Diversity of drosophilid flies on Kume-jima, a subtropical island: comparison with diversity on Iriomote-jima
Abstract
The drosophilid fauna was studied on Kume-jima, a subtropical island located in the central part of the Ryukyu archipelago, and compared with the fauna of Iriomote-jima located near the south-western end of the archipelago. The number of species collected from Kume-jima was 37, much fewer than that recorded from Iriomote-jima (95 species). The number of subtropical species was particularly reduced on Kume-jima, possibly owing either to this island being more distantly located from the sources of subtropical species (e.g. Taiwan) than Iriomote-jima and/or to winter temperature on Kume-jima being a little lower (by approximately 1.5°C). The number of fungus-feeders was also much reduced on Kume-jima, but the number of fruit-feeders was only slightly reduced. On Kume-jima, fungi seem to be less abundant because forests are smaller, resulting in a smaller number of fungus-feeders. Habitat selection and seasonality were analyzed for species collected using “retainer” type traps baited with banana. For species occurring on both islands, habitat selection differed little between the two islands, whereas the seasonality of some species differed markedly between the two islands.
INTRODUCTION
The number and composition of species inhabiting an island is dependent on various factors, including distance from source areas (e.g. a neighboring continent), the size of the island, and the characteristics of the environments on the island (MacArthur & Wilson 1967; MacArthur 1972; Whittaker & Fernández-Palacios 2007). On continental islands, time since separation from the mainland would also be an important factor. Relative to organisms inhabiting continental regions or neighboring islands, not only the species diversity and composition, but also the morphology, physiology and ecology of organisms on an island sometimes differ (MacArthur & Wilson 1967; Cox & Moore 2005; Whittaker & Fernández-Palacios 2007). In this paper, we report the faunal and ecological characteristics of drosophilid species on Kume-jima, an island located in the central part of the Ryukyu archipelago, Japan, and discuss the factors that determine the species diversity and composition on this island through comparison with similar data from Iriomote-jima, an island located near the south-western end of the same archipelago (Okada 1965; Hirai et al. 2000; Chen & Toda 2001; Chen & Aotsuka 2003, 2004; Itoh et al. 2003). The Ryukyu archipelago consists of continental islands located in a subtropical region, and the fauna and flora of the archipelago comprises complex combinations of tropical, subtropical and temperate elements (Kimoto & Gressitt 1966; Hatusima 1975; Motokawa 2000; Ota 2000; Otsuka & Takahashi 2000). Some islands in this archipelago also have endemic species with specific features (Ota 2000; Otsuka & Takahashi 2000). Of the islands in this archipelago, intensive surveys of drosophilid flies have been undertaken for Iriomote-jima to date, but only fragmental collections have been made for other islands.
MATERIALS AND METHODS
Study areas
Kume-jima is located in a subtropical region (26.2°N, 126.5°E; Fig. 1), and has an area of 59.1 km2, approximately one-fifth that of Iriomote-jima (284.4 km2; 24.2°N, 123.8°E). Relative to Iriomote-jima, the area of forest is much smaller on Kume-jima because of agricultural activities: there is 22.5 km2 of forest on Kume-jima and 269.0 km2 on Iriomote-jima. Forests on Kume-jima are secondary and are dominated by an evergreen broad-leaved tree, Castanopsis sieboldii Nakai. The winter temperature is approximately 1.5°C colder on Kume-jima than on Iriomote-jima, but the average monthly rainfall does not differ much between these two islands (Fig. 2).

Locations of Kume-jima and Iriomote-jima.

Monthly mean temperature and rainfall on (○,□) Kume-jima and (●,▪) Iriomote-jima, based on data from the meteorological observatory on each island.
