Extended-Release Trospium Chloride Improves Quality of Life in Overactive Bladder
ABSTRACT
Objectives: Overactive bladder syndrome (OAB) is a urinary condition that often exerts detrimental effects on an individual's quality of life (QoL). A once-daily, extended-release (ER) formulation of the quaternary amine trospium chloride has recently been developed for the treatment of OAB. The pooled health-related QoL (HRQoL) data from two multicenter, parallel-group, double-blind Phase III studies with trospium chloride ER 60 mg were analyzed.
Methods: Subjects aged ≥18 years with urinary urgency, frequency, and an average of ≥1 urge urinary incontinence episode per day on a 3-day bladder diary were randomized (1:1) to receive once-daily trospium 60 mg ER or placebo for 12 weeks. HRQoL was assessed at baseline and at Week 12 using the King's Health Questionnaire (KHQ) and the OAB questionnaire (OAB-q).
Results: Overall, 1165 subjects were randomized (trospium ER, n = 578; placebo, n = 587). Trospium ER produced significantly greater improvements from baseline than placebo in seven of the nine KHQ domains. At Week 12, the improvement in mean OAB-q HRQoL total score (from approximately 52 at baseline) was significantly greater with trospium ER than with placebo (+25.8 vs. +20.7; P = 0.0003). Improvements from baseline were seen with trospium ER on all eight of the OAB-q symptom bother scales.
Conclusions: Once-daily trospium 60 mg ER improved the QoL of subjects with OAB, as assessed using the KHQ and the OAB-q, in two large Phase III clinical trials.
Introduction
Overactive bladder syndrome (OAB) is a symptom complex composed of urinary urgency, with or without urge urinary incontinence (UUI), usually with increased daytime urinary frequency and nocturia [1]. In the United States alone, 34 million adults are affected, with the prevalence increasing with patient age [2]. The symptoms of OAB may compromise a patient's quality of life (QoL), affecting social, physical, psychological, occupational, and sexual aspects [3–5]. The effect on QoL can result in individuals developing complex coping strategies, including “toilet mapping,” limiting travel, and avoiding social and sexual activities [6,7]. Urinary incontinence has also been associated with loneliness [8], social isolation [9], and nursing home placement of elderly patients [10–12].
Malone-Lee and colleagues have suggested that diagnosis of OAB can be based on symptoms, allowing the majority of patients to be treated within the primary care setting without the need for specialist referral [13–16]. Naturalistic empirical treatment of OAB by primary care physicians can improve OAB symptoms as assessed using patient-reported outcomes (PROs) and bladder diaries [17].
Antimuscarinic agent therapy results in improvements in the core symptoms of OAB: urgency, frequency, UUI, and in some cases, nocturia [18]. The inclusion of PROs in some trials suggests that clinical benefits are paralleled by improvements in QoL [19,20]. Interestingly, in some instances, although objective measures (frequency and bladder capacity) showed improvements, subjects did not perceive a significant benefit in health-related QoL (HRQoL) [19]. Despite the benefits provided by the currently available antimuscarinic agents, in a recent survey of women with OAB, subjects expressed dissatisfaction with OAB therapies, and the desire for more effective, convenient therapies with fewer adverse events [3]. In another survey of patients with urinary incontinence, among those who reported discontinuing drug therapy, the major reasons were poor efficacy, side effects, and cost [21].
Trospium chloride is a quaternary amine antimuscarinic agent that has been available in Europe for more than two decades for the management of OAB. Results from two Phase III clinical trials with the extended-release (ER) formulation indicate that the efficacy of once-daily trospium ER 60 mg is similar to that seen previously with trospium immediate-release (IR) 20 mg twice daily, while typical anticholinergic adverse events occurred at lower rates (dry mouth rates with trospium ER 60 mg were 8.7%, 12.9%, and 10.7%, while constipation rates were 9.4%, 7.5%, and 8.5% in these two independent studies, and in pooled data from the two studies, as described in the prescribing information, respectively [22–24]). The incidence of dry mouth associated with trospium ER compares favorably with the other oral antimuscarinic drugs used to treat OAB [24,25].
