“Price Management” and Its Impact on Hospital Pharmaceutical Expenditure and the Availability of Medicines in New Zealand Hospitals
ABSTRACT
Objectives: In 2002, the Pharmaceutical Management Agency (PHARMAC) began negotiating new price contracts for 90% of hospital pharmaceuticals on behalf of all New Zealand (NZ) public hospitals (“price management”[PM]). The present study was undertaken to examine the impact of 3 years of PM on hospital pharmaceutical expenditure, and the impact of the new contracts on the availability of medicines.
Methods: Annual savings for 29 major public hospitals (financial years 2003/4 to 2005/6) were calculated from the data from 11 hospitals and data from PHARMAC. Inpatient and total hospital pharmaceutical expenditure (IPE, THPE) (2000/1 to 2005/6) were calculated from the data from 23 hospitals. Hospital pharmaceutical expenditure (2000/1 to 2005/6) was compared with community pharmaceutical expenditure (CPE) in NZ, and with THPE in the UK, Canada, Norway, and Sweden. Surveys were undertaken (2004, 2005) to examine any changes in medicine availability resulting from the new contracts.
Results: Annual savings were NZ$7.84 million (m) to NZ$13.45m (2003/4 to 2005/6). Growth in IPE slowed for all hospitals in 2003 to 2004. Mean growth was higher for IPE and THPE than for CPE (8.8%, 9.7% vs. 1.9%). Mean growth in THPE appeared slightly lower in NZ (9.6%) and Norway (7.3%) than in the UK 14%, Sweden 12.5%, or Canada 10.2%. Some availability problems occurred with new contract items (“out-of-stocks”; products perceived as inferior). Problems were usually resolved in weeks, but some took more than a year.
Conclusion: PM was moderately successful saving NZ$8m to NZ$13m (6–8%) in 2003/4 to 2005/6 and slowing growth in IPE in 2003/4. Further research should examine whether the favorable economic effects can be sustained while unfavorable effects are minimized.
Introduction
Pharmaceutical expenditure in primary care and hospitals has risen steadily as a share of Gross Domestic Product (GDP) in recent years, averaging 15% of annual health-care expenditure in the Organization for Economic Cooperation and Development countries (1970–1996) [1]. Drivers of growth are considered to be an increase in the average price of medicines (as new medicines are substituted for older cheaper medicines, and new medicines become available to treat previously untreatable disease) and an increase in the utilization of medicines (as populations increase in age and size) [2–4]. Countries and organizations have used “supply-side” and “demand-side” measures to curb this growth [5,6]. Supply-side measures focus on nego tiations with vendors, e.g., price and/or profit control, pooled procurement, rebates, reference pricing, expenditure ceilings, and positive or negative lists. Demand-side measures focus on decreasing or managing the utilization of pharmaceuticals by prescribers and patients, e.g., educational campaigns, prescribing guidelines, patient copayments, and switching prescription medicines to over-the-counter availability.
Growth in pharmaceutical expenditure in hospitals has been a particular concern in recent years as many new expensive medicines are initiated in this setting [7]. In 2002 to 2003, publicly funded hospital pharmaceutical expenditure comprised 20% to 26% of the total pharmaceutical expenditure in New Zealand (NZ), the UK, Australia, and the United States [8–11]. Hospital pharmaceutical procurement systems vary from country to country with mainly local procurement by individual hospitals in the UK, Sweden, and Australia but with a proportion of medicines (mainly generics) procured nationally, regionally, or statewide [12–14]. In Canada, hospitals use group procurement for pharmaceuticals [15]. Both local and group procurement occur in hospitals in the United States [16]. Norway has had a national scheme for the procurement of hospital pharmaceuticals since 1995 [17]. Before 2002, pharmaceutical procurement in NZ was undertaken by individual hospitals, apart from a short period of group purchasing in the 1980s [18].
Since 1993, PHARMAC, the government's Pharmaceutical Management Agency, has successfully managed pharmaceutical expenditure in primary care mainly through supply-side measures [19,20]. In 2001, the government authorized PHARMAC to manage the pharmaceutical expenditure in public hospitals, and in 2002, they launched a three-part National Hospital Pharmaceutical Strategy for this purpose. PHARMAC's main initiatives were to 1) negotiate new national, as opposed to current local contracts for around 90% of hospital pharmaceuticals (price management [PM]); 2) provide economic assessments of new hospital medicines (assessment of new medicines [ANM]); and 3) coordinate activities for improving the use of medicines in hospitals (quality use of medicines [QUM]) [21]. Pharmacists and other health professionals were concerned about the possible impact of these initiatives, and the impact of the strategy's new contracts on the availability of medicines [21]. Earlier studies examined the impact of PM (first year only), and the ANM and QUM initiatives [22–25]. The aim of the present study was to examine the impact of 3 years of PM on hospital pharmaceutical expenditure, and the impact of new contracts on the availability of medicines.
Methods
Data were sought from chief pharmacists at all hospitals employing a pharmacist in NZ, the major public hospitals, 30 hospitals in 2002, and 29 hospitals thereafter. The hospitals were classified into three types for analysis, with assistance from the Ministry of Health. Tertiary hospitals were those with all specialties on-site including a renal unit. Secondary hospitals were those with most specialties on-site but with some visiting specialists. Rural/special hospitals were small hospitals with only visiting specialists or hospitals for a special group of patients (e.g., psychiatric). Three investigations were developed for the present study: two to examine the economic impact of PM, and one to investigate the effects of the new contracts on the availability of those medicines.
Top 150 Analysis
The aim of this investigation was to determine the impact of price changes resulting from PHARMAC's strategy from 3 years of PM. The Top 150 method was initially discussed and the first year results were reported in an earlier article [23].
