2000). This trend is often initiated by environmental pressures. Teeth with higher crowns last longer with abrasive diets. For instance, a significant increase in the prevalence of hypsodonty in commonly found mammals of many taxonomic groups occurred during the Neogene due to an increased aridity in the environment of Europe (Jernvall and Fortelius 2002). Hypselodonty can be seen as an extreme form of hypsodonty. The crown never stops growing and root formation is postponed indefinitely, but often with small tracts of dentin covered with cementum acting as regions attaching the tooth to the jaw bone with a periodontal ligament.
Within closely related species there can be a variation between brachydont, hypsodont, and hypselodont teeth, indicating that the regulation of crown height is rather flexible. For instance in closely related rodent species, the Mouse (Mus musculus) molar is brachydont, the molars of the Bank vole and the Southern Red-backed vole (Clethrionomys glareolus, Clethrionomys gapperi) are hypsodont, and the molars of the Sibling vole and Meadow vole (Microtus rossiaemeridionalis and Microtus clethrionomys) are hypselodont (Phillips and Oxberry 1972; Tummers and Thesleff 2003). It has been proposed that the switch from hypsodont to hypselodont between the microtine genera of Clethrionomys and Microtus is caused by the maintenance of a regenerative unit, possibly due to a simple mutation (Phillips and Oxberry 1972), or that increased crown height results from delayed termination/cytodifferentiation and that hypselodonty is an extreme outcome of such a delay (von Koenigswald 1982). We have more recently proposed that the increase in crown height is a result of prolonging the period during which the epithelial stem cell niche is maintained (Tummers and Thesleff 2003).
During classic root formation the dental epithelium of the cervical loop undergoes some major structural changes (Fig. 1). The cervical loop is created during crown morphogenesis and with root initiation loses the central core of stellate reticulum and stratum intermedium cells, including the putative epithelial stem cells (Ten Cate 1961; Starkey 1963; Harada et al. 1999; Harada et al. 2002). A double layer of basal epithelium is left that is known as Hertwig's epithelial root sheath (HERS) (Thomas 1995). As HERS proliferates, the growing epithelial sheet becomes discontinuous and forms a fenestrated network lining the root surface known as the epithelial cell rests of Malassez (ERM) (Ten Cate 1996). Through this network the follicular mesenchyme cells can migrate to the dentin surface and differentiate into cementoblasts depositing the cementum. The ERM functions in the induction of cementoblast differentiation and regulation of their function (Thomas 1995; Bosshardt and Schroeder 1996; Kagayama et al. 1998). Fibers of the periodontal ligament are embedded in the cementum and connect the root to the jaw bone. HERS forms in brachydont and hypsodont teeth when root formation is initiated and crown formation ends, and its transition to ERM is generally regarded as a typical feature of root formation. Interestingly, the continuously growing rodent incisor is subdivided into two halves. The labial side is called crown analog because it produces ameloblasts and enamel, whereas the lingual half is called root analog, because its epithelium fragments and forms ERM and cementum is produced. Both root and crown analogs are generated continuously by the apical end of the incisor. It has been suggested that the labial cervical loop of the crown analog in the incisor is a specialized stem cell niche providing the epithelial progeny for the entire incisor and that lingually HERS is formed (Ohshima et al. 2005).
Formation of the cervical loop, the putative epithelial stem cell niche, during early development and its fate in the mouse molar and incisor. During early stages of morphogenesis all teeth go through the same developmental stages (initiation, bud stage, cap stage, and the bell stage) generating the crown. During bud stage a core of loosely arranged epithelium is formed in the center of the bud. During the cap stage the cervical loop is formed, a protrusion from the bud that envelopes the condensed dental papilla mesenchyme. The cervical loop is extended during the bell stage and the inner enamel epithelium starts to differentiate into ameloblasts. During the late bell stage crown morphology is established and cells producing mineralized tissues differentiate terminally. Cell differentiation starts from the cusp tips and extends toward the base. Enamel is deposited by ameloblasts and dentin by odontoblasts. In the mouse molar the cervical loop loses its core of stellate reticulum, the putative stem cells, and forms the HERS, which fragments into ERM, typical of a root. On the labial side of the mouse incisor the cervical loop is maintained as a stem cell niche and it keeps giving rise to ameloblasts. On the lingual side no ameloblasts differentiate and instead ERM is formed. The fate of the lingual cervical loop is unclear, although it has been suggested that HERS is present (Ohshima et al. 2005). ERM, epithelial cell rests of Malassez; HERS, Hertwig's epithelial root sheath; iee, inner enamel epithelium; oee, outer enamel epithelium; sr, stellate reticulum.