The two Phase III trials of trospium ER 60 mg also included PROs to determine whether these improvements seen with trospium ER are accompanied by improvements in the QoL of subjects. This article presents integrated PRO data from the two Phase III clinical trials of trospium ER 60 mg.
Subjects and Methods
Pooled data were analyzed from two 12-week, multicenter, parallel-group, double-blind, placebo-controlled trials conducted at 117 centers in the United States, the methods of which have been published previously [22,23].
Men and women aged ≥18 years with symptoms of OAB for ≥6 months, symptoms of urgency (i.e., at least one “severe” urgency severity rating per 3 days, as measured using the validated Indevus Urgency Severity Scale [26]), minimum urinary frequency of ≥30 toilet voids per 3 days, an average of ≥1 UUI episode per day, average total volume voided ≤3000 ml per day, and ≤250 ml per void were eligible for inclusion. Exclusion criteria included: predominant stress, insensate, or overflow incontinence; neurogenic bladder disorder; significant renal disease; urinary tract infection; and significant bladder outlet obstruction. After a 7-day washout period for non-naïve participants, subjects were randomized (1:1) to receive either trospium ER 60 mg or matching placebo once daily for 12 weeks. The randomization schedule was stratified by the average baseline daily urinary frequency (collected in bladder diaries) to provide balance between the treatment groups with respect to this variable. OAB symptom data were collected using 3-day written bladder diaries at Weeks 1, 4, and 12. The studies were performed according to the principles of the Declaration of Helsinki and its amendments and the principles of Good Clinical Practice, with Institutional Review Board approval. All participants provided written informed consent before taking part in the studies.
The primary end points were the calculated changes in diary-recorded daily urinary frequency and daily UUI episodes. Secondary diary-based end points included severity of urgency associated with toilet voids, volume voided per toilet void, and urge frequency per day. Safety was assessed by monitoring adverse events, clinical laboratory values, physical examination, vital signs, and resting electrocardiograms.
QoL data were obtained at baseline and Week 12 using the King's Health Questionnaire (KHQ) and the OAB Questionnaire (OAB-q). The KHQ is a validated self-administered, OAB symptom-specific QoL questionnaire [27]. The instrument contains 21 questions that are scored in nine domains (General Health Perception, Incontinence Impact, Role Limitations, Physical Limitations, Social Limitations, Personal Relationships, Emotions, Sleep/Energy, and Severity of Urinary Symptoms). Each domain is scored from 0 to 100, with higher scores indicating greater impairment. The threshold of a change from baseline of ≥5 points on KHQ domains has been suggested to indicate change that is clinically meaningful to patients [28]. The OAB-q is a validated 33-item, self-administered symptom bother and HRQoL questionnaire [29]. This tool is designed to assess the effect of OAB symptoms (frequency and urgency) in both continent and incontinent male and female subjects with OAB. The HRQoL scale consists of 25 items forming four subscales (coping, concern/worry, social interaction, sleep). Subscale and total scores were transformed on to a 0 to 100 scale, with higher scores indicating better HRQoL. An additional eight items form the Symptom Bother scale. Higher scores on the Symptom Bother scale indicate increasing symptom bother. A threshold of 10 points has been suggested to represent a minimally important difference on the OAB-q [30]. Both the KHQ and OAB-q were scored according to the questionnaire instructions by research personnel blinded to the patient's treatment allocation.
Efficacy assessments were performed on the intent-to-treat subject sample, which included all subjects who were enrolled (i.e., randomized and dispensed study medication) and had at least one post–baseline evaluation. Efficacy analyses were performed using the last observation carried forward (LOCF) data set (to account for missing values), which consisted of data recorded or carried forward at each visit.