Collections
Insects were collected in four seasons, April, July and October in 2004 and February in 2005 using traps and nets. Trap collections were made using “retainer” (Toda 1977) and “open” type traps. The retainer type traps were deployed in five separate environments: forest stream-side, forest floor, forest canopy, open land, and domestic area. The retainer type traps were baited with banana and dry yeast. Two traps were set in each environment for a week in each season. Traps were set at approximately 0.5–1 m above the ground at all sites except the canopy sites, where traps were set at approximately 10 m above the ground. The open type traps were deployed in four separate environments: forest stream-side, forest floor, open land, and domestic area. The open type traps were baited with banana and dry yeast and/or mushrooms (Flammulina velutipes (Curt. ex Fr.) Sing, Grifola frondosa (Dicks. ex Fr.) S. F. Gray and Pleurotus eryngii (DC. ex Fr.) Quél. Flies that were attracted to the open type traps were collected using nets in the morning and evening for 3 days in each season.
Collections were also made by net sweeping on undergrowth plants, tree trunks, fallen fruits, fallen flowers and mushrooms. Flies found in living flowers were collected using aspirators. Naturally occurring flowers and fruits were collected, placed in vials containing vermiculite, and examined for the emergence of adult flies.
Habitat selection
The habitat selection of each drosophilid species was analyzed based on the results of the collections using the retainer type traps in the five different environments. Unweighted pair-group method using arithmetic average (upgma) analysis (Sneath and Sokal 1973) was performed to group drosophilid species by habitat selection. In this analysis, the similarity of habitat selection between two species was evaluated using the niche overlap index calculated according to the relative circular weighting method of Colwell and Futuyma (1971):



where nij (or ni'j) is the number of individuals of species i (or i′) in sample j, Ni is the total number of individuals of species i, Nj is the total number of individuals in sample j, N is the grand total number of individuals. Species with N < 10 were excluded from this analysis.
RESULTS AND DISCUSSION
Species composition
A total of 39 813 individuals representing 16 genera and 37 species (including a female of an undetermined species of the genus Phortica) were collected from Kume-jima in the present survey. The species diversity was much lower on Kume-jima than on Iriomote-jima, where a total of 95 species were recorded by Okada (1965), Hirai et al. (2000), Chen and Toda (2001), Itoh et al. (2003), and Chen and Aotsuka (2003, 2004) (Appendix I).
To compare the faunal characteristics of the two assemblages, drosophilid species recorded on these two islands were classified into subtropical, temperate and cosmopolitan species. Discrimination between temperate and subtropical species was not easy, since some species are sporadically found in areas where they are not able to maintain their populations year-round. In the present study, species that have not been recorded from the Tohoku district of Japan or still more northerly areas (i.e. at latitudes higher than 37°N) are categorized as subtropical species, those that have been recorded from the Tohoku district or still more northerly areas are categorized as temperate species, and those that are found widely in the world are categorized as cosmopolitan species. The Kanto district is located in a temperate region (i.e. at latitudes between 35 and 37°N), and therefore species occurring there could be categorized as temperate species. However, some species that had been distributed only in subtropical areas in the past have recently been found in urban areas in the Kanto district (particularly in Tokyo), probably due to urbanization or global warming. In the present study, therefore, species that are distributed at least in the Tohoku district are categorized as temperate species. This classification is supported by information on the cold tolerance of these species; that is, among drosophilid species examined for cold tolerance, those categorized as temperate species are always more cold-tolerant than those categorized as subtropical species (Kimura 2004).
The drosophilid fauna of Kume-jima comprised 23 subtropical, ten temperate and three cosmopolitan species (Table 1), whereas the fauna of Iriomote-jima comprises 78 subtropical, 12 temperate and five cosmopolitan species. Thus, the diversity of subtropical species was much lower on Kume-jima. Immigration of subtropical species may be more frequent on Iriomote-jima than on Kume-jima because the former is closer to Taiwan, which has a large diversity of subtropical species. Alternatively, the extinction of subtropical species may be more frequent on Kume-jima, where the winter temperature is a little lower (by about 1.5°C) than on Iriomote-jima. In contrast, the number of temperate species differed little between Kume-jima and Iriomote-jima, but the species composition differed to some extent: four species occurred only on Kume-jima and six species occurred only on Iriomote-jima. We cannot explain this difference.