This last notion is questioned by the existence of a special type of continuously growing or hypselodont teeth as is represented by the sloth molar. The dentition of the contemporary sloth species is heavily modified, lacking both incisors and canines. Sloth teeth are open-rooted, grow continuously, and at the same time lack enamel (Naples, 1982). In juvenile specimens the tooth erupts as a simple cone. In adult specimens the cap of the dentin is worn off, leaving a hard shell of dentin with a soft pulp in the center. The edges of the dentin get sharper with age due to wear (Naples 1982). Similarly, the dentition of the mouse, as a representative of the rodents, is also heavily modified during evolution, with only two incisors in each jaw, followed by a diastema region lacking teeth, and three molars.
The transgenic K14-Eda mouse has ectodysplasin (Eda) expressed under the keratin 14 promoter leading to an excessive production of Eda throughout the ectoderm from E10 onwards, including the oral and dental epithelium (Mustonen et al. 2003). The constitutive expression of Eda in the dental epithelium leads to the formation of supernumerary molars and the loss of enamel on crown analog of the incisors. This transgenic mouse line therefore has possibly transformed its incisor into a continuously growing root, and serves in this paper as a model system for continuously growing roots. If HERS is required for the production of root epithelium, these teeth would not have a cervical loop and a stem cell niche.
Here we investigate the structure of the cervical loop area of continuously growing roots of a sloth molar that has erupted into the oral cavity and shows the typical cone-shaped morphology of a juvenile stage, and in the incisors of the wild-type and the K14-Eda transgenic mouse to investigate if root formation is truly linked to HERS formation characterized by the loss of the stellate reticulum containing the putative stem cells. Furthermore, we analyzed molecular markers of the incisor stem cell niche and differentiation in the K14-Eda incisor in order to check the state of the stem cell niche and the fate of the epithelial progeny. We show that stem cells exist in continuously growing roots in the sloth molar, in the K14-Eda incisor, and in the root analog side of the wild-type incisor. This indicates that the crown vs. root formation, i.e., the production of enamel vs. cementum, and the differentiation of the epithelial cells into ameloblasts vs. ERM, can be regulated independently from the maintenance of the stem cells, and that the maintenance of stem cells does not indicate an implicit ameloblast fate for the progeny.
MATERIAL AND METHODS
The sloth histological sections are of a Bradypus tridactylus specimen. The teeth have started to erupt and resemble the juvenile stage (Naples 1982). The sections were collected and processed by the Dutch researcher Van den Broek in 1913 and are part of the historical collection of the Hubrecht Laboratory in Utrecht. The K14-Eda mouse is a transgenic mouse that overexpresses the signal molecule Ectodysplasin-A1 under the Keratin14 promoter and has been previously described (Mustonen et al. 2003). Wild-type tissue was used from 1, 4, and 12 days, and 4-week post-natal NMRI mice. K14-Eda tissue was from 4-week-old specimens.
Radioactive in situ hybridization with 35 S labeled RNA probes was performed on serial paraffin sections as described previously (Tummers and Thesleff 2003). Immunohistochemistry was performed on 7-μm-thick paraffin sections. After deparaffination the sections were microwaved for 10 min in 10 mm natrium citrate buffer, pH 6.0, and then treated for 20 min in Proteinase K 7 μg/ml in phosphate-buffered saline (PBS). After washes in PBS the sections were incubated for 1 h in 3% BSA in PBS and then with polyclonal rabbit anti-human keratin (Dako, Glostrup, Denmark, A0575) 1:250 overnight at 4°C. The Vectastain ABC kit was used for detection and the sections were stained with DAB (Vector Laboratories, Burlingame, CA).
