Prevention and Management of Hyperphosphatemia with Sevelamer in Canada: Health and Economic Consequences
ABSTRACT
Background: Sevelamer hydrochloride (Renagel) binds phosphate in patients with end-stage renal disease without the use of exogenous calcium and may reduce the progression of coronary vascular calcification. This intervention was shown to be cost-effective in the United States. This paper presents the Canadian adaptation.
Methods: A discrete event simulation of the long-term cardiovascular implications of 1 year of phosphate binding in a prevalent hemodialysis population was used to estimate the cost-effectiveness of sevelamer use in Canada based on the demographics, comorbidities, physiological and renal characteristics. Initial calcification score and expected changes over 1 year were derived using regression equations developed from a clinical trial and translated to cardiovascular disease risk based on equations developed from a long-term cohort study. Direct medical costs from a Canadian Medicare perspective were taken from Ontario data. Ten replications of 10,000 patients over 13 years (discounting at 3%) were done for the base case and extensive sensitivity analyses were conducted.
Results: The cardioprotective effect of sevelamer over 1 year is estimated to prevent 10 cardiovascular events and gain 18 life-years compared with calcium carbonate in 100 patients over a lifetime. These benefits are obtained at a net cost of CAD$2,096; an incremental cost-effectiveness ratio of CAD$12,384 per discounted life-year gained. Sensitivity analyses showed that the time horizon and efficacy were the most important factors.
Conclusion: The results of this study provide evidence that use of sevelamer in Canada would be economically sound.
Introduction
Patients with end-stage renal disease (ESRD) are at higher risk of cardiovascular disease [1], and evidence of the predictive value of imaging with electron-beam tomography (EBT) has been accumulating [2]. An increasingly disordered calcium and phosphate metabolism is a major contributor to widespread extra-osseous calcification in ESRD and this is exacerbated by the use of calcium-based phosphate binders [3]. Sevelamer hydrochloride (Renagel, Genzyme Corporation, Cambridge, MA) is an effective noncalcium phosphate binder that has been shown to reduce coronary and aortic calcification [4]. In this brief report, we present a Canadian adaptation of a published economic model that assesses the clinical and economic consequences of the choice of phosphate binder [5].
Methods
The model (Fig. 1) is a discrete event simulation of the changes in calcification score that occur over 1 year, depending on phosphate binder and patient characteristics. The resulting cardiovascular risk and its clinical and economic sequelae are also estimated. At the start, each patient is assigned characteristics by sampling distributions for demographics, physiological parameters, including calcification score, and comorbidities. The patient is copied to ensure only the phosphate binder differs: one copy receives sevelamer, the other a calcium-based binder. Changes in cardiac calcification, physiologic and renal parameters are derived using published regression equations [5]. The long-term occurrence of cardiovascular disease [6], management costs, and survival are accrued. The risk of a cardiovascular event is modeled using regression equations developed based on the only available longitudinal data set that includes information on calcification and cardiovascular risk in patients with ESRD [6–8]. In addition to the EBT score, the regression equation includes diabetes, C-reactive protein levels, diastolic blood pressure, sex, smoking, hypertension, and total cholesterol. The likelihood of a cardiovascular event being fatal is based on the longitudinal data set (22% for an initial and 41% for a subsequent event), and the specific event type is assigned based on the observed distributions.
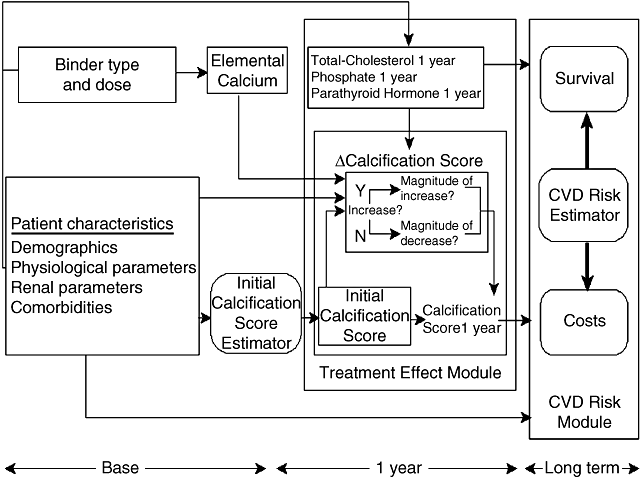
Simplified structure of the Disease Simulation Model. CVD, cardiovascular disease.
