Testing the reliability of the JEOL FEGSEM 6500F electron microprobe for quantitative major element analysis of glass shards from rhyolitic tephra
Sarah E. Coulter (e-mail: [email protected]), Jonathan R. Pilcher (e-mail: [email protected]), Valerie A. Hall (e-mail: [email protected]) and Gill Plunkett (e-mail: [email protected]), School of Geography, Archaeology and Palaeoecology, Queen's University Belfast, Belfast BT7 1NN, UK; Siwan M. Davies (e-mail: [email protected]), School of the Environment and Society, Department of Geography, Swansea University, Swansea, UK
Abstract
Coulter, S. E., Pilcher, J. R., Hall, V. A., Plunkett, G. & Davies, S. M. 2009: Testing the reliability of the JEOL FEGSEM 6500F electron microprobe for quantitative major element analysis of glass shards from rhyolitic tephra. Boreas, 10.1111/j.1502-3885.2009.00113.x. ISSN 0300-9483.
Electronprobe microanalysis is now widely adopted in tephra studies as a technique for determining the major element geochemistry of individual glass shards. Accurate geochemical characterization is crucial for enabling robust tephra-based correlations; such information may also be used to link the tephra to a specific source and often to a particular eruption. In this article, we present major element analyses for rhyolitic natural glass standards analysed on three different microprobes and the new JEOL FEGSEM 6500F microprobe at Queen's University Belfast. Despite the scatter in some elements, good comparability is demonstrated among data yielded from this new system, the previous Belfast JEOL-733 Superprobe, the JEOL-8200 Superprobe (Copenhagen) and the existing long-established microprobe facility in Edinburgh. Importantly, our results show that major elements analysed using different microprobes and variable operating conditions allow two high-silica glasses to be discriminated accurately.
Since early work by Thorarinsson (1944) and others, tephra layers have become a valuable tool in Quaternary Science for the correlation and chronological control of widely spread palaeoenvironmental records. The identification of cryptotephras, i.e. those which are invisible to the naked eye (Lowe & Hunt 2001; Gehrels et al. 2006), in terrestrial, marine and ice-core sequences has enabled temporal tie-points, or marker horizons, to be drawn between disparate sites of palaeoenvironmental interest. This has facilitated precise comparison between spatially diverse environments at, for example, times of significant climate change during the late Quaternary (e.g. Turney et al. 2004). Tephrochronology provides a means for testing the spatial synchroneity of environmental change independent of fluctuations in 14C that limit the chronological precision of 14C-dated sequences (e.g. Lowe et al. 1999; van den Bogaard & Schmincke 2002; Plunkett 2006; Newnham et al. 2007), and has progressed the establishment of a chronology of events for the Last Glacial–Holocene period in Europe (Turney et al. 2001; Davies et al. 2002). Recent research on the Greenland ice-cores (Grönvold et al. 1995; Mortensen et al. 2005; Davies et al. 2008) is yielding more precise ages for prehistoric tephra layers using ice-core dating (Andersen et al. 2006; Svensson et al. 2006, 2008; Vinther et al. 2006).
