Life-history traits related to diapause in univoltine and bivoltine populations of Ypthima multistriata (Lepidoptera: Satyridae) inhabiting similar latitudes
Abstract
In most temperate insects, diapause strategies and voltinism generally exhibit latitudinal clines, supporting the concept that they represent adaptations to climate. In contrast, in the satyrine butterfly Ypthima multistriata Butler, local populations with different voltinism patterns are geographically intermingled, suggesting that life-history traits related to diapause may differ even between geographically and phylogenetically close populations. In this study, we experimentally examined the critical photoperiod for diapause induction and the larval developmental period in two univoltine and two bivoltine populations of Y. multistriata, all of which inhabit virtually the same latitude (34.652–34.750°N). We found that the critical photoperiod for diapause induction was longer in the univoltine populations than in the bivoltine populations. Moreover, the larval period under the long day length treatment was different among populations in both sexes, although significant differences were also detected between populations with the same voltinism. These results indicate that in Y. multistriata, life-history traits related to diapause can not be attributed merely to climatic conditions such as temperature or day length, which depend largely on latitude. Therefore, we suggest that biotic elements, such as leaf toughness, as well as abiotic elements should be taken into account in attempts to explain the enigmatic pattern of geographic variation in the diapause strategies of Y. multistriata.
INTRODUCTION
Most insects have evolved integrated responses that regulate ecologically important phenotypic characters to suit temporally and spatially heterogeneous environments. The life histories of insects living in temperate climates are shaped by the seasonality of abiotic and biotic environmental elements, because only a portion of the year is suitable for growth and reproduction (Tauber et al. 1986). Because the period available for growth and reproduction of insects varies with latitude, life-history traits that are closely related to diapause strategy, such as photoperiodic response, diapause intensity and developmental rate, generally show geographical clines (Danilevskii 1965; Bradshaw 1976; Kidokoro & Masaki 1978; Ishihara & Shimada 1999; Masaki 1999; Ishihara 2000). Moreover, in widely distributed species, voltinism also shows a geographic cline as a result of local adaptation: the lower the latitude the greater the number of generations produced per year (Mousseau & Roff 1989; Gomi & Takeda 1990; Ishihara 1998). Thus, it is generally accepted that geographic variation in life-history traits related to diapause and voltinism is a consequence of climatic adaptations.
Unlike most temperate insect species, the satyrine butterfly Ypthima multistriata Butler shows enigmatic variations in voltinism that are difficult to explain simply by climatic adaptations (Fukuda et al. 1984; Takei 2009a; Noriyuki et al. 2010). In western Japan, the number of Y. multistriata generations each year ranges from one with adult emergence in June or midsummer, to two with adults emerging in June and September, to several with adults emerging intermittently from May to October (Fukuda et al. 1984). The number of generations does not show latitudinal variation, however, and local populations with a particular voltinism pattern are not always distributed adjacently (Noriyuki et al. 2010). Such non-clinal geographic variation in voltinism implies that life-history traits related to diapause may differ even between geographically close populations. Specifically, it is hypothesized that even in areas of similar day length and temperature, both the critical photoperiod and the larval developmental period may be longer in univoltine populations than in bivoltine populations. This hypothesis is in marked contrast to the conventional view of climatic adaptation according to which the critical photoperiod varies regularly with latitude for a wide variety of insects. Therefore, the importance of climatic conditions in the life-history evolution of Y. multistriata can be evaluated by comparing populations inhabiting similar latitude but having different voltinism.
In addition, by examining geographically close populations with diverse voltinism patterns, the evolutionary lability of these life-history traits can be evaluated. The evidence for phylogenetic constraints on the adaptation of insect seasonality is so far surprisingly mixed (Bradshaw & Holzapfel 2006; Agrawal & Stinchcombe 2009; Forrest & Miller-Rushing 2010); thus, the importance of such constraints is still controversial. Some authors have reported that genetic variations in critical photoperiod are maintained to some extent, and that adaptive genetic change can therefore take place in response to environmental fluctuation (Bradshaw & Holzapfel 2001; Ozaki & Ohbayashi 2001; van Asch et al. 2007). In contrast, other authors have emphasized that the evolutionary lability of insect seasonality may be limited because life-history traits are subject to certain unavoidable constraints (Etterson & Shaw 2001; Singer & Parmesan 2010). In Y. multistriata, a previous phylogenetic analysis (Noriyuki et al. 2010) revealed that voltinism has diverged between even geographically and phylogenetically close populations, suggesting that geographic patterns of voltinism might have evolved mainly in response to the specific ecological selective regime experienced by each population. Therefore, we can eliminate phylogenetic effects on the evolution of life-history traits related to voltinism in these populations of Y. multistriata.