The Top 150 method involved the chief pharmacists at 11 NZ hospitals calculating a projected saving (or cost) for their Top 150 items of pharmaceutical expenditure for year two (financial year 2003/4), three, and four from price changes, and volumes used in year one (2002/3), two, and three. Exact calculations were not possible because prestrategy prices between the suppliers of pharmaceuticals and the hospitals were confidential. Therefore, 13 hospitals, representing the three types of hospitals and different geographic localities, were approached, and 11 hospitals provided the data. Projections were for 2003/4 to 2005/6 (July 1–June 30). Net adjustments were added, calculated from the figures obtained from PHARMAC, i.e., additional savings from any rebates, bonuses, and discounts on invoices, minus the cost of compensation payments. PHARMAC estimated the compensation payments from the wholesalers' and suppliers' information. (Compensation payments were amounts paid by a hospital to a pharmaceutical supplier of an item on a new contract, for purchasing a noncontract brand in excess of agreed limits, i.e., discretionary variance (DV) limits. Limits were usually 0% to 5% of the total expenditure on that item). Compensation payments were $5000 per breach in 2003/4 to 2004/5, and NZ$1000 per breach in 2005/6. An assumption was made that projected savings from price changes in year one would continue similarly in subsequent years. Projected savings and net adjustments were used to estimate the annual savings for 2003/4 to 2005/6 using the formulae in Table 1.
| July 1, 2003–June 30, 2004(NZ$) | July 1, 2004–June 30, 2005(NZ$) | July 1, 2005–June 30, 2006(NZ$) | |
|---|---|---|---|
| Projected savings (PS)* | |||
| 5,456,045 | 2,299,070 | 2,268,663 | |
| (4,728,978–6,481,507) | (2,121,159–2,607,636) | (1,795,943–2,620,405) | |
| = PS1 | = PS2 | = PS3 | |
| Rebates (R)† | 1,060,900 | 1,402,225 | 1,688,600 |
| Bonuses and discount oninvoices (B)† | 1,457,654 | 2,129,341 | 1,738,453 |
| Compensation payments (C)† | 130,500 | 75,000 | 5,000 |
| (58,000–203,000) | (289,500–98,000) | (0–6,000) | |
| Net adjustments (NA) = R + B − C | 2,388,054 | 3,456,566 | 3,422,053 |
| (2,315,554–2,460,554) | (3,433,566–3,503,566) | (3,421,053–3,427,053) | |
| = NA1 | = NA2 | = NA3 | |
| Estimated annual savings* | 7,844,099 | 11,211,681 | 13,445,831 |
| (7,044,532–8,942,061) | (10,283,703–12,592,709) | (12,067,133–15,136,601) | |
| = PS1 + NA1 | = PS1 + PS2 + NA2 | = PS1 + PS2 + PS3 + NA3 | |
- * By savings per bed-day.
- † Figures from the Pharmaceutical Management Agency.
Some accuracy checks were made. Chief pharmacists were asked to recalculate the savings 1) for items where new contract prices (not confidential) were incorrectly listed and 2) for items with “outlier results” (showing substantial savings or costs compared with other hospitals). Items with new contracts were called “section H items” because they are listed in section H of NZ's pharmaceutical schedule.
Median savings per hospital bed (or per bed-day) were used to calculate the projected savings for 29 hospitals from the projected savings for 11 hospitals (data were not normally distributed). The chief pharmacists provided information on bed numbers, and the Ministry of Health on bed-days [26]. Median savings per bed (or bed-day) for each type of hospital were multiplied by the number of missing beds (or bed-days) and added to known values to give an estimate of the projected savings for 29 hospitals. (Missing beds/bed-days were the total number of beds/bed-days from the 18 hospitals that were not providing data). A sensitivity analysis was undertaken. The lowest and highest savings per bed (or bed-day) for each type of hospital were multiplied by the missing beds (or bed-days) and added to known values to give an upper and lower limit of a range for each type of hospital. Projected savings and a range were calculated for the 29 hospitals in total. (Note that this methodology differs slightly from that used in the earlier article [23]. Medians, highest and lowest savings per bed/bed-days, were applied only to missing bed/bed-days in the present study, but to all bed/bed-days in the earlier article). Items with new contract prices were identified from current section H lists, categorized using the World Health Organization's anatomical therapeutic chemical (ATC) classification system, ranked in order of projected savings, and tabulated by hospital type [27].
Hospital Pharmaceutical Expenditure (HPE) Analysis
The aim of this investigation was to determine whether the PM part of the strategy would impact on growth in hospital pharmaceutical expenditure (inpatient and/or total). Because national hospital pharmaceutical expenditure figures were not routinely collected by the Ministry of Health, figures were sought directly from the hospitals. (Only two official estimates have been recently published: NZ$140m in 2003 [8] and NZ$174m in 2007 [28]).
Chief pharmacists at all 29 hospitals were asked to provide data on total hospital pharmaceutical expenditure (THPE) and inpatient pharmaceutical expenditure (IPE) for the financial years: 2000/1 to 2005/6 (July 1–June 30). IPE was calculated from THPE by subtracting the sum of expenditure on outpatient prescriptions and expenditure on pharmaceuticals supplied to other hospitals/institutions. The terms total hospital pharmaceutical expenditure 29 (THPE29) and inpatient pharmaceutical expenditure 29 (IPE29) were used to denote pharmaceutical expenditure for all 29 hospitals. One rural/special hospital had missing data for 2005/6 through contracting out a small part of their dispensing to three community pharmacies. Missing data were calculated by costing the medicines supplied, and the original figure was updated. A subset of hospitals provided a full 6-year data set. Data were found to be normally distributed, so mean expenditure per bed-day (and confidence limits) were calculated for each type of hospital and each year. An analysis of variance (ANOVA) test was undertaken on bed-day data.