The application of tephrochronology for resolving environmental, climatic and archaeological change is thus extremely powerful. The potential of a tephra layer, however, can only be fully realized if accurate, quantitative, geochemical data are available for the glass shard component, which embodies the chemical signature of the erupted melt (Barker 1983). High-silica tephras usually result from explosive eruptions and siliceous glass shards are less dense than basic shards making it more likely that these, rather than basaltic shards, will be propelled into the upper atmosphere. Explosive basaltic eruptions, facilitated by meltwater in Iceland, are known to have deposited low- silica tephra glass shards offshore (Larsen & Eiríksson 2008). Examples of this are infrequent, but include the widespread Vedde Ash and Saksunarvatn Ash (Birks et al. 1996). It is the rhyolitic component, however, that typically travels the furthest and is widespread. This dispersal pattern is borne out by study of distal tephras in Ireland (Pilcher & Hall 1992), Germany (van den Bogaard & Schmincke 2002) and Scandinavia (Pilcher et al. 2005; Wastegård 2005). The trace- and rare-earth element compositions of glass shards may be assessed, but in most cases major elements are analysed first. Often, an identification is based solely on the latter, and this may usually be sufficient to discriminate between tephras. The widespread application of tephra studies makes it crucial that geochemical analyses are carried out using equipment and protocols that promote consistently reliable data, and care must be taken when selecting a laboratory in which to carry out tephra-derived glass analyses. An earlier investigation, reported by Hunt & Hill (1996), compared the analyses on a Lipari obsidian from seven different European electron microprobe centres. Their work highlighted inter-laboratory discrepancies for this rhyolitic glass great enough to mask potential tephra distinctions, and showed that sodium measurements were the least accurate (Hunt & Hill 1996) – a finding explicit in some earlier papers by Froggatt (1983, 1992). Despite the recommendations of Hunt & Hill (1996), further tephra facility-based data comparisons have not been reported in the literature apart from the work of Potts et al. (2002) on TB-1, a basaltic glass fused and prepared by the U.S. Geological Survey.
The aim of this work is to compare data from four different microprobe facilities; this is the first study of its kind since 2002. Importantly, we assess the capabilities of the JEOL FEGSEM 6500F, at Queen's University Belfast, for generating accurate tephra geochemical data using two rhyolitic glass standards. We hope to demonstrate that the results generated for the JEOL FEGSEM 6500F are comparable not only with those of the microprobe it replaces (JEOL-733 Superprobe), but also those of another major tephra-orientated microprobe, the Cameca SX-100 (NERC Tephrochronology Analytical Unit, University of Edinburgh) and with the JEOL-8200 Superprobe (Copenhagen).
The JEOL FEGSEM 6500F system operates differently from most tephra-analytical facilities, as it uses both energy-dispersive and wavelength-dispersive spectrometry to generate major element data. Data from individual instruments using contrasting spectrometers are often combined, e.g. the wavelength-dispersive JEOL JXA733 Superprobe with energy-dispersive systems; JEOL JXA-5A+Link Systems LZ-5 detector (Schmitz & Smith 2004) and JEOL JXA-84+PGT Prism 2000 (Shane et al. 2003). The reliability of analyses that have been generated using a single instrument operating both types of spectrometer has not been considered in the published literature.
Electron probe microanalysis
Electron probe microanalysis (EPMA) is a common technique for quantifying the major element composition of tephra-derived components, including glass. EPMA can obtain data from glass shards that appear physically homogeneous and unweathered, making it a valuable tool for the characterization of tephras. This technique works by directing a focused beam of electrons onto a sample, generating X-rays. The X-rays produced are of particular energies and wavelengths relating directly to individual elements, and their intensities are a measure of specific element abundance (Hunt & Hill 1993). Two types of analysis are available for the detection and recording of X-ray signals: energy-dispersive spectrometry (EDS) and wavelength-dispersive spectrometry (WDS). Both EDS and WDS systems are widely used in tephra studies (e.g. Grönvold et al. 1995; Dwyer & Mitchell 1997; Davies et al. 2005; Mortensen et al. 2005; Pilcher et al. 2005; Mascarenhas-Pereira et al. 2006).