Only direct medical costs were considered (Table 1). Acute hospital costs (including all accommodations; ancillary services such as pharmacy, laboratory, imaging, radiology; diagnostic and surgical procedures; and inpatient physician services) were estimated from data obtained from the Ontario Case Costing Initiative for each type of event defined by International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), developed for morbidity classification in Canada (ICD-10-CA) diagnosis codes. Unit costs for the physician services, profiled based on length of stay, case complexity level, ambulance use, surgical procedure codes and disposition, were obtained from the Ontario Physician Fee Schedule [9]. Because costs of post-discharge care for patients with ESRD suffering a cardiovascular event were not available, only acute inpatient costs are considered here. Nevertheless, this is explored in sensitivity analyses. Because calcium acetate is not widely used in Canada, the results focus on sevelamer (CAD$1.7750/g for 800 mg tabs) versus calcium carbonate (CAD$0.0472/g). A weighted average daily cost was calculated for 40-mg tablets of cholesterol-lowering drugs using the Ontario Drug Benefit Formulary [10]. The characteristics of the patient population used for the base case are summarized in Table 1.
| Model parameter | ||||
|---|---|---|---|---|
| Hospitalization (mean LOS in days; mean cost in 2005 CAD$) | Alive at discharge | Died in hospital | ||
| LOS | Cost | LOS | Cost | |
| Congestive heart failure | 9 | 9,199 | 12 | 18,961 |
| Coronary artery disease | 6 | 11,627 | 8 | 22,429 |
| Cerebrovascular disease | 10 | 12,370 | 12 | 18,524 |
| Aortic disease | 11 | 24,947 | 8 | 29,150 |
| Peripheral arterial disease | 9 | 13,280 | 16 | 25,005 |
| Demographics | Mean or % | Interquartile range | ||
| Age (years) | 54 | 40–67 | ||
| Sex (males) | 60% | |||
| Race (white) | 85% | |||
| Smoking (smokers) | 6% | |||
| Physiological parameters | ||||
| Total-cholesterol (mmol/L) | 4.75 | 3.95–5.50 | ||
| HDL-cholesterol (mmol/L) | 1.16 | 0.90–1.36 | ||
| Diastolic blood pressure (mmHg) | ||||
| Hypertension | 85 | 74–95 | ||
| No hypertension | 73 | 64–81 | ||
| Renal parameters | ||||
| Serum phosphate (mmol/L) | 1.84 | 1.52–2.15 | ||
| Serum calcium (mmol/L) | 2.34 | 2.25–2.44 | ||
| Parathyroid hormone (pg/mL) | 318 | 80–436 | ||
| Vintage (months) | ||||
| Diabetes | 28 | 10–24 | ||
| No diabetes | 79 | 16–120 | ||
| Comorbidities | ||||
| Diabetes by age group | ||||
| ≤29 years | 0% | |||
| 30–49 years | 2% | |||
| 50–64 years | 23% | |||
| ≥65 years | 18% | |||
| Hypertension by sex | ||||
| Males | 80% | |||
| Females | 63% | |||
| CVD history by diabetes and age | ||||
| Diabetes | ||||
| ≤29 years | 0% | |||
| 30–49 years | 46% | |||
| 50–64 years | 73% | |||
| ≥65 years | 88% | |||
| No Diabetes | ||||
| ≤29 years | 3% | |||
| 30–49 years | 11% | |||
| 50–64 years | 31% | |||
| ≥65 years | 58% | |||
| Treatment | ||||
| Dose sevelamer (mg) | 6,136 | 3,781–8,607 | ||
| Dose calcium acetate (mg) | 4,443 | 2,549–5,766 | ||
| Dose calcium carbonate (mg) | 3,470 | 2,651–4,323 | ||
| Baseline EBT calcification score | 1,500 | 79–1,652 | ||
- LOS, length of stay; HDL, high-density lipoprotein; EBT, electron-beam tomography.