IPE29 and THPE29 were calculated for all 29 hospitals from the data from the subset of hospitals that were able to provide data each year. Mean expenditure per bed-day was multiplied by the number of missing bed-days for each type of hospital, and added to known data. (Missing bed-days were the total number of bed-days from the hospitals that did not provide data in a particular year). A sensitivity analysis was undertaken by multiplying the missing bed-days for each type of hospital, by the upper and lower confidence intervals around the mean. These were added to the known values to calculate the upper and lower limits of the range. A range was not calculated if a data set for a hospital type was complete for that financial year. Tables and graphs of IPE were prepared and examined for trends. An assumption was made that IPE trends in the 11 hospitals would reflect trends in all 29 hospitals, so a post hoc analysis of data from the Top 150 analysis (11 hospitals) for 2002/3 to 2004/5 was undertaken to identify any reasons for the trends.
IPE29 and THPE29, representing expenditure in the major hospitals, were considered a proxy measure for IPE and THPE for all NZ public hospitals. Community pharmaceutical expenditure (CPE) figures for NZ were obtained for the years 2000/1 to 2005/6 from the official statistics [29]. CPE, IPE29, and THPE29 growth were compared. In addition, an Internet search was undertaken for the official statistics (in the English language) on hospital pharmaceutical expenditure from developed countries. THPE29 was compared with the data obtained. Explanations for trends were sought from pharmaceutical experts and the literature.
Availability of Medicines Surveys
The aim of this investigation was to determine the impact of the new contracts under the PM part of the strategy on the availability of medicines. A survey was administered to chief pharmacists at all 29 hospitals in July 2004 and repeated in July 2005. The respondents were asked 1) for examples of where section H contracts had resulted in the loss of access to useful pharmaceutical items; 2) how hospitals had overcome the loss of these items; 3) for examples of section H items considered by hospitals as inferior to items they were replacing; and 4) for examples of useful new pharmaceutical items becoming available as a result of the new section H contracts. Results, and any unsolicited comments, were examined.
Results
Top 150 Analysis
Eleven of the 13 hospitals originally approached provided data, i.e., five tertiary, three secondary, and three rural/special hospitals. Two hospitals were unable to participate because of time/data retrieval constraints. The annual savings were calculated from the projected savings and net adjustments as described earlier.
Projected savings for the 11 hospitals were NZ$3,439,749 (2003/4), $1,464,182 (2004/5), and $1,349,342 (2005/6). Savings were projected for all types of hospital, and as a proportion of expenditure, were higher for rural/special (R/S) and secondary (S) than tertiary (T) hospitals each year: T5.3%, S7.4%, R/S9.6% (2003/4); T2.5%, S2.7%, R/S4.5% (2004/5); and T1.9%, S2.4%, R/S2.3% (2005/6).
The main drivers of the projected savings on section H items by the ATC category varied. In 2003/4, they were agents for infections (antibiotics) (55% of savings); the nervous system (anesthetics, antipsychotics) (29%); musculoskeletal system (bisphosphonates) (6%); and blood/blood-forming organs (plasma substitutes) (5%). In 2004/5, they were agents for infections (antivirals, antibiotics) (50%); the nervous system (analgesics) (20%); alimentary tract (proton pump inhibitors) (10%). In 2005/6, they were agents for the alimentary tract (5HT3-antagonists) (45%); antineoplastic/immunomodulating agents (taxanes) (28%); and infections (antibiotics) (17%).
Using savings per bed-day, projected savings (range) for 29 hospitals were estimated to be NZ$5,456,045 (4,728,978–6,481,507) in 2003/4; $2,299,070 (2,121,159–2,607,636) in 2004/5; and $2,268,663 (1,795,943–2,620,405) in 2005/6 (Table 1). Estimates were very similar whether calculated using savings per bed or per bed-day, but the latter were considered more accurate, reflecting activity rather than capacity.
Net adjustments were calculated as NZ$2,388,054 (2,315,554–2,460,554) for 2003/4 (NA1); $3,456,566 (3,433,566–3,503,566) for 2004/5 (NA2); and $3,422,053 (3,421,053–3,427,053) for 2005/6 (NA3) (Table 1). Rebates, bonuses, and discount on invoices increased from a total of NZ$1,438,609 in 2002/3 (part year) to $3,427,053 by 2005/6. Compensation payments decreased from NZ$130,500 in 2003/4 to NZ$5,000 by 2005/6.
Annual savings for 29 hospitals, by savings per bed-day, were estimated as NZ$7,844,099 (7,044,532–8,942,061) for 2003/4; $11,211,681 (10,283,703–12,592,709) for 2004/5; and $13,445,831 (12,067,133–15,136,601) for 2005/6 (Table 1).
Hospital Pharmaceutical Expenditure Analysis
Twenty-three (79%) of the 29 hospitals contacted (representing 87% of the total bed-days) provided hospital pharmaceutical expenditure data for 2000/1 to 2005/6: 5 tertiary, 11 secondary, and 7 rural/special hospitals. Five hospitals provided the data for part of the period and one was unable to provide the data because of time/technology constraints. IPE29 and THPE29 were calculated from IPE23 and THPE23 as outlined earlier. The ANOVA test detected no significant change in bed-days during the period 2000/1 to 2005/6 (around 2.5 m per year for all 29 hospitals). Figure 1 shows the effect of PM on IPE29 and THPE29 from 2000/1 to 2005/6.