For histology the tissues were sectioned at 4 and 7 μm thickness, deparaffinized and stained with hematoxylin–eosin. The histological structures were identified based on definitions and examples in Ten Cate's Oral Histology (Nanci 2003).
RESULTS
The sloth molar
Figure 2A shows the general histology of a frontal section of the sloth molar (Bradypus tridactylus) from an unspecified stage, showing a conical-shaped molar of which the tip has erupted into the oral cavity, similar to the juvenile stage (Naples 1982). This molar is characterized by a prominent thick cap of dentin at the tip. This cap was not covered by enamel typical of the crown of brachydont and hypsodont teeth. Also the side surface of the tooth seemed to lack enamel and we confirmed this with a close-up of a representative area (Fig. 2B). The sloth molar lacked enamel-producing ameloblasts and the surface of this molar was entirely covered with dentin and cementum, with occasional cementoblasts visible within the cementum, all typical features of a root surface (Fig. 2C). The sloth molar therefore lacked any enamel from the tip to the base of the tooth and instead had acquired a root surface.
Histological structure of continuously growing sloth molar. (A) The frontal section shows the general structure of the sloth molar with open root and a massive core of dental mesenchyme topped off with a thick cap of dentin. B and C are higher magnifications of the boxes in A. (B) A continuous layer of polarized odontoblasts is evident as well as thick layers of dentin and cementum. Neither ameloblasts nor enamel is observed. The arrow shows a cementoblast inside the cementum. (C) At the apex of the root a cervical loop, i.e. the putative epithelial stem cell niche, is present. Some stellate reticulum cells are visible in the core. This cervical loop is magnified in D and a schematic representation shows the structure of the cervical loop and the basal lamina that separates the epithelium from the mesenchyme. Scale bars are 1 mm in A and 200 μm in B and C.
The general overview (Fig. 2A) showed a thin epithelial structure at the base of the tooth, where normally the HERS is found in brachydont roots. However, a close-up of this area showed that the typical structure of the HERS, consisting only of inner and outer enamel epithelium, was not found in the sloth. Instead we found that the cervical loop was maintained and it contained a core of cells surrounded by inner and outer enamel epithelium (Fig. 2C).
Histological structure of the cervical loop of the wild-type incisor
The mouse incisor is subdivided into two domains, the labial crown analog and the lingual root analog. The crown analog is characterized by an enamel surface whereas the lingual side has a cementum surface. An overview of the apical end of the incisor was dominated by the presence of the prominent labial cervical loop and a reduced epithelial structure on the lingual side. It has recently been suggested that the lingual aspect of the incisor consists of HERS instead of a cervical loop (Ohshima et al. 2005) and therefore we closely examined the structural organization of the epithelium on the lingual side (Fig. 3B). We observed that there are indeed two epithelial cell layers, apparently representing the inner and outer enamel epithelium, but also that a small core of stellate reticulum is retained between these layers. We checked this at older stages as well, and this phenotype did not change from 1 day post-natal to 4 weeks post-natal. In HERS this core is lost; hence the lingual side of the mouse incisor has maintained the cervical loop structure although diminished in size. The labial cervical loop is much enlarged as described previously due to a large amount of stellate reticulum in the core of the cervical loop and here the inner enamel epithelium proliferates actively and subsequently differentiates into ameloblasts (Fig. 3C).