EDS analysis has been favoured for its speed. All of the X-ray energies produced from a sample are measured simultaneously. The X-ray photons are converted into photoelectrons by the detector; these form a charge pulse which is translated into voltage by a preamplifier and relates to the energy of the analysed element (Goldstein et al. 2003: pp. 301–302). Alternatively, WDS uses crystals to diffract the X-rays generated by the sample, and these in turn are counted in a gas-filled chamber (Goldstein et al. 2003: p. 324). It has been demonstrated that, during analysis, the peak count rate of alkali metals, sodium and potassium, falls away due to mobilization of the elements, and this results in an over-representation of the other elements present (Froggatt 1992; Hunt & Hill 2001). These factors have been related to the amount of time the sample is exposed to the beam as well as the beam diameter and strength (Hunt & Hill 1993, 2001), and can be minimized through analysing the alkali metals first or using a ‘cold finger’ (which reduces sample temperature) (Froggatt 1992). When using EDS, a balance must be sought between accuracy and precision: analysis using a narrow beam and a longer count rate reduces output accuracy because of sodium ‘mobility’, but produces a more precise count. In contrast, by increasing beam diameter and reducing exposure time, precision is lost (Hunt & Hill 2001). The alternative method, WDS, measures individual wavelengths under a higher beam current, increasing the resolution of data acquired. It has the capacity to monitor sodium loss during longer count rates. In this case, a narrower beam may be employed (promoting accuracy), while the drawback of a longer count rate (alkali mobility) may be accounted for and a correction factor applied. Integral ZAF (Sweatman & Long 1969) and PAP (Pouchou & Pichoir 1991) matrix correction algorithms are used to resolve differences between X-rays generated in the sample and in the standard. The algorithms also correct for absorbance and fluorescence, which relate to X-ray beam intensity generated in the sample and X-rays produced by atoms other than the element which is being measured, respectively.
JEOL FEGSEM 6500F
The JEOL FEGSEM 6500F at Queen's University Belfast comprises a combined EDS and WDS system consisting of a JEOL 6500F field emission SEM with Oxford Instruments INCA energy/wave spectrometers. The integrated field emission gun maintains exceptional beam stability under a high vacuum. A further advantage of this system is that the most abundant elements can be measured quickly and accurately through EDS, while simultaneous analyses of less abundant, or volatile, elements selected for sequential WDS are performed. In contrast to the usual analytical procedures, this electron microprobe is set up to measure Na, Mg, Mn and Ti through WDS, and the remaining elements through EDS. All elements, apart from Na, are counted with a 20 s peak and 10 s background. Because of its mobility, count times for Na are reduced to 10 s peak and 1 s background and this element is measured first, further reducing Na loss. Each live analysis lasts 120 s. Taking into account EDS deadtime (percentage of time that the system is not available to accept pulses being processed from the X-ray detector), the total time for an analytical run is approximately 160 s. The accelerating voltage (15 kV) and raster beam current (5 nA) were kept consistent. Magnification, which determines the size of the analytical area, can be altered depending on the size and thickness of the exposed glass shard. Only analyses derived using × 6000 magnification (∼7 × 9 μm) are presented here; however, previous work has shown that Na remains stable up to × 20 000. Nine major elements are measured routinely, each initially calibrated against a corresponding mineral standard.
Methods
Two high-silica natural glass standards, A-ALK (alkaline rhyolitic obsidian) and A-THO (tholeiitic obsidian), were provided by Dr Karl Grönvold (University of Iceland) for the standardization of ice-core tephra analyses (e.g. Mortensen et al. 2005). Single analyses on these standards had previously been generated using XRF at the University of Iceland by Karl Grönvold. Relatively large samples (∼500 μm2–1000μm2) of A-ALK and A-THO were mounted in Araldite, ground and polished. Slides were carbon-coated and then thin lines of carbon tape or dag were applied across the resin and holder to prevent the sample from electrostatically charging during analysis. Glass standard analyses on the new JEOL FEGSEM 6500F were carried out over two separate sessions in July 2007 and January 2008. The Belfast JEOL-733 Superprobe, Edinburgh Cameca SX-100 and Copenhagen JEOL-8200 Superprobe are dedicated WD systems. The operating conditions for all four electron microprobes are listed in Table 1. Both the Belfast JEOL-733 Superprobe and the JEOL FEGSEM 6500F were run under consistent operating conditions. This procedure is in contrast to that used for the Cameca SX-100 at Edinburgh, where different operators have experimented with various set-ups over the past four years. It is important that the results generated from different facilities and under dissimilar operating conditions are shown to be consistent, as tephra-derived glass analysis data sets are often compared in isolation of these factors. Therefore, we have presented all analyses that we carried out on the Edinburgh microprobe that reflect the range of routine conditions under which it has been run in recent years (Table 1A–C). Furthermore, no attempt has been made to standardize the operating conditions of the other three microprobes, which have been run at their optimum set-up. A set of mineral standards was used to calibrate major element measurements. These standards vary for each microprobe. At the start of each session, analyses of an in-house glass standard were carried out to test the accuracy and consistency of element measurements. In both Edinburgh and Belfast the same natural volcanic glass standard, Lipari, derived from glassy lava flows in the Aeolian Islands, was used. Any microcrystalline inclusions are generally visible and the composition includes all of the major elements commonly found in tephra-derived glass shards, making Lipari a suitable standard (Hunt & Hill 1996, 2001). These analyses provide an additional test for comparability and are therefore presented alongside the A-ALK and A-THO data.