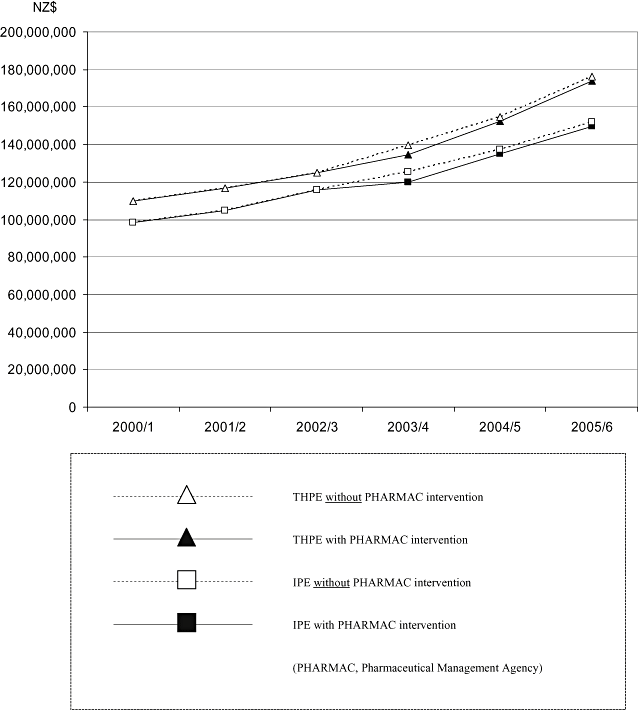
Impact of price management on inpatient pharmaceutical expenditure (IPE29) and total hospital pharmaceutical expenditure (THPE29).
IPE29 increased from approximately NZ$98m in 2000/1, to almost NZ$150m in 2005/6 (Table 2, Fig. 2): from NZ$59 to NZ$97m for all six tertiary hospitals; NZ$31 to NZ$44m for all 12 secondary hospitals; and from NZ$8.6 to NZ$9.4m for all 11 rural/special hospitals. Growth in IPE29 slowed in 2003/4 for all types of hospitals. A sensitivity analysis indicated a range of up to ±7% around the point estimates (Table 1).
| Financial year† | All hospitals (29) | Tertiary (6) | Secondary (12) | Rural/special (11) | ||||
|---|---|---|---|---|---|---|---|---|
| Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | |
| 2000/1 | 98,179,005 | 58,575,524 | 31,049,449§ | 8,554,032 | ||||
| (93,569,886–102,492,178)‡ | (55,206,932–61,943,193) | (7,313,505–9499,535) | ||||||
| 2001/2 | 104,883,699 | 6.8 | 63,249,742 | 8.0 | 32,635,416§ | 5.1 | 8,998,541 | 5.2 |
| (99,745,838–110,023,793) | (59,274,637–67,223,966) | (7,835,785–10,164,411) | ||||||
| 2002/3 | 115,637,834 | 10.3 | 70,728,921 | 11.8 | 35,535,995§ | 8.9 | 9,372,918 | 4.2 |
| (109,237,198–122,237,659) | (65,316,206–76,141,791) | (8,384,997–10,559,873) | ||||||
| 2003/4 | 120,117,326 | 3.9 | 75,289,031§ | 6.4 | 35,915,528§ | 1.1 | 8,912,767 | −4.9 |
| (119,342,609–120,944,024) | (8,138,050–9,739,465) | |||||||
| 2004/5 | 135,160,797 | 12.5 | 83,881,619§ | 11.4 | 41,617,991§ | 15.9 | 9,661,114 | 8.4 |
| (133,466,041–136,705,492) | (7,966,359–11,205,810 | |||||||
| 2005/6 | 149,621,374 | 10.7 | 96,664,653 | 15.2 | 43,511,942 | 4.6 | 9,444,778 | −2.2 |
| (138,020,984–160,925,066) | (86,961,306–106,367,237) | (43,128,289–43,895,654) | (7,931,388–10,662,174) | |||||
- * Calculated using mean expenditure per bed-day for missing bed-days for each type of hospital.
- † New Zealand financial year runs from July 1 of one year through June 30 of the next year.
- ‡ Range, calculated using upper and lower confidence limits for mean expenditure per bed-day for any missing bed-days.
- § No missing data.
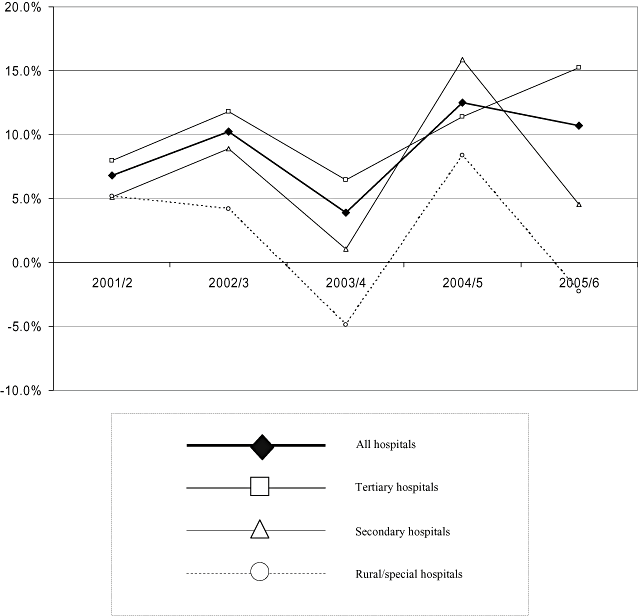
Percentage growth in inpatient pharmaceutical expenditure, 29 hospitals (IPE29).