The base-case runs for 13 years because most hemodialysis patients have died by then. Costs and benefits occurring beyond 1 year are discounted at 3% per year. Results are based on 10 replications of 10,000 patients. Sensitivity analyses considered sevelamer's efficacy, effect of calcification on cardiovascular risk, cholesterol-lowering treatment practices, treatment cost, event costs, time horizon, discount rate, patients' age, baseline calcium, and phosphate levels. Duration of risk reduction was explored as well.
Results
After 1 year of sevelamer use, calcification increased in only 36% of patients compared with 57% on calcium carbonate and calcification scores were 14% lower. In 100 patients, 5 fewer (32 vs. 37) suffered an initial cardiovascular event and 5 fewer a subsequent event [32 vs. 38 (Difference does not equal 5 events due to rounding)] over their lifetime, representing a 13% reduction in cardiovascular risk. Survival increased from 8.98 years to 9.22 years (discounted gain: 0.1777 years). The majority of the cost was related to inpatient management of cardiovascular disease (73% for sevelamer; 98% for calcium carbonate). One year of sevelamer use accounted for 26% of the cost compared with less than 1% for calcium. Statins made up the remaining 1% of the cost in both groups. The savings of CAD$160,402 in cardiovascular costs largely offsets the increased binder costs, leading to a cost-effectiveness ratio of CAD$12,384 per discounted life-year gained (dLYG).
Univariate sensitivity analyses (Fig. 2, Panel A) indicated that results were very sensitive to time horizon and efficacy but not to cholesterol-lowering treatment practice, costs of hospitalizations, inclusion of subsequent care costs, or patient characteristics. The results of the multivariate sensitivity analyses—which indicate the probability that sevelamer is cost-effective at different possible values of the maximum acceptable cost-effectiveness ratio appropriate for decision-making—are summarized in Figure 2, Panel B.
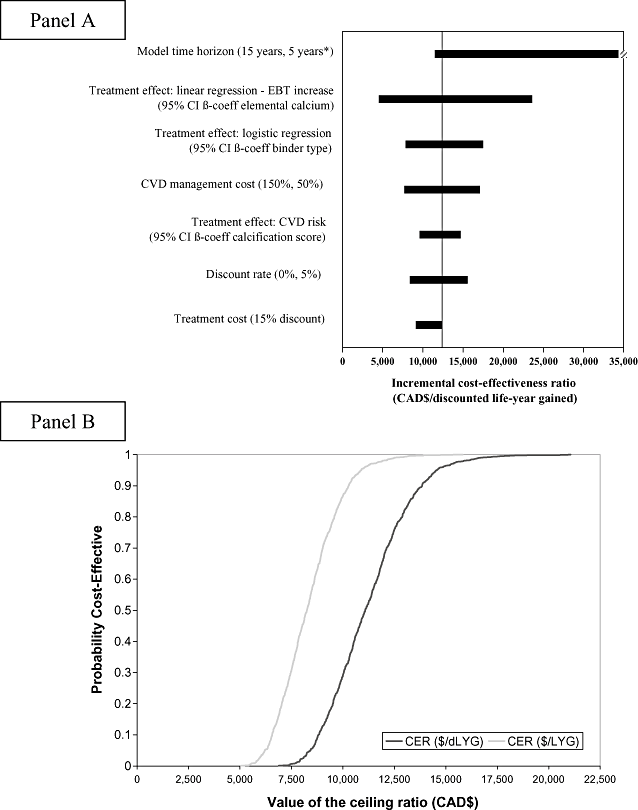
Panel A: Univariate sensitivity analyses. The solid vertical line represents the base case incremental cost-effectiveness of sevelamer versus calcium carbonate. Horizontal bars indicate range obtained for each variable (*: shorter time horizons are not depicted for clarity. Two years: CAD$88,767/dLYG). Panel B: Cost-Effectiveness Acceptability Curve, derived by simultaneously varying the treatment effect [95% confidence interval (CI) for the β-coefficients in: 1) logistic regression providing the probability a patient will have an increase in calcification score; 2) linear regression predicting the magnitude of the increase in calcification score; 3) Cox-proportional hazards regression predicting cardiovascular risk] and the cardiovascular disease (CVD) management cost (±50%). EBT, electron-beam tomography.