In this study, we experimentally examined the critical photoperiod and larval developmental period in two univoltine and two bivoltine populations of Y. multistriata. We chose three populations on the Ieshima Islands, which, despite their close geographical proximity, comprise two univoltine and one bivoltine population (Fig. 1). We also chose a second bivoltine population in Kyoto, which is at similar latitude to the Ieshima Island populations (Table 1). We then discuss in this paper the evolutionary lability of life-history traits of Y. multistriata and the factors other than climatic conditions that might have affected diapause strategies in the species.
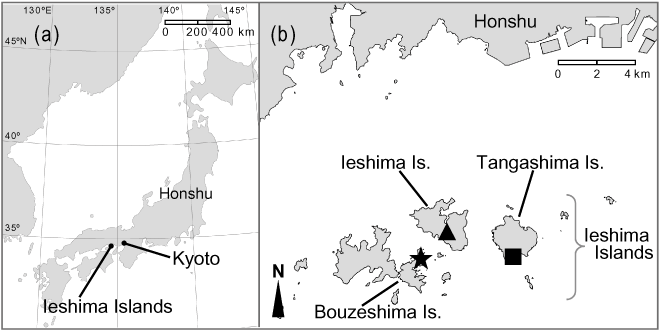
Sampling localities of Ypthima multistriata in western Japan. (A) All populations are located at nearly the same latitude, and the bivoltine population at Kyoto is about 110 km east of the Ieshima Islands. (b) Sampling localities in the Ieshima Islands, southern Hyogo Prefecture. The Tangashima ( ) and Bouzeshima populations (★) are univoltine, whereas the Ieshima population (▴) is bivoltine. In southern Hyogo Prefecture, some univoltine populations also occur on the mainland (Honshu).
) and Bouzeshima populations (★) are univoltine, whereas the Ieshima population (▴) is bivoltine. In southern Hyogo Prefecture, some univoltine populations also occur on the mainland (Honshu).
| Locality | Latitude (°N) | Longitude (°E) | Voltinism | Annual mean temperature (°C) | Annual precipitation (mm) | No. of females captured | No. of reproductive females |
|---|---|---|---|---|---|---|---|
| Kyoto | 34.750 | 135.819 | Bivoltine | 14.6 | 1394.5 | 12 | 11 |
| Ieshima Is. | 34.667 | 134.526 | Bivoltine | 15.3 | 1084.3 | 2 | 2 |
| Tangashima Is. | 34.657 | 134.576 | Univoltine | 15.3 | 1095.7 | 12 | 9 |
| Bouzeshima Is. | 34.652 | 134.512 | Univoltine | 15.4 | 1082.6 | 12 | 12 |
- † Climate data are from Mesh Climate Data 2000, distributed by the Japan Meteorological Agency (2002).
MATERIALS AND METHODS
Study species
Ypthima multistriata is distributed widely in temperate areas from China to western Japan, although throughout the distribution range in Japan each population is small and highly localized (Fukuda et al. 1984; Ministry of the Environment 2006). In nature, larvae are thought to feed on several species of Poaceae and Cyperaceae, because they show a feeding preference for these grasses in the laboratory (Nihira 2004). Larvae enter diapause and overwinter at the third instar (Fukuda et al. 1984). In western Japan, four types of voltinism are observed in Y. multistriata (Fukuda et al. 1984; Takei 2009a; Noriyuki et al. 2010): univoltine with adult emergence in June or midsummer, bivoltine and multivoltine. These different voltinism patterns in Y. multistriata are considered to be fairly discrete because of the highly localized distribution of this species in Japan. Bivoltine populations of Y. multistriata occur more frequently throughout its distribution range in Japan than populations with other voltinisms (Noriyuki et al. 2010). Univoltine populations with adult emergence in June occur only in southern Hyogo and Okayama Prefectures, whereas univoltine populations with adult emergence in midsummer (from mid-June to early August) are scattered from the eastern to the southern limit of the species' distribution range in Japan (Takei 2009a,b). No adult butterflies have ever been collected from univoltine populations except in June or midsummer despite intensive sampling efforts (Takei 2004; Togari 2004). On Tsushima Island there are multivoltine populations in which adults emerge intermittently from May to October (Fukuda et al. 1984).