THPE29 increased from approximately NZ$110m in 2000/1, to almost NZ$174m in 2005/6 (Table 3, Fig. 3): from NZ$63 to NZ$108m for all six tertiary hospitals; from NZ$37 to NZ$52m for all 12 secondary hospitals; and from NZ$10 to NZ$13m for all 11 rural/special hospitals. THPE29 growth appeared higher for all hospitals in total in 2004/5 to 2005/6, but appeared to slow for rural/special hospitals in 2002/3 and 2005/6, and for secondary hospitals in 2003/4 and 2005/6. A sensitivity analysis indicated a range of up to ±6% around the point estimates (Table 2).
| Financial year† | All hospitals (29) | Tertiary (6) | Secondary (12) | Rural/special (11) | ||||
|---|---|---|---|---|---|---|---|---|
| Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | Expenditure (NZ$) | % Growth | |
| 2000/1 | 109,834,053‡ | 62,776,971 | 36,656,768§ | 10,400,314 | ||||
| (105,077,940–114,192,948) | (59,980,396–65,573,161) | (8,440,776–11,963,019) | ||||||
| 2001/2 | 116,636,018 | 6.2 | 67,525,811 | 7.6 | 37,769,876§ | 3.0 | 11,340,331 | 9.0 |
| (111,057–120,821,145) | (64,253,093–70,797,998) | (9,034,375–12,253,271) | ||||||
| 2002/3 | 126,556,315 | 8.5 | 74,505,672 | 10.3 | 41,229,171§ | 9.20 | 10,821,471 | −4.6 |
| (120,300,824–132,811,887) | (69,688,566–79,322,561) | (9,383,087–12,260,155) | ||||||
| 2003/4 | 136,746,451 | 8.1 | 82,739,181§ | 11.1 | 42,472,227§ | 3.00 | 11,535,043 | 6.6 |
| (135,716,338–137,914,834) | (10,504,930–2703,426) | |||||||
| 2004/5 | 155,770,199 | 13.9 | 91,780,602§ | 10.9 | 50,667,639§ | 19.3 | 3,321,958 | 15.5 |
| (153,303,755–157,985,292) | (10,855,514–15,537,051) | |||||||
| 2005/6 | 173,706,715 | 11.5 | 108,200,607 | 17.9 | 52,491,521 | 3.6 | 13,014,587 | −2.3 |
| (163,789,846–184,075,225) | (100,706,614–116,244,438) | (52,007,639–52,975,414) | (11,075,593–14,855,373) | |||||
- * Calculated using mean expenditure per bed-day for missing bed-days for each type of hospital.
- † New Zealand financial year runs from July 1 of one year through June 30 of the next year.
- ‡ Range, calculated using upper and lower confidence limits for mean expenditure per bed-day for any missing bed-days.
- § No missing data.
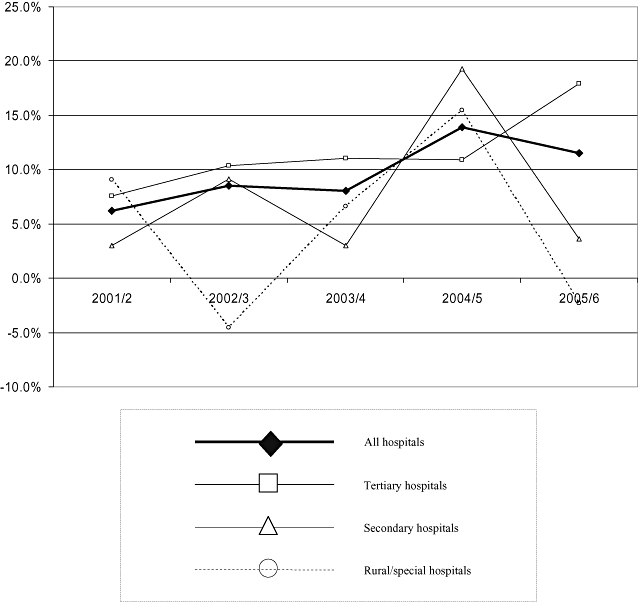
Percentage growth in total hospital pharmaceutical expenditure, 29 hospitals (THPE29).
The post hoc analysis identified the likely major contributors to the NZ$20m increases in IPE29 in 2002/3 to 2004/5 (Table 2). Expenditure increased by almost NZ$9m for antineoplastic/immunomodulating agents; by NZ$2.4m for agents for the nervous system; and by $NZ1.2m for agents for blood/blood-forming organs.
There appeared to be a higher mean growth rate per annum for hospital pharmaceutical expenditure in NZ, IPE29 (8.8%) and THPE29 (9.7%), than for CPE (1.9%) (Table 4, Fig. 4). Growth was lower for CPE for all years except 2003/4. In 2003/4, the growth rate for IPE29 was marginally lower (5% for CPE vs. 3.9% for IPE29). THPE was identified for Canada (2001–2003), the UK and Norway (2001–2004), and Sweden (2001–2005) (Table 5). With the exception of Norway in 2002 (−1.4%), THPE increased yearly for each country. Norwegian contacts could provide no specific reason for this decrease [30]. Mean growth in THPE in NZ (9.6%) and Norway (7.3%) appeared slightly lower than in the other countries (UK 14%; Sweden 12.5%; and Canada, three published figures, 10.2%) (Table 5, Fig. 5). Statistical analysis was considered inappropriate because this aggregate data may not compare identical cohorts each year.
| Financial year* | IPE29 | THPE29 | Community Pharmaceutical Expenditure | |||
|---|---|---|---|---|---|---|
| (NZ$m) | % Growth | (NZ$m) | % Growth | (NZ$m) | % Growth | |
| 2000/1 | 98.18 | — | 109.83 | — | 514.77 | −0.2 |
| 2001/2 | 104.88 | 6.8 | 116.64 | 6.2 | 503.35 | −2.2 |
| 2002/3 | 115.64 | 10.3 | 126.56 | 8.5 | 509.19 | 1.2 |
| 2003/4 | 120.12 | 3.9 | 136.75 | 8.1 | 534.81 | 5.0 |
| 2004/5 | 135.16 | 12.5 | 155.77 | 13.9 | 565.22 | 5.7 |
| 2005/6 | 149.62 | 10.7 | 173.71 | 11.5 | 564.39 | −0.1 |
| Mean annual percentage growth | 8.8 | 9.7 | 1.9 | |||
- * New Zealand financial year runs from July 1 of one year through June 30 of the next year.