| Acceleratingvoltage (kV) | Beamcurrent (nA) | Beamdiameter (μm) | Correction | No. of analyses | |||
|---|---|---|---|---|---|---|---|
| Lipari | A-ALK | A-THO | |||||
| JEOL FEGSEM 6500F | 15 | 5 | 9 (raster) | ZAF | 24 | 31 | 20 |
| JEOL-733 | 15 | 10 | 8 (defocused) | ZAF | 45 | 15 | 35 |
| Cameca SX-100 | |||||||
| A | 20 | 4 | 10 (raster) | PAP | 21 | 55 | 47 |
| B | 10 | 10 | 5 | PAP | 41 | 10 | 9 |
| C | 15 | 5 | 5 | PAP | 15 | 7 | 9 |
| JEOL-8200 | 15 | 15 | 8 (defocused) | ZAF | 0 | 21 | 22 |
Results and discussion
Analyses were performed using the electron microprobes and the operating conditions listed in Table 1, means and standard deviations are illustrated in Fig. 1, and a summary of these is presented in Table 2. Multiple analyses were performed on a single Lipari shard, five A-ALK and five A-THO shards (Table 1). By investigating two glass standards of similar silica composition, we can assess rigorously the performance of the electron microprobes in distinguishing tephras using relatively minor and/or more volatile elements: CaO, Na2O and K2O.
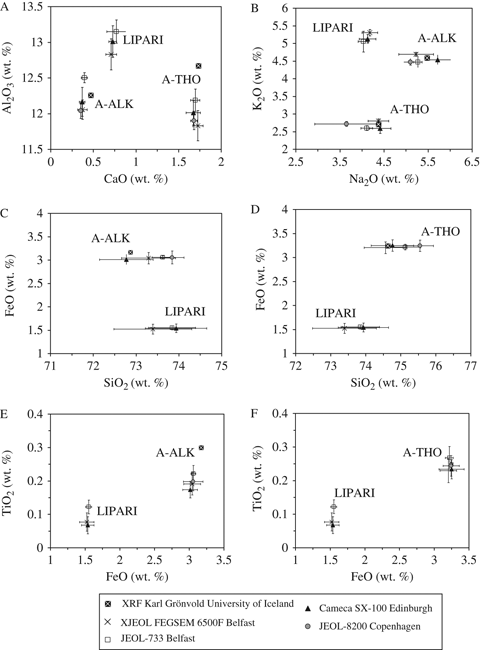
Biplots of (A) CaO–Al2O3; (B) Na2O–K2O; (C & D) SiO2–FeO; and (E & F) FeO–TiO2 for glass standards Lipari, A-ALK and A-THO. Total Fe expressed as FeO. The results are un-normalized.