The butterflies used for our experiment were from four sites (four populations) in the Kinki district, western Japan: Kizugawa city in Kyoto Prefecture (Kyoto), and Ieshima (Ieshima), Tangashima (Tanga) and Bouzeshima (Bouze) Islands. The Kyoto and Ieshima populations are bivoltine, and the Tanga and Bouze populations are univoltine with adult emergence in June (Fig. 1). All populations are located at nearly the same latitude, although the Kyoto population is about 110 km from the other three populations (Table 1). Because all populations are at altitudes of less than 50 m, altitude effects on their life-history traits should be negligible. Annual mean temperature and precipitation at each sampling locality are shown in Table 1.
Experimental design
The critical photoperiod and the larval period were examined by the following experimental procedure. Female butterflies were collected on 18 June 2009 at Ieshima and Bouze, on 19 June 2009 at Kyoto and on 20 June 2009 at Tanga. Twelve females were collected from each of these three populations and then transported individually in plastic jars (200 mL), which were carried in an insulated box with frozen gel packs. Only two females, however, were collected from the Ieshima population because of the small size of that population. In the laboratory, each collected female was kept in a plastic jar (200 mL) covered with a piece of polyethylene netting and held at 25°C under a 16 h light : 8 h dark (LD 16:8) regime for several days. Each butterfly was provided a small wad of tissue paper saturated with a sports drink at 50% concentration (Pocari Sweat, Otsuka Pharmaceutical Group, Tokyo, Japan) on which to feed. No host plant material was added to the jars because the female butterflies readily laid their eggs on the netting or on the plastic walls of the jar. We regarded females that produced at least 30 eggs under these conditions as reproductive females, and then used larvae from those butterflies for our experiments. The eggs from each mother were kept in a Petri dish (9 cm in diameter, 3 cm high) under the same standard conditions as described above until eclosion. To examine larval photoperiodic responses, approximately ten newly hatched larvae from each mother were transferred to individual plastic jars (120 mL) and kept at 25°C under three different photoperiods (i.e. 10 larvae × 3 photoperiods = 30 larvae from each mother): LD 13:11 (short day length), LD 14.5:9.5 (intermediate day length) and LD 16:8 (long day length). However, approximately five hatched larvae from each mother of the Tanga and Bouze populations were used in the short and intermediate day length treatments, because our preliminary experiment indicated that all individuals from univoltine populations entered diapause under these conditions. As food for the larvae, a few fresh leaves of Oplismenus undulatifolius Roemer et Schultes, collected in the Botanical Garden of Kyoto University (135°47′E, 35°02′N), were provided every second day. Several studies have demonstrated that the leaf toughness of grasses is critical to larval performance in grass-feeding butterflies, including Satyridae (Nakasuji & Kimura 1984; Braby 1994; Seko & Nakasuji 2006). Specifically, larval performance is higher in grass species with soft leaves than in those with tough leaves. In regard to this point, O. undulatifolius leaves are softer than leaves of other Poaceae species (S. Noriyuki, unpubl. data 2007). Thus, O. undulatifolius can be regarded as suitable food for larvae from any population of Y. multistriata. Indeed, Nihira (2004) reported that O. undulatifolius is suitable for the development of butterflies of genus Ypthima as well as for many other Japanese satyrids. Third instar larvae that had not fed on any O. undulatifolius leaves for more than 15 days were considered to have entered diapause. As expected, no individuals of any population under the long day length treatment, and all individuals of all populations under the short day length treatment, entered diapause (Fig. 2), so the proportion of individuals that entered diapause under the intermediate day length treatment was compared among populations. Because the proportion of diapaused individuals from the same mother was not statistically independent, we used nested logistic regression analysis for mothers within populations to compare the proportion of diapaused individuals among populations. Similarly, the larval period in each sex (the number of days required from hatch to pupation) under the long day length treatment was compared among populations using nested analysis of variance (nested anova) for mothers within populations. All statistical analyses were conducted using IMP Discovery Software (SAS Institute Inc., Cary, NC, USA).
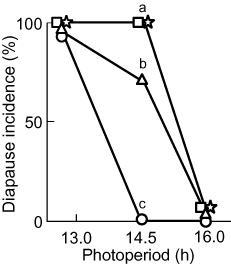
Diapause induction in four populations of Ypthima multistriata at 25°C in relation to photoperiod. Bivoltine populations, Kyoto (○) and Ieshima (▵); univoltine populations, Tanga (□) and Bouze (⋆). Note that the photoperiod response was identical in the Tanga and Bouze populations. Different letters indicate significant differences among populations (nested logistic regression analysis with Bonferroni adjustment, P < 0.05). Sample sizes under the short, intermediate and long day length treatments were, respectively, 98, 26 and 76 in the Kyoto population; 5, 17 and 18 in the Ieshima population; 16, 18 and 70 in the Tanga population; and 28, 21 and 64 in the Bouze population.