- [The headings for Table 4 have been changed after online publication 16-May-2008].
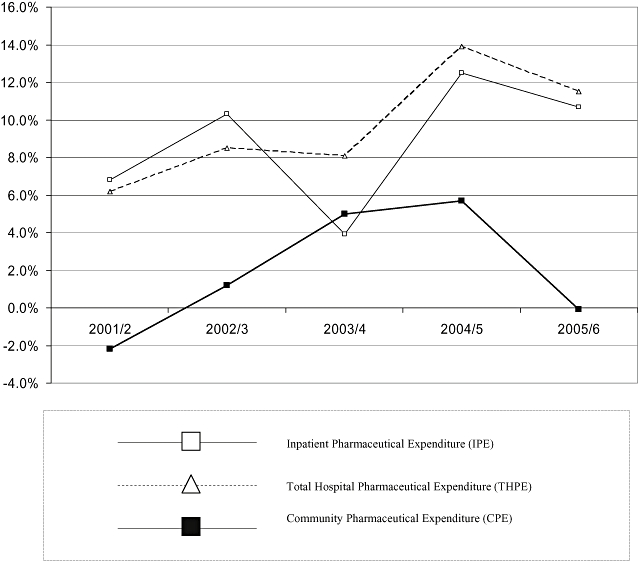
Percentage growth of inpatient and total hospital pharmaceutical expenditure for 29 major hospitals (IPE29, THPE29) and CPE, New Zealand, 2000/1 to 2005/6.
| New Zealand | UK [9] | Canada [34] | Sweden [35] | Norway [36] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expenditure (NZ$m) | % Growth | Expenditure (£m) | % Growth | Expenditure (CA$m) | % Growth | Expenditure (MSEK) | % Growth | Expenditure (NOKm) | % Growth | |
| 2001 | 109.8 | 6.2 | 1677 | 11.1 | 1204.8 | 8.0 | 2325 | 8.0 | 1376 | −1.4 |
| 2002 | 116.6 | 8.5 | 1876 | 11.8 | 1337.2 | 11.0 | 2547 | 9.5 | 1515 | 10.1 |
| 2003 | 126.6 | 8.1 | 2186 | 16.5 | 1494.2 | 11.7 | 2870 | 12.7 | 1624 | 7.2 |
| 2004 | 136.7 | 13.9 | 2548 | 16.5 | 3432 | 19.6 | 1839 | 13.2 | ||
| 2005 | 155.8 | 11.5 | 4033 | 17.5 | ||||||
| Mean annual percentage growth | 9.6 | 14.0 | 10.2 | 12.5 | 7.3 | |||||
- * Financial year commences July 1 in New Zealand; commences January 1 in other countries.
- † No figures available for 2005 for the UK, Canada, Sweden, and Norway, or for 2004 for Canada.
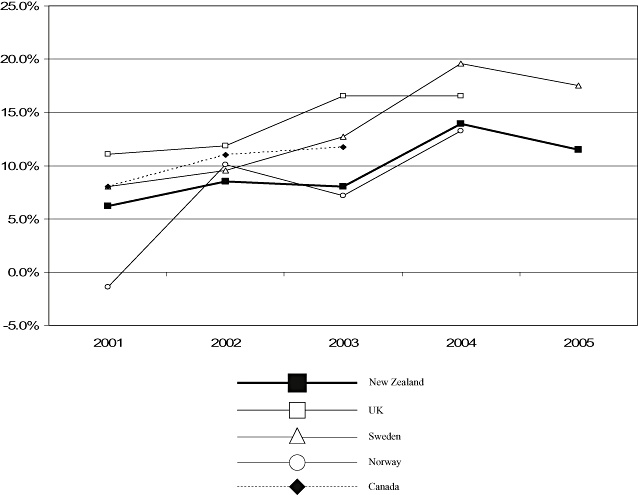
Percentage growth in total hospital pharmaceutical expenditure in five countries, 2001 to 2005.
Availability of Medicines Surveys
Twenty-eight hospitals (97%) responded in 2004, and 25 (86%) in 2005. Results indicated that the contracts had both favorable and unfavorable effects on the availability of medicines.
Respondents commented on the favorable aspects of the new contracts: reduced prices for previously expensive medicines; the availability of some new injections that were previously difficult to source; and some new items for primary care becoming available in hospitals at the same advantageous prices. Nevertheless, some respondents from secondary hospitals in smaller towns cited the difficulty of obtaining thehospital brand when their wholesaler tended to stock the community brand.
Unfavorable effects were discrepancies in brand availability between hospital and community; a perceived loss of access to some medicines (lack of choice of products, pack sizes, and some temporary shortages); and some products perceived as “inferior.” Some brand discrepancies required an explanation to patients (where hospital brands differed from those funded for primary care, e.g., glyceryl trinitrate sprays and salbutamol inhalers). Loss of access and choice was perceived because purchasing an alternative to the section H brand could result in being charged a compensation payment. Examples cited were a lack of a pleasant tasting, color-free version of paracetamol liquid; a lack of hydrocortisone cream packs greater than 15 g; and a lack of small packs of some tablets. Respondents reported some temporary shortages of section H brands of injections and tablets. Access problems appeared to be resolved in weeks, overall, but some problems took more than a year. Respondents overcame “loss of access” by using an alternative (sometimes more expensive) medical entity or another brand but keeping expenditure within the DV limits; repackaging items to overcome large pack sizes; and using short-dated tablets briefly after their expiry in emergency situations. The respondents were unsure whether “out-of-stocks” resulted from PHARMAC's sole-supply contracts, or from issues related to international markets. Some thought the strategy had led to an increased numbers of medicines only available as unlicensed medicines, and to increased sourcing of medicines from overseas. Others thought this trend began before the strategy.