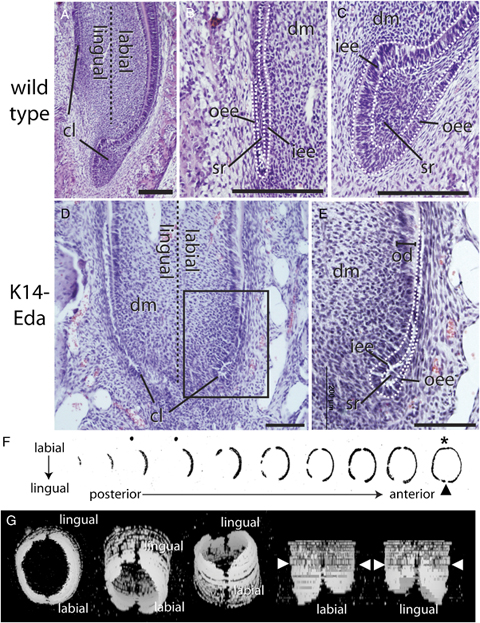
Fate of the epithelial stem cell niche in the wild-type mouse incisor and K14-Eda transgenic mouse incisor. (A) The apical end of a 1 day post-natal wild-type incisor with the large labial cervical loop and the smaller lingual cervical loop. (B) Magnification of the lingual cervical loop showing a small core of stellate reticulum cells surrounded by inner and outer enamel epithelium. (C) The specialized enlarged structure of the labial cervical loop with a large core of stellate reticulum cells surrounded by outer and inner enamel epithelium. The inner enamel epithelium is starting to differentiate into preameloblasts. (D) The apical end of the K14-Eda incisor. (E) Higher magnification of the labial cervical loop shows a significantly reduced core of stellate reticulum and lack of pre-ameloblasts. (F) A pan-keratin antibody immunohistochemistry of the frontal sections of the K14-Eda incisor shows that from posterior to anterior the lateral cervical loops appeared first, with the labial cervical loop (asterix) closing more anteriorly, and the lingual loop did not close in a small area (arrowhead). (G) Three-dimensional reconstructions of the images in F confirmed lateral cervical loops protruding. The arrowheads show the start of epithelial fragmentation. Scale bars are 200 μm in A–C and 100 μm in D and E.
Histological structure of the cervical loop in the K14-Eda incisor
Previously we have shown that the K14-Eda incisor lacks enamel on its labial aspect (Mustonen et al. 2003). We therefore investigated here the fate of the cervical loop area of the K14-Eda incisor to determine whether HERS was formed or the cervical loop was maintained. Eda is highly expressed throughout the dental epithelium in the K14-Eda incisor at 4 days and 5 weeks post-natal (data not shown). We observed that the lingual and labial aspects of this incisor looked strikingly similar (Fig. 3D) and resembled the lingual aspect of the wild incisor. A few cells of stellate reticulum were present in the cervical loop between the inner and outer enamel epithelium (Fig. 3E). Moreover, we also observed that the progeny of this cervical loop did not differentiate into elongated ameloblasts on the labial side but instead the epithelium fragmented and generated ERM typical of root surface.
Three-dimensional (3D) reconstruction of the apical end of the K14-Eda incisor
To determine whether the stem cell niche is localized to a certain region or is a continuous structure in the K14-Eda incisor, we analyzed the spatial location of the cervical loop area at the tooth base. 3D reconstructions of serial sections revealed that the cervical loop was not limited to the most labial or lingual aspects in the wild-type incisor but runs around the entire base of the tooth. In the following we refer to the cervical loop area situated between the lingual and labial aspect as the lateral cervical loop. In the wild-type incisor the labial cervical loop appeared first in frontal sections that go from posterior to anterior because the lingual loop is located more toward the tip (Fig. 3A). Frontal sections of the K14-Eda incisor where the epithelium was labeled with a pan-keratin antibody showed a different picture with the lateral cervical loops appearing first on the most posterior sections (Fig. 3F). The most labial aspect remained open for a very long time but eventually closed (Fig. 3F– asterix). The lingual aspect of the cervical loop however never fully closed in the K14-Eda incisor. We confirmed our findings by making a 3D reconstruction of the apical end of the incisor (Fig. 3G). We confirmed that the lateral cervical loops protruded posteriorly and that the labial cervical loop closes more anteriorly compared with the sections containing the lateral cervical loops. Also the transition was clearly visible from cervical loop epithelium to fragmented epithelium, i.e., ERM in this reconstruction. Interestingly, on the lingual side, a small area of a few cells width did not see closure of the cervical loop. Instead this area remained free from epithelium and immediately undergoes the transition into ERM.