Statistical Analysis
In each individual study, pooled investigator centers that included at least 40 patients were created from the original investigational centers. This pooling was performed before the study blind being broken in either study. These same pooled centers were used in the integrated analysis, and thus were used in analyses that required a “center” effect in the models. Change from baseline in QoL scores (for each domain and overall) were analyzed using rank analysis of variance models, including effects for treatment, pooled center, and their interaction; when the interaction terms were not significant (P > 0.10), the interaction terms were dropped from the efficacy analysis models. P-values ≤0.05 were considered statistically significant.
Results
Overall, 1165 subjects were randomized to receive either trospium ER 60 mg (n = 578) or placebo (n = 587). Baseline characteristics (Table 1) and QoL domain scores (Table 2) were comparable for the two treatment groups. The study was completed by 521 placebo recipients (88.8%) and 506 trospium ER recipients (87.5%; Fig. 1). The primary reasons for premature discontinuation were adverse events (placebo, 19 subjects [3.2%]; trospium ER, 30 subjects [5.2%]) and withdrawal of consent (placebo, 18 [3.1%]; trospium ER, 16 [2.8%]). The treatment groups were generally similar with regard to the premature discontinuation rates and reasons.
| Characteristic | Placebo (n = 587) | Trospium ER (n = 578) |
|---|---|---|
| Gender, n (%) | ||
| Female | 505 (86.0) | 484 (83.7) |
| Male | 82 (14.0) | 94 (16.3) |
| Mean age ± SE, years | 58.9 ± 0.50 | 60.4 ± 0.54 |
| Race, n (%) | ||
| White | 493 (84.0) | 503 (87.0) |
| Black | 58 (9.9) | 44 (7.6) |
| Hispanic | 21 (3.6) | 21 (3.6) |
| Asian | 7 (1.2) | 5 (0.9) |
| Other | 8 (1.4) | 5 (0.9) |
- ER, extended release; SE, standard error.
| Domain | Placebo | Trospium ER | P-value |
|---|---|---|---|
| King's Health Questionnaire | |||
| General health perceptions | n = 426 | n = 406 | 0.9084 |
| 32.04 (0.58) | 31.77 (0.60) | ||
| Incontinence impact | n = 556 | n = 530 | 0.5115 |
| 81.12 (0.92) | 80.06 (1.00) | ||
| Role limitations | n = 539 | n = 507 | 0.6286 |
| 63.02 (1.06) | 62.23 (1.10) | ||
| Physical limitations | n = 528 | n = 503 | 0.8227 |
| 60.23 (1.11) | 59.97 (1.14) | ||
| Social limitations | n = 521 | n = 500 | 0.7346 |
| 42.16 (1.13) | 42.13 (1.21) | ||
| Personal relationships | n = 387 | n = 363 | 0.4250 |
| 55.38 (1.53) | 57.07 (1.60) | ||
| Emotions | n = 509 | n = 488 | 0.7585 |
| 46.85 (1.19) | 47.36 (1.24) | ||
| Sleep/energy | n = 540 | n = 519 | 0.6524 |
| 57.50 (1.11) | 56.71 (1.12) | ||
| Severity | n = 559 | n = 535 | 0.8707 |
| 34.07 (0.28) | 34.09 (0.31) | ||
| Overactive bladder questionnaire HRQoL scale | |||
| Total score | n = 559 | n = 535 | 0.6231 |
| 51.79 (0.88) | 52.11 (0.92) | ||
| Concern/worry | n = 547 | n = 520 | 0.8528 |
| 45.85 (0.99) | 45.52 (1.07) | ||
| Coping | n = 552 | n = 526 | 0.1428 |
| 47.23 (1.02) | 49.23 (1.05) | ||
| Social interaction | n = 559 | n = 532 | 0.6234 |
| 74.35 (0.99) | 74.69 (1.04) | ||
| Sleep | n = 550 | n = 528 | 0.8868 |
| 47.93 (1.08) | 47.89 (1.11) | ||