Nevertheless, some of the perceived “loss of access” attributed to the strategy resulted from other PHARMAC activities such as community pharmacy contracts, e.g., the uncoated paracetamol tablets that some patients found difficult to swallow. Because of a “sole-supply” contract for community pharmaceuticals, uncoated tablets became the only reasonably priced source for hospitals in 2003. Coated tablets, no longer subsidized for community prescribing, resumed a list price twice than previously charged. The issue was resolved in 2005 when a community contract was agreed for both brands at similar low prices.
Some products were perceived as inferior, e.g., three hospitals considered “look-alike” ampoules of an antiemetic and a muscle relaxant (both kept close to patients in theaters) inferior and a potential hazard. One hospital used an alternative antiemetic for safety reasons, incurring considerable extra costs. Eleven respondents considered the new long-acting morphine tablets inferior to the previous brand, saying patients reported reduced pain relief, and that there was a lack of information on the possible rectal use of the tablets. Eight respondents thought the section H paracetamol liquid an inferior product and four, that children disliked the taste. These last two issues were reported to be resolved later when the DV limits were removed and other brands could be purchased without penalty. Four hospitals reported dissatisfaction with the hospital brand of glyceryl trinitrate spray, citing: a stronger mist when administering it; difficult for arthritic patients to use; difficult to see when the spray was empty; and no placebo was available to demonstrate to new users.
Some unsolicited comments were made: “in general things haven't really changed for us for better or worse” (tertiary hospital); “overall, I think the concept is good but the administration of it is a nightmare, e.g., to make sure we don't accidentally buy the wrong brand!” (secondary hospital); and “we had good access (to pharmaceuticals) before, but now we do have price advantages” (secondary hospital). One respondent emphasized the difficulties for smaller hospitals if the supplier had “draconian terms of trade” like large minimum orders or limits on the numbers of orders per month (rural/special hospital). Another summarized “overall, I don't feel that section H has been particularly helpful or unhelpful. It does limit choices and means you have to be very alert to ensure you purchase the contracted brands or risk the dreaded fine; as often there is only one brand to choose from, I do not see that PHARMAC can really dictate prices to any great extent on the more expensive items” (rural/special hospital).
Discussion
In 2002, PHARMAC launched the National Hospital Pharmaceutical Strategy to manage hospital pharmaceutical expenditure in NZ. The present study examined the impact of 3 years of PM, one part of the strategy, on hospital pharmaceutical expenditure, and the impact of the new contracts on the availability of medicines. The study found that PM was moderately successful. Savings of NZ$8m to NZ$13m (6–8%) per annum were made (2003/4 to 2005/6), and growth in IPE appeared to slow down for all types of hospitals in the year after the launch of the strategy (2003/4). Growth in hospital pharmaceutical expenditure in NZ appeared higher than growth in CPE, and mean growth in hospital pharmaceutical expenditure in NZ (and Norway) appeared slightly lower than in the UK and Sweden. Some availability problems occurred with items on new contracts such as “out-of-stocks” or products considered inferior by respondents. These problems were usually resolved in weeks, but some took more than a year.
The Top 150 analysis estimated an annual savings of NZ$7.84m to NZ$13.45m per annum for the years ending June 30, 2004 to June 30, 2006, 6% to 8% of the estimated hospital pharmaceutical expenditure. The annual savings were derived from the projected savings and net adjustments. As expected moderate savings, rather than increased costs, were projected on the Top 150 items of pharmaceutical expenditure compared with pre-strategy years for all types of hospitals each year. Projected savings were greater in the first year of the strategy (around NZ$5.5m) than in subsequent years (around NZ$2m), and greater as a proportion of expenditure for the smaller hospitals. The latter was expected because smaller hospitals probably had less attractive contracts in place before the strategy (possibly because lower volumes of use resulted in weaker bargaining power). Net adjustments were calculated (rebates, bonuses, and discounts on invoices less compensation payments) and represented modest additional savings each year, for example NZ$3,422,053 in 2005/6. In 2003/4, the net adjustments were lower than the projected savings in monetary terms, but were higher in subsequent years. Compensation payments were small in comparison with other adjustments and decreased each year possibly because compliance with contracts improved as hospitals became increasingly motivated to avoid these payments.
Savings appear to have been achieved by targeting contracts to high-volume pharmaceuticals. Savings were predominantly from antibiotics, anesthetics, antipsychotics, bisphosphonates, and blood substitutes (first year); antivirals, analgesics, and gastrointestinal agents (second year); and antibiotics, antipsychotics, antiemetics, and cytotoxics (third year). Because generic versions of some hospital medicines began to be marketed during this period, some savings might have occurred regardless of the strategy. Nevertheless, the impact of this was considered to be small because hospitals using some generic versions of antibiotics and bisphosphonates before the strategy still indicated substantial savings on these particular items. It is likely that PHARMAC was able to negotiate lower prices for new generics than prices previously available to the hospitals.