Molecular markers of the stem cell niche
In the wild-type incisor, notch1 has been shown to be specifically expressed in the stellate reticulum and stratum intermedium cells of the labial cervical loop (Harada et al. 1999) (Fig. 4A, and B). We showed that Notch1 was also expressed in the central cells of the lingual cervical loop of the wild-type incisor (Fig. 4C) as well as in the lateral cervical loop area (data not shown). Also in the K14-Eda incisor notch1 was expressed in the central epithelial cells of the cervical loop (Fig. 4D, and E) resembling the lingual wild-type pattern (Fig. 4C). In the K14-Eda incisor Fgf10 was expressed in the mesenchyme directly surrounding the cervical loop similar to the wild type (Fig. 4F). Fgf3 expression is restricted in the wild-type incisor to the labial mesenchyme and this pattern was similar in the K14-Eda incisor (Fig. 4G). Lunatic fringe is a marker for the transit-amplifying epithelial cells of the inner enamel epithelium (Harada et al. 1999) and it was expressed in the K14-Eda incisor similar to the wild type in the inner enamel epithelium of the labial cervical loop (Fig. 4H).
Molecular regulation of the epithelial stem cell niche. (A) Notch1 expression in the wild-type incisor (4 dpn) is confined to the stellate reticulum and stratum intermedium of the labial as well as the lingual aspects. (B) Magnification of the labial cervical loop with a large core of stellate reticulum. (C) Magnification of the lingual cervical loop and although much smaller than the labial loop in B, stellate reticulum cells are present as indicated by notch1 expression at the core. (D) Notch1 expression in the K14-Eda incisor. (E) The K14-Eda labial cervical loop is much smaller than in the wild type resembling the wild-type lingual cervical loop B. (F–H) Other markers of the stem cell niche are normal in the K14-Eda incisor. (F) Fgf10 is expressed in the supporting mesenchyme. (H) Lfng is expressed in the inner enamel epithelium. (G) Fgf3 is only expressed on the labial side of the K14-Eda incisor similar to the wild type. In the sections of the K14-Eda incisor the lingual cervical loop is absent due to sectioning exactly through the cervical loop free zone as described in Fig. 3F and H; however, Notch1, Lfng and Fgf10 are present in neighboring and lateral sections.
Differentiation in wild-type and K14-Eda incisor
We compared cell differentiation in the K14-Eda and wild-type incisor by means of histology and markers for different cell types. The labial aspect of the K14-Eda incisor showed an identical picture to the lingual side of the wild-type incisor with a layer of odontoblasts, dentin, cementum, and periodontal ligament typical of a root. The transformation of the crown analog into a root analog in the K14-Eda incisor was confirmed by frontal sections labeled with a pan-keratin antibody. The distinctive cap of tall ameloblasts was obvious in the wild-type incisor on the labial aspect, and fragmented ERM covered the lingual side (Fig. 5D), whereas ERM surrounded the entire circumference of the K14-Eda incisor (Fig. 5E). The absence of ameloblasts was confirmed by the differentiation marker jagged1, which is normally expressed in differentiating ameloblasts (Harada et al. 1999), and it was absent in the epithelium of the K14-Eda incisor (Fig. 5F). Bsp1 is a marker for cementoblasts and odontoblast differentiation (Yamashiro et al. 2003), and in the K14-Eda incisor cementoblasts were present on both the lingual and labial aspects of the incisor (Fig. 5G) while in the wild type they were restricted to the lingual side.
Cell differentiation in the wild-type and K14-Eda incisor. (A) The labial aspect or crown analog of the wild-type incisor with the typical layer of elongated epithelial ameloblasts producing enamel and the mesenchymal odontoblasts generating dentin. (B) The root analog side has no ameloblasts or enamel. Dentin produced by odontoblasts is covered with cementum and the periodontal ligament attaches the cementum surface to the bone. (C) The labial aspect of the K14-Eda incisor is similar to the lingual root analog of the wild type in B. (D) Immunohistochemical staining with a pan-keratin antibody in the frontal sections of the wild-type incisor shows ameloblasts on the labial side and fragmented ERM epithelium on the lingual side. (E) Similar frontal section of the K14-Eda incisor shows fragmented ERM epithelium surrounding the entire tooth. (F,G) Sagittal sections of the K14-Eda incisors. (F) Jagged1 expression is missing in the epithelial compartment indicating the lack of preameloblasts, although jagged1 is still expressed normally in differentiating odontoblasts (arrowheads). (G) Bsp1 is expressed in odontoblasts (arrowheads) and cementoblasts (arrows) in a similar pattern on both lingual and labial side indicating that the labial side has adopted the lingual root phenotype. Scale bars are 200 μm.