| SiO2 | Al2O3 | TiO2 | FeO* | MnO | MgO | CaO | Na2O | K2O | Total | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipari | |||||||||||
| JEOL FEGSEM 6500F | 73.39 | 12.83 | 0.08 | 1.52 | 0.08 | 0.04 | 0.71 | 4.18 | 5.31 | 98.14 | 24 |
| (0.91) | (0.22) | (0.03) | (0.10) | (0.04) | (0.02) | (0.06) | (0.18) | (0.09) | (1.24) | ||
| JEOL-733 | 73.84 | 13.15 | 0.12 | 1.55 | 0.06 | 0.77 | 4.03 | 5.05 | 98.57 | 45 | |
| (0.55) | (0.16) | (0.02) | (0.04) | (0.02) | (0.11) | (0.12) | (0.30) | (0.60) | |||
| Cameca SX-100+ | |||||||||||
| A | 74.36 | 13.01 | 0.06 | 1.56 | 0.08 | 0.05 | 0.72 | 4.41 | 5.08 | 99.31 | 21 |
| (0.32) | (0.20) | (0.01) | (0.09) | (0.03) | (0.01) | (0.03) | (0.07) | (0.11) | (0.46) | ||
| B | 73.49 | 12.97 | 0.07 | 1.54 | 0.07 | 0.04 | 0.73 | 4.03 | 5.17 | 98.18 | 41 |
| (0.68) | (0.24) | (0.03) | (0.10) | (0.07) | (0.01) | (0.05) | (0.10) | (0.11) | (0.82) | ||
| C | 74.53 | 13.12 | 0.08 | 1.52 | 0.05 | 0.04 | 0.72 | 4.00 | 5.09 | 99.18 | 16 |
| (0.32) | (0.17) | (0.02) | (0.07) | (0.07) | (0.01) | (0.06) | (0.07) | (0.14) | (0.42) | ||
| JEOL 8200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 |
| A-ALK | |||||||||||
| XRF | 72.87 | 12.26 | 0.30 | 3.17 | 0.07 | 0.07 | 0.47 | 5.48 | 4.59 | 99.29 | |
| JEOL FEGSEM 6500F | 73.29 | 12.06 | 0.19 | 3.04 | 0.07 | 0.05 | 0.37 | 5.23 | 4.69 | 99.00 | 31 |
| (0.63) | (0.14) | (0.03) | (0.12) | (0.07) | (0.02) | (0.06) | (0.39) | (0.06) | (0.66) | ||
| JEOL-733 | 73.62 | 12.50 | 0.22 | 3.06 | 0.06 | 0.39 | 5.27 | 4.47 | 99.60 | 15 | |
| (0.25) | (0.07) | (0.02) | (0.03) | (0.01) | (0.04) | (0.10) | (0.14) | (0.27) | |||
| Cameca SX-100+ | |||||||||||
| A | 72.66 | 12.12 | 0.17 | 3.00 | 0.08 | 0.06 | 0.36 | 5.86 | 4.54 | 98.85 | 55 |
| (0.63) | (0.18) | (0.02) | (0.10) | (0.04) | (0.02) | (0.03) | (0.17) | (0.13) | (0.78) | ||
| B | 73.07 | 12.24 | 0.17 | 3.05 | 0.11 | 0.04 | 0.38 | 5.24 | 4.58 | 98.89 | 10 |
| (0.42) | (0.13) | (0.03) | (0.13) | (0.09) | (0.01) | (0.03) | (0.05) | (0.09) | (0.48) | ||
| C | 73.25 | 12.42 | 0.19 | 3.03 | 0.05 | 0.04 | 0.38 | 5.32 | 4.48 | 99.20 | 7 |
| (0.58) | (0.22) | (0.01) | (0.10) | (0.05) | (0.02) | (0.06) | (0.03) | (0.16) | (0.78) | ||
| JEOL 8200 | 73.84 | 12.04 | 0.20 | 3.06 | 0.08 | 0.04 | 0.35 | 5.10 | 4.48 | 99.20 | 21 |
| (0.28) | (0.11) | (0.03) | (0.14) | (0.05) | (0.01) | (0.02) | (0.14) | (0.09) | (0.44) | ||
| A-THO | |||||||||||
| XRF | 74.63 | 12.67 | 0.25 | 3.24 | 0.10 | 0.11 | 1.73 | 4.37 | 2.71 | 99.86 | |
| JEOL FEGSEM 6500F | 74.56 | 11.83 | 0.23 | 3.20 | 0.07 | 0.08 | 1.73 | 4.37 | 2.80 | 98.89 | 20 |
| (0.61) | (0.21) | (0.04) | (0.12) | (0.06) | (0.02) | (0.06) | (0.23) | (0.06) | (0.77) | ||
| JEOL-733 | 75.12 | 12.19 | 0.27 | 3.22 | 0.10 | 1.70 | 4.11 | 2.60 | 99.30 | 35 | |
| (0.44) | (0.16) | (0.03) | (0.06) | (0.01) | (0.05) | (0.13) | (0.07) | (0.61) | |||
| Cameca SX-100+ | |||||||||||
| A | 74.63 | 11.98 | 0.23 | 3.24 | 0.12 | 0.10 | 1.67 | 4.55 | 2.53 | 99.09 | 47 |