| Overactive bladder questionnaire symptom bother scale | |||
| Total symptom bother | n = 559 | n = 536 | 0.8463 |
| 65.18 (0.71) | 65.26 (0.78) | ||
| Frequent urination during the daytime hours | n = 559 | n = 536 | 0.2658 |
| 4.57 (0.04) | 4.62 (0.04) | ||
| An uncomfortable urge to urinate | n = 559 | n = 536 | 0.6725 |
| 4.32 (0.04) | 4.32 (0.05) | ||
| A sudden urge to urinate with little or no warning | n = 559 | n = 535 | 0.5986 |
| 4.30 (0.05) | 4.31 (0.06) | ||
| Accidental loss of small amounts of urine | n = 559 | n = 536 | 0.5388 |
| 4.09 (0.05) | 4.13 (0.06) | ||
| Night time urination | n = 558 | n = 536 | 0.1713 |
| 4.17 (0.06) | 4.07 (0.06) | ||
| Waking up at night because you had to urinate | n = 559 | n = 535 | 0.8091 |
| 4.24 (0.06) | 4.24 (0.06) | ||
| An uncontrollable urge to urinate | n = 559 | n = 536 | 0.9451 |
| 4.25 (0.05) | 4.25 (0.05) | ||
| Urine loss associated with a strong desire to urinate | n = 559 | n = 536 | 0.5675 |
| 4.13 (0.06) | 4.18 (0.06) |
- ER, extended release; HRQoL, health-related quality of life; SE, standard error.

Disposition of subjects. ER, extended release.
Among the 2454 subjects considered for enrollment who did not meet eligibility criteria, the most common reasons were: urinary frequency average <10/day (approximately 25%); average total daily volume voided >3000 ml (12%); other medical conditions (e.g., bladder surgeries, irritable bowel disease, renal disease, interstitial cystitis, neurogenic bladder; 12%); investigator decision or patient schedule (11%); <7 UUI episodes/week (7%); and inability to complete diary correctly (3%).
Full details of the individual study results are reported elsewhere [22,23]. A statistically significantly (P < 0.01) greater improvement in both primary end points—changes in the average number of diary-recorded daily toilet voids and daily UUI episodes—was observed in the trospium ER group at Week 1 when compared with placebo, and was maintained to study end. The trospium ER group also demonstrated statistically significantly greater improvements in urgency severity and volume voided compared with placebo at Weeks 1, 4, and 12. Treatment-emergent adverse events that were judged by the investigator to be at least possibly related to study medication and occurring in ≥1.5% of subjects in either treatment group were dry mouth (placebo, 3.7%; trospium ER, 10.7%), constipation (placebo, 1.5%; trospium ER, 8.5%), dry eyes (placebo, 0.2%; trospium ER, 1.6%), flatulence (placebo, 0.5%; trospium ER, 1.6%), and headache (placebo, 2.4%; trospium ER, 1.4%).
The treatment groups were similar at baseline with respect to QoL measurements. Following treatment for 12 weeks, patients treated with trospium ER demonstrated statistically significantly greater improvements (decreased scores from baseline) than patients treated with placebo for all KHQ domains except General Health Perceptions and Personal Relationships (Fig. 2).
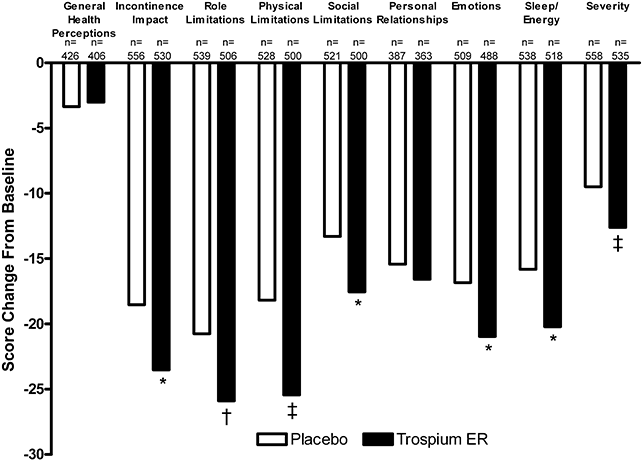
Mean change from baseline in King's Health Questionnaire domain scores at Week 12. Rank analysis of variance model. A decrease from baseline indicates improved quality of life. ER, extended release. *P < 0.05; †P < 0.01; ‡P < 0.001 versus placebo.