| DT | MBS | Adult collection | Larval collection | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trap baited with | Sweeping on undergrowthplants | Fallen fruits and flowers | Livingflowers | Fungi | Tree trunks | Fruits | Flowers | |||||
| Banana | Mushrooms | |||||||||||
| Stegana kanmiyai | S | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Phortica sp. ♀ | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Leucophega angusta | T | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 6 | |
| Le. orientalis | T | M | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| Microdrosophila elongata | S | 1 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | |
| Mi. submarginata | S | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | |
| Liodrosophila bicolor | S | F | 3 | 0 | 82 | 6 | 0 | 0 | 0 | 0 | 0 | 91 |
| Lissocephala subbicolor | S | FG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Mulgravea asiatica | S | 2 | 1 | 31 | 4 | 0 | 0 | 0 | 0 | 0 | 38 | |
| Colocasiomyia alocasiae | S | LF | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 18 |
| Co. xenalocasiae | S | LF | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 37 |
| Dichaetophora lindae | S | 0 | 0 | 32 | 3 | 0 | 0 | 0 | 0 | 0 | 35 | |
| Mycodrosophila planipalpis | T | M | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| My. subgratiosa | S | M | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Paramycodrosophila pictula | S | M | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Phorticella htunmaungi | S | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Scaptomyza pallida | T | H | 3 | 1 | 18 | 2 | 0 | 0 | 0 | 0 | 0 | 24 |
| Sm. clavata | S | H | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Scaptodrosophila bryani | S | F | 1 329 | 18 | 10 | 777 | 0 | 0 | 0 | 0 | 0 | 2 134 |
| Sd. coracina | T | 219 | 66 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 290 | |
| Sd. dorsocentralis | S | F | 1 189 | 1 | 3 | 30 | 0 | 0 | 0 | 0 | 0 | 1 223 |
| Hirtodrosophila unicolorata | S | M | 0 | 0 | 0 | 0 | 0 | 31 | 0 | 0 | 0 | 31 |
| Drosophila busckii | C | M+H | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| D. suzukii | T | F | 226 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 233 |
| D. takahashii | S | F | 26 823 | 255 | 29 | 515 | 0 | 0 | 0 | 14 | 13 | 27 649 |
| D. melanogaster | C | F | 447 | 0 | 1 | 11 | 0 | 0 | 0 | 0 | 0 | 459 |
| D. simulans | C | F | 647 | 2 | 0 | 26 | 0 | 0 | 0 | 6 | 0 | 681 |
| D. ficusphila | S | F | 247 | 1 | 9 | 35 | 0 | 0 | 0 | 0 | 0 | 292 |
| D. bipectinata | S | F | 975 | 7 | 7 | 131 | 0 | 0 | 0 | 0 | 0 | 1 120 |
| D. sp. aff. asahinai | S | F | 1 956 | 227 | 222 | 249 | 0 | 0 | 2 | 0 | 11 | 2 667 |
| D. triauraria | T | F | 48 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 53 |
| D. elegans | S | LF | 0 | 0 | 3 | 0 | 17 | 0 | 0 | 0 | 0 | 20 |
| D. daruma | T | 206 | 0 | 46 | 12 | 0 | 0 | 0 | 0 | 0 | 264 | |
| D. bizonata | T | M | 549 | 676 | 46 | 2 | 0 | 0 | 1 | 0 | 0 | 1 274 |
| D. albomicans | S | F | 883 | 9 | 11 | 196 | 0 | 0 | 0 | 2 | 2 | 1 103 |
| D. annulipes | T | F+B | 29 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 30 |
| D. quadrilineata | S | F | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Total | 35 789 | 1277 | 570 | 2015 | 72 | 33 | 6 | 25 | 26 | 39 813 | ||
- Distribution (DT): S, subtropical; T, temperate; C, cosmopolitan. Major breeding site (MBS): F, fruit; FG, figs; LF, living flowers; M, mushrooms (fungi); H, herbage; B, bamboo shoots.
Of the subtropical species, there are seven species that have been collected from the main islands of Japan and Iriomote-jima but were not found on Kume-jima. Among them, six species are only rarely found on Iriomote-jima and/or the main islands (Toda 1984; Hirai et al. 2000; Beppu 2006), suggesting that their niches are small. Thus, they may be unable to maintain their populations on a small island like Kume-jima where their niches could be lost from time to time. The temperate species Liodrosophila aerea is rather common on both Iriomote-jima and the main islands (Toda 1984; Hirai et al. 2000; Beppu 2006) but, interestingly, is not distributed on Kume-jima.