As anticipated, the present study indicated some slowing in growth in hospital pharmaceutical expenditure during the period. In 2003 to 2004, the year after the launch of the strategy IPE slowed for all types of hospitals. Furthermore, there were decreases in IPE for the smaller hospitals (rural/special) in 2002 to 2003 and 2003/4 and in THPE in 2002/3. This larger impact on the smaller hospitals was anticipated as discussed earlier. The study indicated that, with the exception of IPE29 in 2003/4, hospital pharmaceutical expenditure grew faster than CPE. The post hoc analysis suggested growth in hospital pharmaceutical expenditure was mainly due to expenditure growth on antineoplastic/immunomodulating agents. Some growth was expected, because a list of anticancer treatments, to which hospitals were expected to provide access, was agreed in 2002. (A “Pharmaceutical Cancer Treatment Scheme” was launched in 2002, an initiative to improve equity of access to cancer treatments in NZ, but not part of the National Hospital Pharmaceutical Strategy). Before 2002, hospitals used a locally agreed range of anticancer treatments, and anecdotal reports suggest some hospitals rarely used the newer more expensive treatments. From 2002, these hospitals were obliged to provide the more expensive anticancer treatments for certain cancers, and this may have contributed to some degree to the growth in hospital pharmaceutical expenditure. Growth also occurred with other newer pharmaceuticals, but would likely have been greater had PM not been in place, because many of these had price reductions under this scheme. Increased use of new, expensive pharmaceuticals is a well-known driver of expenditure growth. PHARMAC, aware of this, has some initiatives in place to moderate this. These were discussed in earlier articles [22,25]. THPE appeared to grow more slowly in NZ and Norway than the UK, Sweden, in the period (Canada had only three values to compare). National pooled procurement for hospital medicines in both countries may have contributed to this [21,30]. Nevertheless, caution must be exercised in comparing the countries' aggregate data because differences in health systems and other confounding factors exist.
The availability of medicines surveys found some modest availability effects resulting from the new contracts. Favorable effects were the reduced cost of some medicines and access to some new medicines. Unfavorable effects were brand choice restrictions, out-of-stock and short-dated items, and products perceived as inferior. Problems were mainly resolved in weeks or months, but some took more than a year. Unfavorable effects have occurred with other pooled procurement schemes, and frustration (from choice restrictions) has led to decreased contract compliance over time [18,31]. In contrast, the present study showed increased compliance over time, similar to that achieved by group purchasing organizations in the United States (hospitals agree to a high level of compliance but are allowed to purchase a small percentage of noncontract items) [32]. The threat of compensation payments under the strategy appears to have motivated staff in NZ hospitals to comply with the contracts. Nevertheless, quicker responses on “inferior” products would increase confidence in PHARMAC's abilities to manage hospital pharmaceuticals over the longer term.
The two main difficulties in undertaking the study were the lack of access to pre-strategy prices because of confidentiality agreements, and the lack of national figures on hospital pharmaceutical expenditure. These were overcome with the help of the hospital pharmacists in calculating the projected savings and providing annual pharmaceutical expenditure data. In examining the impact of both price decreases and increases among that group of medicines, the Top 150 analysis differed from previous studies that examined only the impact of price decreases on the items subject to new contracts [18,33].
The main limitation to this study was that a randomized controlled trial could not be undertaken because the PM intervention was applied simultaneously to all hospitals. Therefore, the effects of price changes were examined using a pre-post design; hospital pharmaceutical expenditure data were collected annually; and a survey of opinions was undertaken to examine the effects on availability. A further limitation was the use of processed rather than raw data in the Top 150 analysis. Hence, accuracy checks were used to minimize error (recalculations were requested for “outliers” or wrongly noted contract prices). In addition, sensitivity analyses were undertaken for both the Top 150 and HPE analyses to account for uncertainty when subgroup results were extrapolated. The availability surveys had some possible sources of bias. Potential respondents were closely followed up to maximize response rates and reduce bias from missing responses. Nevertheless, bias from inaccurate recall or positive or negative views about PHARMAC could have been incorporated because they are difficult to eliminate. A further limitation was the short time frame of the study (4 years). A longer study may have captured more changes. The generalizability of the study could be limited. PM may be more (or less) acceptable in different countries or settings. The restricted choices with pooled procurement may be less acceptable where prescribers have traditionally had more freedom of choice (e.g., Europe) or may be difficult to organize where there are inconsistent funding streams, shortages of trained staff, or political unrest (the developing world).
Although the PM part of the National Hospital Pharmaceutical Strategy appeared to be successful, further studies should be undertaken to examine whether the low prices are maintained in the longer term and whether “availability” issues increase or decrease.
Conclusion
PM, part of PHARMAC's National Hospital Pharmaceutical Strategy launched in 2002 to manage hospital pharmaceutical expenditure in NZ, was moderately successful. Savings of NZ$8m to NZ$13m (6–8%) per annum were made, and growth in IPE appeared to slow for the year after the launch of the strategy for all types of hospitals, and for some hospitals in subsequent years. Hospital pharmaceutical expenditure appeared to have risen more steeply during the period than CPE. Hospital pharmaceutical expenditure in NZ (and Norway) appeared to grow more slowly than in the UK and Sweden. Some availability problems occurred with items on new contracts under the strategy such as out-of-stocks and products perceived by respondents as inferior. Problems were usually resolved in weeks, but some took more than a year. Further research is needed to ensure that favorable economic effects can be sustained from PM while keeping unfavorable effects on the availability of medicines to a minimum. Similar centralized approaches to managing pharmaceutical expenditure may be worth considering in other countries or regions.
The authors thank the chief pharmacists at NZ's public hospitals for providing data and completing the questionnaires; the community pharmacists that supplied some missing data related to one hospital; PHARMAC for providing data on additional savings/costs; and the Ministry of Health for providing information on bed numbers and helping classify the hospitals. There are no conflicts of interest to declare.
Source of financial support: None.