Trospium ER was also associated with significantly greater improvements in QoL than placebo as assessed using the OAB-q (3, 4). At Week 12, the improvement from baseline in mean OAB-q HRQoL total score was significantly greater with trospium ER than with placebo (+25.8 vs. +20.7; P = 0.0003) (Fig. 3). Trospium ER also produced significantly greater improvements than placebo on the Concern/Worry (P < 0.0001), Coping (P = 0.0009), and Sleep (P = 0.01) domains of the OAB-q (Fig. 3). In addition, trospium ER demonstrated significantly greater improvements (decreased scores from baseline) than placebo in all domains of the OAB-q Symptom Bother scale (P < 0.05) (Fig. 4). The mean change in total OAB-q Symptom Bother/Severity score was −31.5 with trospium ER and −23.7 with placebo at Week 12 (P < 0.0001).
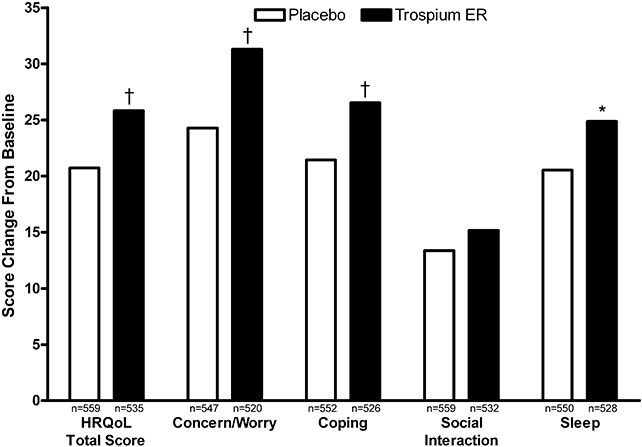
Mean change from baseline in Overactive Bladder Questionnaire–HRQoL Total and domain subscale scores at Week 12. Rank analysis of variance model. An increase from baseline indicates improved quality of life. ER, extended release; HRQoL, health-related quality of life. *P < 0.05; †P < 0.001 versus placebo.
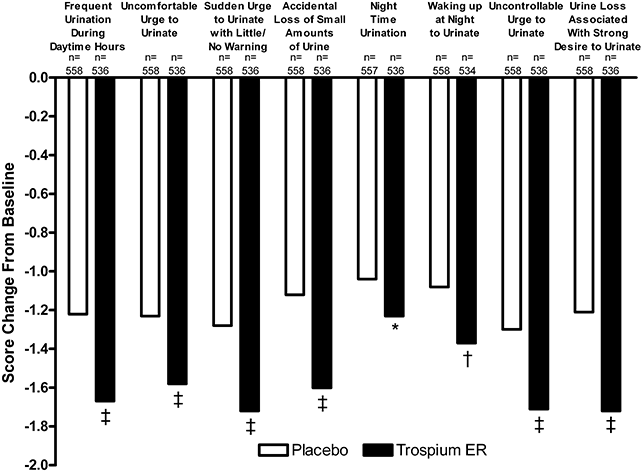
Mean change from baseline in Overactive Bladder Questionnaire–Symptom Bother domain scores at Week 12. Rank analysis of variance model. A decrease from baseline indicates improved quality of life. ER, extended release. *P < 0.05; †P < 0.01; ‡P < 0.001 versus placebo.