An undescribed species, Drosophila sp. aff. asahinai, has been found on this island and surrounding islands including Okinawa-jima (M. Watada, unpublished data). This species and its sibling species are allopatrically distributed in this archipelago and neighboring regions: D. lacteicornis occurs on Iriomote-jima and neighboring islands; D. sp. aff. asahinai on Kume-jima, Okinawa-jima and neighboring islands; D. asahinai on Amami-oshima and surrounding islands; D. rufa on the main islands of Japan, Korea and neighboring islands; and D. tani on the Chinese continent (Okada 1964, 1965; Cheng & Okada 1985; Hirai et al. 2000; Beppu 2006; M. Watada, unpublished data). In this archipelago, allopatric patterns of species distributions have been identified for terrestrial vertebrates such as amphibians, reptiles and mammals, which have a low capacity to travel across the sea (Ota 1998, 2000; Otsuka & Takahashi 2000). However, drosophilids have a rather high capacity to disperse (Kimura 1992), so it is interesting that this group (i.e. the rufa species complex) has undergone speciation in this archipelago, whereas the other groups of drosophilids have not.
Only one individual of the cosmopolitan species D. simulans has been collected so far on Iriomote-jima (Itoh et al. 2003); however, this same species was rather abundant on Kume-jima, and also on the Bonin Islands, another archipelago located in a subtropical region (Toda 1976; Toda et al. 1987). It is interesting that D. simulans is almost absent from Iriomote-jima.
Feeding habits
Larval feeding habits are presented in Table 1 on the basis of the results from previous studies (Kimura et al. 1977; Nishiharu 1980; Hirai et al. 2000). Fermenting fruit and fungi would be major breeding sites on both Kume-jima and Iriomote-jima. In addition, some species breed on living flowers, fresh figs and herbage (decayed plant parts). The numbers of fruit-feeding, fungus-feeding and herbage-feeding species were compared between Kume-jima and Iriomote-jima. In this analysis, cosmopolitan species and species for which the breeding site was unknown or ambiguous were excluded. Although the breeding sites of some Hirtodrosophila and Mycodrosophila species have not been confirmed, species of these two genera are categorized as fungus-feeders since no exceptions have been observed. In addition, Dichaetophora sp. aff. emeishanensis, cited as an undescribed species of Lordiphosa in Hirai et al. (2000), has unknown feeding habits, but is assumed to be a herbage-feeder on the basis of the feeding habits of its relatives (Kimura et al. 1977; Nishiharu 1980). Figure 3 shows the results of the feeding habit analysis. There were slightly fewer fruit-feeders on Kume-jima than on Iriomote-jima, but much fewer fungus-feeders on Kume-jima. This difference may be attributable to differences in the abundance of resources. Fungi seem to be less abundant on Kume-jima, where forests are smaller and more arid. In fact, fungi were seldom observed during our collections on Kume-jima. On the other hand, fruiting trees and bushes were rather abundant on Kume-jima, even in lightly wooded areas and human-associated environments, which would explain the similar numbers of fruit-feeders. Herbage-feeders were rare on these two subtropical islands, but they are abundantly observed in temperate areas (Kimura et al. 1977; Nishiharu 1980; Toda 1984; Beppu 2006). This is because herbaceous plants are scarce on the forest floor in subtropical areas, where evergreen trees dominate, but abundant in temperate regions, where deciduous trees dominate.
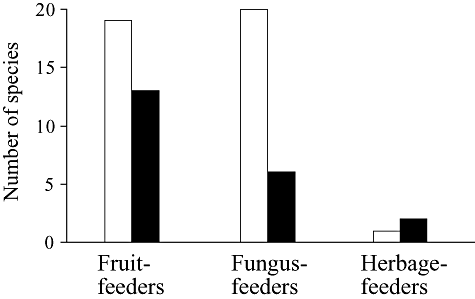
Numbers of fruit-feeding, fungus-feeding and herbage-feeding species on (▪) Kume-jima and (□) Iriomote-jima.