DISCUSSION AND CONCLUSIONS
The rodent incisor is functionally and morphologically subdivided into the labial crown analog and the lingual root analogue. Each side shows a typical differentiation pattern where the crown analog is covered by enamel deposited by ameloblasts and the root analog is covered by cementum deposited by cementoblasts. It has been suggested that the large cervical loop at the labial aspect of the incisor represents the sole epithelial stem cell niche supplying epithelial stem cells for the growth of all aspects, and that HERS typical of roots in molars, forms lingually and is responsible for root formation there (Ohshima et al. 2005). However, we found no presence of HERS at the lingual side; instead there were stellate reticulum cells present in the core of the lingual cervical loop, as was confirmed by the notch1 expression in these cells. Moreover, the cervical loop was shown to be a continuous structure around the base of the incisor.
Similarly, no HERS typical of brachydont teeth (Ten Cate 1996) was found in the continuously growing sloth molar or the K14-Eda incisor. Both these teeth were covered by a typical root surface consisting of dentin covered by cementum, and they had cervical loops in their apical ends that had maintained stellate reticulum cells in the center. Moreover, the cervical loop was present in all sections of the apical aspect, indicating that it is present in the entire circumference of the base of the tooth. A similar situation is present in the continuously growing molar of the sibling vole where the cervical loop is not restricted to a specific local area (Tummers and Thesleff 2003). The continuously growing molar of the guinea pig shows a morphology comparable to that of the vole (Hunt 1958).
The 3D reconstruction of the apical end of the K14-Eda incisor allowed the observation of the cervical loop structures and this indicated that the labial cervical loop had a similar sized core of stellate reticulum as the cervical loop at other aspects of the tooth (Fig 3F). In addition, the lateral cervical loops were seen to protrude slightly from the apical end and the labial cervical loop was folded inwards. At the most lingual aspect the lateral loops did not meet and close. The total lack of epithelium here may indicate that the tooth is subdivided into individual sections representing a lineage from stem cell to differentiated cell similar to that of the crypt of the gut (Crosnier et al. 2006). Lack of the cervical loop in a specific area of the incisor therefore means local depletion of stem cells and eventually all epithelial structures deriving from those stem cells, because no replenishment takes place from neighboring areas. The husbandry of the K14-Eda transgenic mice shows that the reduced cervical loop of the K14-Eda incisor is indeed a functional stem cell niche because the incisors need to be clipped regularly to prevent misalignment due to constant regeneration of this tooth. In conclusion, our data clearly showed that the HERS is not an obligatory structure for root formation, that no specialized stem cell niche existed in the sloth molar or mouse incisor that is restricted to a local area, and that a cervical loop with a reduced core of stellate reticulum cells can still act as a stem cell niche.
It is known that Fgf10 is important for the maintenance of the stem cell niche in the incisor (Harada et al. 1999; Harada et al. 2002), and we have suggested that Fgf10 signaling is maintained in all continuously growing teeth to maintain the epithelial stem cell niche based to the similarities in the continuously growing molar of the sibling vole and the rodent incisor (Tummers and Thesleff 2003). It has also been proposed that the disappearance of Fgf10 signaling leads to the transition from crown to root formation due to a loss of the dental epithelial stem cell compartment (Yokohama-Tamaki et al. 2006). Based on our observations we would however like to suggest that although lack of Fgf10 can lead to a reduction of the stem cell niche and switch to root fate as can be seen in the mouse molar (Tummers and Thesleff 2003), differentiation into root can also take place in the presence of a functional epithelial stem cell niche. In the K14-Eda incisor, Fgf10 and Fgf3 expression was continued and although the amount of stellate reticulum, containing the putative stem cells, was reduced, it was not lost. At the same time the stem cell niches in the K14-Eda incisor and sloth molar give rise to root epithelium, suggesting that maintenance of the stem cells has no default effect on the differentiation of the progeny, which apparently can differentiate into either ameloblasts or ERM. It does not seem that the size of the niche determines the fate of the progeny, because the enlarged cervical loops in the K14-noggin transgenic mouse form no enamel (Plikus et al. 2005). We propose that in the wild-type incisor the labial cervical loop is enlarged due to the functional requirement to produce a large amount of ameloblast progeny, whereas the lingual cervical loop merely provided progeny for fragmented epithelium of the ERM.