Discussion
The present results indicate that the symptomatic improvements observed in the Phase III trials with trospium ER 60 mg are accompanied by significant improvements in HRQoL when compared with placebo, as assessed using the validated KHQ and OAB-q measures, over the 12-week study. Patients in the trospium ER group had significantly greater improvements from baseline in scores on the Incontinence Impact, Role Limitations, Physical Limitations, Social Limitations, Emotions, Sleep/Energy, and Severity domains of the KHQ, compared with placebo recipients. Similarly, on the OAB-q HRQoL scale, trospium ER produced significantly greater improvements from baseline than placebo in the Total Score and scores for the Concern/Worry, Coping, and Sleep domains. Improvements from baseline on all eight items of the OAB-q Symptom Bother Scale were significantly greater with trospium ER than with placebo, indicating a benefit with regard to subjects' own individual assessments of how troubling their symptoms are to them. These findings correspond with measures of efficacy; at Week 12, trospium ER produced significantly greater improvements from baseline than placebo on the coprimary efficacy end points, mean number of toilet voids/day (−2.7 vs. −1.9; P < 0.001), and mean number of UUI episodes/day (−2.4 vs. −1.8; P < 0.001), as well as secondary efficacy end points such as volume voided/toilet void (+30.6 vs. +18.4 ml; P < 0.001) [31]. The findings of this pooled analysis of the QoL data are also similar to the QoL responses observed in the two individual studies included in this analysis.
The finding of improvements in sleep is particularly interesting, given the impact that adequate sleep can have on all aspects of people's lives. The improvements observed in sleep may reflect the corresponding improvement reported on the OAB-q Symptom Bother domains “Night time urination” and “Waking up at night to urinate,” with patients able to experience more restful sleep because they are less bothered by the need to urinate during the night.
While objective assessment tools can be useful in measuring a biologic response to treatment of conditions such as OAB, they do not reflect whether these changes correspond to noticeable improvements in patients' daily lives. For this reason, PROs—encompassing HRQoL (as reported here) as well as symptom bother (such as assessment of urgency), patient satisfaction, and patient adherence—have become increasingly important in clinical trials of treatments for symptom-based conditions such as OAB, as they provide a more comprehensive method of monitoring treatment benefits than traditional objective clinical outcomes [32]. The International Continence Society has recommended that therapeutic interventions aimed at improving the symptoms of OAB should also be assessed for their effects on HRQoL [33].
Our data are not only consistent with those for other antimuscarinic agents in showing an improvement in disease-specific HRQoL (including other large-pooled analyses utilizing the KHQ where a similar degree of difference between placebo and active treatment was observed [34,35]), but are also consistent with studies using the IR form of trospium chloride. Zinner et al. showed that 12 weeks of treatment with trospium chloride IR was associated with a significant improvement in the Travel, Emotional Health, and Social Relationships QoL subscales on the Incontinence Impact Questionnaire [36]. Fuertes et al. also showed improved QoL with trospium chloride IR using a generic HRQoL instrument, the EuroQoL scale [37].
The degree of effect of trospium ER reached the thresholds suggested to represent a minimally important difference on the OAB-q [30], and change that is clinically meaningful to patients on KHQ domains [28]. However, it should be noted that in this analysis, these thresholds were also reached by patients receiving placebo; this has also been observed in placebo-controlled studies of other antimuscarinics in OAB using these instruments [34,35,38]. The placebo effect on symptoms such as urinary incontinence and OAB is well known [39]. Reasons are unclear but may be related to factors such as interactions with health-care providers during clinical study participation, and increased awareness of voiding habits and potential risk factors [39]. The placebo effect is greater with subjective parameters than with objective measures (such as voided volume) [39]; thus, it is not surprising that we saw a considerable placebo effect in the QoL assessments. Given the particular susceptibility of subjective measures such as QoL to the influence of the strong placebo effect observed in OAB studies, it is important to consider QoL data evaluating treatment effect in the context of more objective measures of treatment effect. Despite the high level of placebo response observed in this analysis, the effects of trospium ER were still significantly greater on most items and domains of the QoL scales, consistent with the significant superiority versus placebo observed on the more objective efficacy parameters, including volume voided/toilet void.