For 1 year of sevelamer treatment to yield incremental cost-effectiveness ratios below CAD$25,000/dLYG, the reduction in cardiovascular risk would have to persist for at least 6.6 years; for CAD$50,000/dLYG, about 3.4 years; and for CAD$100,000/dLYG, only 1.8 years.
Discussion
Despite uncertainty regarding the implications of coronary calcification, it seems prudent to minimize it in patients with ESRD because a major contributor may be the additional calcium load from calcium-based phosphate binders. Use of sevelamer instead is estimated to prevent about 10 cardiovascular events and to save 18 life-years per 100 treated patients, at a favorable cost-effectiveness relative to calcium carbonate of under CAD$12,500/dLYG.
There are some limitations to these estimates. The Canadian adaptation of the sevelamer economic model focuses on costs. The trial and epidemiological data supporting the key functional relations in the model should be generally applicable, however. The link between calcification and cardiovascular risk is based on only one study, although it is consistent with the findings from studies in individuals without ESRD. Moreover, coronary calcification was recently demonstrated to be a significant predictor of all-cause mortality in subjects new to hemodialysis [11]. Although calcification has been shown to be a strong independent predictor of cardiovascular and all-cause mortality in ESRD, it remains to be proven that interventions that prevent calcification will actually result in lower cardiovascular risk. This can only be shown conclusively on the basis of long-term intervention studies that specifically address calcification and its relation to long-term outcomes. Although not conclusive, the initial results look promising: treatment with sevelamer was associated with a significant survival benefit as compared to calcium-containing phosphate binders in multivariate analyses in subjects new to hemodialysis [11].
The predicted changes in EBT scores are also based on only one clinical trial in 200 patients. The effect of 1 year of sevelamer treatment is to change cardiovascular risk at that point. The only presumption made about future effect is that the predictive ability of the calcification score is not itself affected by treatment, i.e., the impact of a change in score is correctly reflected by the respective failure-time curves. This type of assumption is commonly required when intermediate outcomes are used. Only long-term data can demonstrate this conclusively.
The economic implications estimated in this study are conservative in that only acute inpatient costs are included for the management of cardiovascular events. There were insufficient data to reliably estimate subsequent care costs in patients with ESRD who are already receiving intense care. The consequences in terms of fractures and vascular access problems are also not included, further contributing to the conservative nature of the estimates. Indeed, in a study comparing patients on sevelamer to controls not receiving this binder, a 50% reduction was observed in all-cause first hospitalization over 17 months of follow-up leading to an annual savings of more than $16,500 per patient, on average [12].
In summary, randomized controlled trials have shown that the use of sevelamer compared to calcium-based binders moderates valvular and vascular calcification, predictors of cardiovascular and all-cause mortality in ESRD. As long-term follow-up data were not yet available, understanding the implications of this physiological effect from a clinical and economic perspective requires predictive equations. Development of such a model cannot wait for the accumulation of follow-up data because payers and policymakers must decide today whether or not to reimburse for this binder, and individual physicians must make treatment decisions based on the available evidence. Although subject to the uncertainties inherent in modeling long-term outcomes based on short-term clinical trial results, this Canadian adaptation—which, like the original model, is based on the assumption that coronary calcification is a serious problem exacerbated by exogenous calcium—suggests that use of sevelamer should be considered an economically acceptable approach to treating hyperphosphatemia in patients on hemodialysis.
Source of financial support: This work was supported in part by a grant from Genzyme Canada to Caro Research. Genzyme collaborated in helping set the specifications for the study but had no role in methodological decisions or interpretation of results. They were also allowed to review and comment on this manuscript but were explicitly forbidden from exerting any editorial control.