We do not suggest that modification of Eda signaling is the mechanism used in the sloth tooth to acquire the continuously growing root phenotype and lack of enamel. The acquisition of this phenotype may occur at different regulatory levels. Recent studies on the regulation of the asymmetric development of the mouse incisor have revealed a central role for follistatin, an inhibitor of TGFβ signaling. The expression of follistatin in the lingual epithelium prevents enamel formation by inhibiting the inductive effect of BMPs on ameloblast differentiation (Wang et al. 2004). Interestingly, follistatin also inhibits the proliferation of the epithelial cells in the cervical loop, but this effect is due to inhibition of the positive effect of activin on stem cell proliferation (Wang et al. 2007). Recombinant Eda protein induces the expression of follistatin as well as another BMP inhibitor CCN2 and prevents BMP-induced ameloblastin expression in vitro, showing that the lack of enamel in the K14-Eda mice may result from inhibited BMP signaling (Pummila et al. 2007). These studies indicate that the maintenance of stem cells and their differentiation are regulated by different molecular mechanisms supporting the findings we have presented here. Taken together the different models show that there are many possible ways to create a sloth tooth phenotype.
In conclusion, there appears to be regulatory flexibility in the decision between crown and root fate that is independent of the depletion of the stem cells in the niche. The differentiation compartment and stem cell compartment of the niche can be regulated independently, giving rise to multiple patterns (Fig. 6): the brachydont pattern with low crown and high roots, the hypsodont pattern with high crown and low roots, the crown hypselodont pattern with a continuously growing crown domain and root domain, and the exclusively hypselodont root pattern. In the brachydont tooth, the disappearance of the stem cells coincides with the switch to root fate of the epithelial progeny during late development. Hypsodonty can be seen as a simple extension of the brachydont pattern where the stem cells are maintained longer during crown development, and root formation is postponed leading to a higher crown. In sharp contrast, the fate of root and crown domains in continuously growing teeth is probably already determined during early development, and is independent of the maintenance of the stem cells. We propose that the diversity of tooth patterns is possible because the differentiation of the progeny of the epithelial stem cells in the cervical loop is not restricted to one specific fate, the ameloblast cell lineage, but also root epithelium can form.

Diversity in tooth patterning is due to independent regulation of the epithelial stem cell cells and differentiation of its progeny in the stem cell niche. In brachydont teeth HERS is formed after completion of crown morphogenesis and growth of the root is limited to a typical length. During crown morphogenesis the stem cell niche is formed and a signaling event leads to the formation of HERS simultaneously with the disappearance of the stem cells. In hypsodont teeth root formation is delayed and crown formation is extended, which is accompanied by the maintenance of the stem cell niche. Root initiation is accompanied by the loss of stem cells, which results in the formation of HERS. In continuously growing hypselodont teeth such as the sloth molar and the rodent incisor, stem cells are present in the stellate reticulum of the cervical loop and the stem cells can give rise to either ameloblasts or ERM. Hence, the fate of the epithelial progeny is independent of the maintenance of the stem cells. The flexibility of regulation allows the diversity in tooth types and classic spatial association of root with jaw and crown with oral cavity becomes pointless. ERM, epithelial cell rests of Malassez (fragmented root epithelium); scn, stem cell niche; HERS, Hertwig's epithelial root sheath.
Acknowledgments
We thank Raija Savolainen, Merja Mäkinen, and Riikka Santalahti for their excellent technical assistance, and we thank the Hubrecht Laboratory for supplying the sloth sections. This work was supported by the Academy of Finland and the Sigfrid Juselius Foundation.