| (0.40) | (0.16) | (0.02) | (0.20) | (0.04) | (0.02) | (0.08) | (0.09) | (0.10) | (0.46) | ||
| B | 74.83 | 12.03 | 0.27 | 3.35 | 0.11 | 0.09 | 1.71 | 4.02 | 2.79 | 99.27 | 9 |
| (0.50) | (0.14) | (0.04) | (0.11) | (0.09) | (0.01) | (0.05) | (0.06) | (0.07) | (0.52) | ||
| C | 75.42 | 12.17 | 0.24 | 3.19 | 0.09 | 0.08 | 1.65 | 4.08 | 2.67 | 99.62 | 9 |
| (0.29) | (0.16) | (0.02) | (0.10) | (0.03) | (0.01) | (0.11) | (0.07) | (0.10) | (0.34) | ||
| JEOL 8200 | 75.55 | 11.90 | 0.24 | 3.24 | 0.09 | 0.08 | 1.68 | 3.65 | 2.72 | 99.17 | 22 |
| (0.39) | (0.12) | (0.03) | (0.12) | (0.04) | (0.02) | (0.04) | (0.72) | (0.07) | (0.63) | ||
- n=Number of analyses.
- * Total Fe expressed as FeO.
- + A=20 kV/4 nA/10 μm; B=10 kV/10 nA/5 μm; C=15 kV/5 nA/5 μm.
Seven of the nine major elements analysed are compared in Fig. 1 (MgO and MnO concentrations were negligible and are therefore not illustrated). A moderate degree of scatter exists for each element within all sets: Lipari, A-ALK and A-THO. The XRF results represent single bulk analyses and their significantly higher measurements for Al2O3 and TiO2 for A-THO and A-ALK, respectively, suggest minor heterogeneity in these samples (Fig. 1A, E). All three samples are natural glasses and therefore it is reasonable to expect some degree of geochemical heterogeneity; however, this natural variation is insufficient to blur their distinction from each other. The surface area of these glasses is relatively large and measurements were taken at random points. Slight heterogeneity in the samples probably accounts for some of the analytical variability demonstrated by each of the four microprobes; however, clear trends in the data sets indicate that there is also probe variability. The most significant scatter is shown in values for SiO2, Al2O3 and Na2O, similar to the findings of Hunt & Hill (1996)– each microprobe records variability in these elements. Hunt & Hill (1996) recorded the differences between the maximum and minimum values for each element generated by the seven laboratories and these ranged from 0.89% to 11.3% for SiO2, from 0.34% to 12.33% for Na2O and from 0.33% to 2.7% for Al2O3. Measurements from the JEOL-733 Superprobe indicate a bias toward higher Al2O3 and TiO2 concentrations, but individual analyses are usually within the range recorded by the Cameca SX-100 (Fig. 1A) and the JEOL-8200 (Fig. 1E, F). Similarly, a bias towards increased SiO2 is identified in the JEOL-8200 and JEOL-733 Superprobe results. The JEOL FEGSEM 6500F and Cameca SX-100 define a greater, yet consistent, spread in measurements of this element (Fig. 1C, D), totalling 2.23% (A-ALK), 2.18% (A-THO) and 2.14% (A-ALK), 1.96% (A-THO), respectively. The JEOL FEGSEM 6500F demonstrates less variability in values of K2O and these tend to be at the higher end of the compositional envelopes for Lipari, A-ALK and A-THO (Fig. 1B).