Habitat selection
Species that were abundantly collected in the retainer type traps were analyzed for habitat selection (Table 2). They are classified into several groups by upgma analysis as shown in Figure 4. These species had basically similar habitat selection on Iriomote-jima if they occurred there (Hirai et al. 2000). However, Scaptodrosophila bryani, D. takahashii, D. bipectinata and D. annulipes, which were rare in the forests of Iriomote-jima, were rather abundantly collected in the forests of Kume-jima. This may be due to the forests on Kume-jima occupying only small areas.
| Stream-side | Forest floor | Forest canopy | Open lands | Domestic areas | Total | |
|---|---|---|---|---|---|---|
| Ph. sp. ♀ | 0 | 0 | 1 | 0 | 0 | 1 |
| Pc. htunmaungi | 0 | 0 | 2 | 0 | 0 | 2 |
| Sm. pallida | 0 | 0 | 0 | 1 | 0 | 1 |
| Sd. bryani | 26 | 107 | 187 | 608 | 84 | 1 012 |
| Sd. coracina | 2 | 44 | 121 | 20 | 12 | 199 |
| Sd. dorsocentralis | 97 | 172 | 318 | 374 | 123 | 1 084 |
| D. busckii | 0 | 0 | 0 | 2 | 0 | 2 |
| D. suzukii | 7 | 100 | 83 | 19 | 1 | 210 |
| D. takahashii | 205 | 1174 | 5488 | 14 857 | 866 | 21 890 |
| D. melanogaster | 5 | 1 | 11 | 39 | 269 | 325 |
| D. simulans | 10 | 26 | 125 | 197 | 168 | 526 |
| D. ficusphila | 5 | 21 | 180 | 27 | 2 | 235 |
| D. bipectinata | 86 | 204 | 196 | 86 | 146 | 718 |
| D. sp. aff. asahinai | 60 | 396 | 719 | 228 | 18 | 1 421 |
| D. triauraria | 0 | 1 | 3 | 34 | 3 | 41 |
| D. daruma | 10 | 0 | 0 | 1 | 3 | 14 |
| D. bizonata | 15 | 266 | 14 | 7 | 1 | 303 |
| D. albomicans | 120 | 258 | 29 | 29 | 59 | 495 |
| D. annulipes | 0 | 3 | 9 | 17 | 0 | 29 |
| D. quadrilineata | 0 | 0 | 0 | 0 | 1 | 1 |
| Total | 648 | 2773 | 7486 | 16 546 | 1756 | 29 209 |

upgma analysis for drosophilid species collected using retainer type traps on Kume-jima. The habitats of these species were inferred from the data given in Table 2.
Seasonality
Species that were abundantly collected by the retainer type traps were also analyzed for seasonality (Fig. 5). Species were divided into three categories: (i) species that were abundant only in April (D. takahashii); (ii) species that were abundant only in October (Sd. dorsocentralis, D. suzukii, D. bipectinata and D. bizonata), and (iii) species that were abundant for longer periods (the remaining species). The species of the last group were further subdivided into five groups: species that were more abundant in April (Sd. coracina and D. sp. aff. asahinai), species that were more abundant in October (Sd. bryani), species that were more abundant in July (D. albomicans), species that were rare in July (D. melanogaster and D. simulans), and species that were abundant in April and October (D. ficusphila). Scaptodrosophila bryani, D. bizonata and D. albomicans showed similar seasonality on Iriomote-jima (Hirai et al. 2000). Scaptodrosophila dorsocentralis and D. bipectinata showed abrupt expansion of populations on both islands, but their expansion was delayed 2 or 3 months on Iriomote-jima. On the other hand, D. takahashii had very different seasonality on Iriomote-jima. These seasonal patterns should be analyzed with respect to the seasonality of the species' resources and enemies.

Seasonal changes in the number of individuals collected using retainer type traps on Kume-jima. A, April; J, July; O, October; F, February.