RESULTS
The proportion of individuals that entered diapause was significantly different among populations under the intermediate day length (nested logistic regression analysis with Bonferroni adjustment, whole model: d.f. = 32, χ2 = 88.615, P < 0.001; population: d.f. = 3, χ2 = 77.027, P < 0.001; mother (population): d.f. = 29,χ2 = 0.016, P = 1.000; Fig. 2). Under this treatment, all individuals from the univoltine populations (Tanga and Bouze) entered diapause, whereas approximately 65% of individuals from the Ieshima population and no individuals from the Kyoto population entered diapause. In all populations, no individuals under the long day length but all individuals under the short day length entered diapause (Fig. 2).
Population was a significant factor affecting the duration of the larval period under the long day length in both sexes (nested anova, Table 2). The effect of mothers within each population was also significant in males, but not in females (Table 2). Significant differences were also detected between populations with the same voltinism (nested anova after Bonferroni adjustment, P < 0.05; Fig. 3). Specifically, the larval period was longer in the Ieshima population than in the Kyoto population in males, and it was longer in the Tanga population than in the Bouze population in both sexes (Fig. 3).
| Sex | Source | d.f. | SS | MS | F | P |
|---|---|---|---|---|---|---|
| Male | Population | 3 | 11 823.09 | 3941.03 | 13.77 | 0.0001 |
| Mother (population) | 28 | 8 012.70 | 286.17 | 1.91 | 0.0110 | |
| Residual | 93 | 13 900.02 | 149.46 | |||
| Female | Population | 3 | 9 463.70 | 3154.57 | 8.72 | 0.0003 |
| Mother (population) | 28 | 10 124.42 | 361.59 | 1.34 | 0.1625 | |
| Residual | 72 | 19 458.45 | 270.26 |
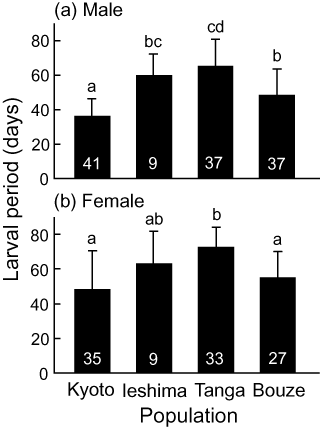
Larval developmental period (mean ± SD) of (A) males and (B) females in four populations of Ypthima multistriata at 25°C under the long day length (16 h light : 8 h dark) regime. The sample size of each treatment is shown on each bar. Different letters indicate significant differences among populations (nested anova with Bonferroni adjustment, P < 0.05).
DISCUSSION
Most studies of insect seasonality have focused on geographical variation, along latitudinal and altitudinal clines, in the critical photoperiod and voltinism, supporting the interpretation of climatic adaptations (Danilevskii 1965; Bradshaw 1976; Masaki 1999). In contrast, our results demonstrated that even among populations distributed at similar latitudes, life-history traits related to diapause differed considerably in Y. multistriata. Temperature and precipitation as well as day length are virtually the same for all three populations in the Ieshima Islands (Table 1), suggesting that climatic adaptations, conventionally considered the key driver of the evolution of insect seasonality, can not account for life-history traits related to diapause strategy in Y. multistriata.
The phylogenetic analysis results (Noriyuki et al. 2010) strongly suggest that geographic variation in voltinism in Y. multistriata evolved independently and rapidly in response to the specific ecological selective regime encountered by each population. Accordingly, we suggest that the observed geographic variation in life-history traits also evolved flexibly. Our inference that life-history traits related to diapause are evolutionarily labile is supported by the fact that genetic shifts in the critical photoperiod of the pitcher-plant mosquito Wyeomyia smithii Coquillett have occurred over as short a time span as 5 years in response to global warming (Bradshaw & Holzapfel 2001).
Natural selection is predicted to favor a longer critical photoperiod in a univoltine population than in a bivoltine population at a similar latitude and altitude, to preclude production of a maladapted generation that might have difficulty reaching the diapause stage before winter (Tauber et al. 1986; Danks 1987). Our results in Y. multistriata are consistent with this prediction (Fig. 2). Even though the larval period in the Bouze population was relatively short under the long day length (Fig. 3), all individuals from the Bouze and Tanga populations entered diapause under the intermediate day length (Fig. 2), thus allowing a life cycle with one generation per year to be maintained in the Bouze and Tanga populations under natural conditions.