It was expected that measurements of sodium would show greatest variation given the mobility of this element, and this is confirmed by the results (Fig. 1B). Results from the JEOL FEGSEM 6500F show variability of <1% for Na2O, indicating better precision than for most of the laboratories reported in Hunt & Hill (1996). The Cameca SX-100 Na2O measurements cluster more at the higher end of the compositional envelopes. We believe this higher estimation to be a consequence of the higher accelerating voltage and lower beam current conditions used to generate the data set (Table 1, condition A; Table 2), which has also resulted in lower mean SiO2 values (Table 2). This interpretation is based on the theory that Na2O loss results in a relative increase in SiO2 (e.g. Hunt and Hill 2001), and hence stable Na2O measurements should create no relative increase in SiO2. The results for the Cameca SX-100 set-up A contrasts with the output from set-up C where both the operating conditions and the corresponding measurements for Na2O are in line with those of the other three microprobes (Table 2).
Glass standards A-THO and A-ALK can be distinguished clearly through CaO and K2O analyses, and reasonably well through Na2O measurements, by all four electron microprobes. This is encouraging, because earlier inter-laboratory comparisons highlighted discrepancies which were significant enough to hamper the discrimination of similar glasses (Hunt & Hill 1996). JEOL FEGSEM 6500F results fall within the natural and analytical variability reflected in the output from the other three facilities. Using the method described in Borchardt et al. (1972), similarity coefficients were calculated to test the comparability of the four facilities. A result of 0.95–1.00 is indicative of a correlation. The similarity coefficients recorded in Table 3 for analyses on A-ALK and A-THO are all >0.95, demonstrating good comparability between the results from all four electron microprobes.
| JEOLFEGSEM6500F | JEOL-733 | CamecaSX-100 | JEOL8200 | |
|---|---|---|---|---|
| A | ||||
| JEOL FEGSEM 6500F | 1 | |||
| JEOL-733 | 0.97 | 1 | ||
| Cameca SX-100 | 0.98 | 0.97 | 1 | |
| JEOL 8200 | 0.98 | 0.97 | 0.96 | 1 |
| B | ||||
| JEOL FEGSEM 6500F | 1 | |||
| JEOL-733 | 0.97 | 1 | ||
| Cameca SX-100 | 0.97 | 0.98 | 1 | |
| JEOL 8200 | 0.96 | 0.97 | 0.96 | 1 |
Conclusions
By using natural glass standards which are subject to some heterogeneity we have compared the accuracy and precision of the electron microprobes based on real conditions. The results show that the JEOL FEGSEM 6500F, as well as the other three microprobes in this study, clearly enables the glass standards A-ALK and A-THO to be distinguished. Importantly, this new equipment has produced analyses that are directly comparable with those generated by three other well-established systems (Table 3), demonstrating that it is reliable. We acknowledge that analytical precision could be tested further through the analyses of homogeneous synthetic glasses and we recommend that a new inter-laboratory test be initiated to include the JEOL FEGSEM 6500F, JEOL-8200 Superprobe, Cameca SX-100 and other European tephra-analytical centres that use EPMA.
Acknowledgments
Acknowledgements. – This work is part of a Chrono funded project at Queen's University Belfast. Many thanks to the following people responsible for running the electron microprobes and who helped to generate much of the data presented in this article: Stephen McFarland (Belfast), Anthony Newton and David Steele (Edinburgh), Berit Wenzell (Copenhagen). We gratefully acknowledge NERC for supportng our use of the electron microprobe facility at the Tephrochronology Analytical Unit, University of Edinburgh. The analyses undertaken in Copenhagen were part of the Copenhagen Ice Core Dating Initiative, which was supported by a grant from the Carlsberg Foundation. We are grateful to Karl Grönvold for supplying the glass standards A-ALK and A-THO and for carrying out the XRF analyses. We thank the reviewers, Stefan Wastegård and David Lowe, for their useful comments on an earlier draft.