ACKNOWLEDGMENTS
We would like to thank Drs M. Yafuso, H. Shima, O. Yata and K. Araya for their kind guidance; Dr M. J. Toda for identification of some species; Dr M. Watada for unpublished information on the distribution of Drosophila sp. aff. asahinai; and Mr F. Sato for his kind help with the field information. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 17570010).
Appendix
APPENDIX I
| DT | MBS | DT | MBS | ||
|---|---|---|---|---|---|
| Stegana bacilla †† | S | Paramycodrosophila pictula | S | M | |
| St. izu †† | S | Phorticella bistriata | S | ||
| St. kanmiyai | S | Pc. flavipennis | S | ||
| St. nigripennis | S | Pc. htunmaungi | S | ||
| St. ornatipes | S | Zaprionus bogoriensis | S | ||
| Amiota kimurai ‡ | S | Scaptodrosophila bryani | S | F | |
| A. nagatai ‡ | S | Sd. coracina | T | ||
| A. okinawana | S | Sd. dorsocentralis | S | F | |
| A. planata ‡ | S | Sd. kyushuensis | S | ||
| A. sinuata | S | Sd. subtilis | T | ||
| A. speculum | S | Sd. sp. 1 | S | ||
| Phortica magna | S | Sd. sp. 2 | S | ||
| Ph. sp. aff. orientalis | S | Sd. sp. 3 | S | ||
| Leucophenga argentata | S | Hirtodrosophila elliptosa | S | M | |
| Le. concilia ¶ | S | Hi. latifrontata | S | M | |
| Le. confluens ¶ | S | Hi. longecrinita | S | M | |
| Le. digmasoma | S | Hi. manonoensis | S | M | |
| Le. iriomotensis ¶ | S | Hi. nudinokogiri | S | M | |
| Le. maculata ¶ | T | M | Hi. seminigra | S | M |
| Le. meijerei | S | Hi. seminokogiri | S | M | |
| Le. multipunctata ¶ | S | Hi. unicolorata | S | M | |
| Le. obsucura ¶ | S | Hi. sp. aff. paramanona | S | M | |
| Le. orientalis ¶ | T | M | Hi. sp. aff. seminigra | S | M |
| Le. ornata | T | Hi. sp. aff. yakushimana | S | M | |
| Le. regina ¶ | S | Hi. sp. 1 | S | M | |
| Le. umbratula ¶ | S | Drosophila ancora | S | ||
| Cacoxenus perspicax | S | D. busckii | C | M+H | |
| Microdrosophila elongata † | S | D. subpulchrella | T | F | |
| Mi. maculata | S | D. suzukii | T | F | |
| Mi. matsudairai † | S | D. takahashii | S | F | |
| Mi. pleurolineata | S | D. melanogaster | C | F | |
| Mi. submarginata † | S | D. simulans § | C | F | |
| Mi. sp. aff. cristata | S | D. ficusphila | S | F | |
| Liodrosophila aerea | T | F | D. ananassae | C | F |
| Lio. bicolor | S | F | D. bipectinata | S | F |
| Lio. globosa | S | F | D. bocki | S | F |
| Lissocephala binotata | S | FG | D. lacteicornis | S | F |
| Lis. subbicolor | S | FG | D. elegans | S | LF |
| Mulgravea asiatica | S | D. daruma | T | ||
| Colocasiomyia alocasiae | S | LF | D. bizonata | T | M |
| Co. xenalocasiae | S | LF | D. albomicans | S | F |
| Dichaetophora lindae | S | D. annulipes | T | F+B | |
| Di. okadai | T | D. immigrans | C | F | |
| Di. sp. aff. emeishanensis | S | H | D. quadrilineata | S | F |
| Mycodrosophila bicolor | S | M | D. ruberrima | S | F |
| My. subgratiosa | S | M | D. angor | S | |
| My. sp. 1 | S | M | D. sp. aff. angor | S | |
| My. sp. 2 | S | M |
- See Table 1 for abbreviations. Species were recorded by Hirai et al. (2000) (unless otherwise noted), †Okada (1965), ‡Chen and Toda (2001), §Itoh et al. (2003), ¶Chen and Aotsuka (2003) and ††Chen and Aotsuka (2004).