As the only quaternary amine antimuscarinic currently approved for the treatment of OAB, trospium has a number of additional properties that make the agent suitable for prescription to a wide range of patient groups [40]. These include a low propensity to cross lipid membranes such as the blood–brain barrier. This may contribute to a low rate of central nervous system adverse events, minimizing the potential for negative effects of such adverse events on QoL. In addition, no clinically relevant metabolic drug-drug interactions are anticipated with trospium, based on in vitro data [24]. Trospium is metabolized by ester hydrolysis and excreted by the kidneys through a combination of tubular secretion and glomerular filtration [24]. In contrast, the tertiary amines are metabolized hepatically via the cytochrome P450 system. Two Phase III clinical trials of the new formulation have demonstrated that trospium ER provides similar efficacy to that of the IR (20 mg twice daily) formulation, but with a lower incidence of dry mouth, which should positively impact QoL [22,23].
Persistence in the use of antimuscarinics as a class is poor [41,42], and many hypotheses have been put forward to explain this. It is reasonable to believe that the more tolerable a medication is, the more likely the patient is to continue using it. Side effects were the second most common reason for drug discontinuation (after poor efficacy) in a survey of 1447 OAB patients in the United States [21]. Tolerability must, therefore, be accompanied by efficacy to maximize adherence. Patient satisfaction with treatment was significantly associated with compliance in another survey of 345 OAB patients using ER forms of tolterodine or oxybutynin [43]. In this survey, a significant determinant of patient satisfaction with treatment was the impact of OAB symptoms on their lives (P < 0.001) [43], reinforcing the importance of evaluating HRQoL in clinical studies of OAB medications.
It is important to acknowledge the potential limitations of our study. First, this study was of short duration, and a longer follow-up is needed to confirm that the results are maintained during a longer course of treatment. Second, the results were obtained in a group of OAB patients with specific minimum levels of urgency, frequency, and UUI, and may not be generalizable to patients with characteristics other than those studied. However, setting these baseline criteria as requirements for enrollment resulted in a certain severity level among the subjects that is not always seen in OAB studies. Thus, the study population may be representative of patients previously under-represented in some other studies. In addition, we would expect the data from subjects with greater symptom severity at baseline to be generalizable to those with lesser symptom severity. Thirdly, we used only OAB-specific HRQoL instruments, and so it is not possible to compare our findings with those of studies with antimuscarinic agents using generic HRQoL instruments or to compare our baseline data of OAB QoL with those of patients with other conditions. However, our study included two measures of HRQoL using widely used and validated disease-specific questionnaires, and found results comparable to those with other antimuscarinics using the same QoL instruments—tolterodine ER [44], solifenacin [34], and IR or transdermal oxybutynin [4,45] with the KHQ, and solifenacin and darifenacin with the OAB-q [38,46].
Although our study was a post hoc pooled analysis, it is not subject to selection bias, since the data are analyzed for all patients, using the LOCF method, and not to a subgroup of selected patients. In fact, the identical nature of the studies and their robust design (randomized, double-blind, and placebo-controlled) provide an opportunity to examine outcomes in a larger patient cohort, and therefore to produce more reliable results.
This study demonstrates that the symptomatic improvements resulting from treatment with trospium ER translate into improvements in HRQoL, as measured using the KHQ and OAB-q. HRQoL benefits extended to statistically significant improvements in all aspects of subject-perceived symptom bother.
Source of financial support: This work was supported by Allergan, Inc. and Endo Pharmaceuticals (formerly Indevus Pharmaceuticals Inc).
Editorial support for the development of this manuscript was provided by Catherine Rees and Sushma Soni. This support was funded by Allergan, Inc.