It is unclear why the larval period under the long day length was shorter in the Bouze population than in the Tanga population (Fig. 3). Even in populations with the same voltinism, developmental time should be shorter when the period available for growth is relatively limited in a year. Thus, it is possible that variation in the duration of the favorable period for larval growth might have enhanced the variation of the developmental period between the Bouze and Tanga populations, although seasonal fluctuation in the quality and quantity of food sources in Y. multistriata has not been examined. Moreover, developmental time is usually affected by temperature (Tauber et al. 1986). Therefore, the interactive effects of temperature and photoperiod on the larval developmental time should be examined to infer the pattern under field conditions more accurately. Despite these possibilities, other adaptive forces might also have affected the larval period duration in each of these populations, but further field studies are needed to generate a hypothesis that can explain the significant difference in the duration of the larval period between the two univoltine populations.
In contrast to univoltine populations, in bivoltine populations natural selection should favor both a shorter critical photoperiod and a shorter larval period in order to ensure the production of the complete next generation. In fact, the critical photoperiod was shorter in the bivoltine populations than in the univoltine populations (Fig. 2). However, a significant difference in the critical photoperiod was also detected between the bivoltine Ieshima and Kyoto populations (Fig. 2). Moreover, the larval period was not shorter in the Ieshima population than in the two univoltine populations in either sex (Fig. 3). From these results, we speculate that some individuals in the Ieshima population may have only one brood within a season; that is, there may be voltinism polymorphism within the population. It would be difficult to confirm the presence of voltinism polymorphism in the Ieshima population in the field because of the small population size. However, a further examination of the diapause polymorphism under an intermediate day length might elucidate this possibility.
It is not certain which ecological factors might account for the observed geographic distribution of life-history traits. Rather than abiotic factors such as temperature, host-plant quality might be partially responsible for the variation in diapause strategies among neighboring populations in the Ieshima Islands and between them and the Kyoto population, all of which are at approximately the same latitude. Consistent with this argument, in the European corn borer Ostrinia nubilalis Hübner, host plant races (ecotypes) inhabiting geographically proximate areas exhibit various diapause strategies and voltinism (Marçon et al. 1999; Thomas et al. 2003; Coates et al. 2004), suggesting significant effects of host plant species and quality on life-history evolution. Indeed, some studies have reported that host-plant quality influences the induction of diapause in some herbivorous arthropods (Hunter & McNeil 1997; Ito 2003; Ishihara & Ohgushi 2006; Ito & Saito 2006; Takagi & Miyashita 2008). In grass-feeding butterflies, including Satyridae, leaf toughness of the host plant has significant effects on larval growth and survival (Nakasuji & Kimura 1984; Braby 1994; Seko & Nakasuji 2006). Generally, consumption of plant species with tougher leaves has adverse effects on larval performance. Old plant age, dry season and poor soil nutrient status may enhance leaf toughness in grasses, which may reduce larval developmental performance of insects and thus affect their diapause strategy (Braby 1994, 1995). In support of this explanation, the habitats of the univoltine populations in southern Hyogo Prefecture, including the Ieshima Islands, are characterized by low rainfall, acidic swamps, and dry grassland (Takei 2009a). In contrast, the habitat of the bivoltine Ieshima and Kyoto populations is the forest fringe, similar to the typical habitat of other bivoltine populations in western Japan, even though the annual precipitation on Ieshima Island is very similar to that on the adjacent Tangashima and Bouzeshima islands, where univoltine populations are found (Table 1). These differences in vegetation among adjacent islands may reflect the geological heterogeneity of southern Hyogo Prefecture (Geological Survey of Japan 2010). Therefore, the variation of host plant quality, which can be induced by spatial and temporal heterogeneity of the habitat, might affect life-history traits and voltinism in Y. multistriata.
In conclusion, our study revealed diverse life-history traits related to diapause in Y. multistriata among populations living under similar climatic conditions, which are largely latitude dependent. Thus, biotic elements, such as leaf toughness, as well as abiotic elements need to be taken into account to explain the enigmatic geographic distribution of diapause strategies in Y. multistriata. To gain a comprehensive understanding of the evolution of insect seasonality, the elucidation of the roles of non-climatic factors is essential.
ACKNOWLEDGMENTS
We thank Dr M Ishihara and Dr J-Y Ide for critical reading of the manuscript, and we are grateful to the staff at the Botanical Garden of Kyoto University for permission to collect Oplismenus undulatifolius leaves. This study was supported by a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science to S Noriyuki.




