Demography of the Okinawan eusocial wasp Ropalidia fasciata (Hymenoptera: Vespidae) II. Effects of foundress group size on survival rates of colonies and foundresses, and production of progeny
Abstract
Using demography data for the primitively eusocial wasp Ropalidia fasciata collected at 10 survey stations over 5 years, the effects of foundress group size (FGS) on colony survival rate and production of progeny were examined. The distribution pattern for the frequency of nests established by different numbers of foundresses fit a 0-truncated negative binomial distribution. The rate of nest failure up to the beginning of June decreased with FGS, and colony survival rate up to June and September increased with FGS. Although there were large variations among stations and years, the survival rate of colonies established by a single foundress (haplometrotic colonies) was significantly lower than that of colonies established by two or more foundresses (pleometrotic colonies). The number of new adults that emerged per colony up to the end of July increased with FGS, but there was no significant correlation between number of new adults per foundress and FGS. The number of potential foundresses produced per colony tended to increase with FGS, but there was no significant difference among the values produced per foundress for the three FGS categories. The percentage parasitism by an ichneumon parasitoid, Arthula formosana, tended to be higher in colonies established by a small number of foundresses. The relation between FGS and the productivity of the new foundresses was not statistically significant, suggesting that independent founding may be a better strategy for future subordinate foundresses. However, the long colony life span (mean 100 days, maximum 240 days) as compared with the shorter life span of foundresses (40 or 50 days) may be a good condition for the coexistence of many egg-layers in a colony.
INTRODUCTION
The evolution and maintenance of reproductive division of labor in eusocial insects with multi-queen social systems is one of the most debated topics within the field of behavioral ecology (e.g. Itô 1993; Keller 1993; Bourke & Franks 1995). Although the degree of reproductive division among colony members (reproductive skew; Reeve & Keller 1995) is influenced by demographic and environmental factors, studies on the demography of eusocial wasps are small in number as compared with studies of ethological and genetic factors (Gadagkar 2001).
In the first part of the present study (Itô & Kasuya 2005), we presented some demographic data for a “primitively eusocial” wasp, Ropalidia fasciata (Fabricius). A primitively eusocial wasp is one in which there is no clear morphological difference between queens and workers. According to that study, the mean life span of foundresses is approximately 50 days after attendance of nest foundation and that of workers is 20 days after emergence; both values are generally similar to those of other polistine wasps. However, the colony cycle of this species has a notable feature. Although the Okinawan R. fasciata has an annual colony cycle, the longest colony life span is more than 240 days, far longer than the life spans of other temperate polistine wasps (140–150 days). Thus, many colonies continue to produce progeny adults during October, November and December, several months after the death of all foundresses. This fact suggests that the Okinawan R. fasciata is partially bivoltine. The long colony life span and partial bivoltinism in this population may provide good conditions for polygyny, the coexistence of many ovipositors in a colony. Polygyny is considered to be common in R. fasciata (Itô 1993).
However, to evaluate how polygyny operates in this colony, it is necessary to study the effects of foundress group size (FGS) on the survival rates of foundresses and colonies and on the productivity of reproductive individuals per colony and per foundress. We analyzed the relation between FGS and demographic parameters in R. fasciata colonies, using the same data set as used in the first part of this study, and the results obtained are reported herein.
MATERIALS AND METHODS
Species
The primitively eusocial wasp Ropalidia fasciata (Fabricius) is distributed from India to Flores, Indonesia, in the east, and to the Ryukyu Archipelago in the north (Das & Gupta 1989). In Okinawa, new nests are usually established in late March or early April by one or more foundresses (independent founding) under the leaves of a grass, Miscanthus sinensis Anderson, or sugarcane, Saccharum officinarum L. (see also Itô 1993; Itôet al. 1994; Itô & Kasuya 2005).
This species is quite philopatric, that is, the reproductives rarely move to nearby grass fields or sugarcane fields, rather they make their nests within the habitats from which they have emerged. Thus, we found in our previous study that some small grass fields of only 150–200 m2 in area had 30–50 nests every year, but nearby grass fields separated only by a small road had no or only a few nests during study years (Itô & Kasuya 2005).
In Okinawa, females emerge from eggs laid in September, October and sometimes November, when almost all nests lacks foundress, to become foundresses in the next spring (Itô 1996). In the present paper, as in the first part of this report, we defined adult females emerging during and after September as potential reproductives of the next year. Some females that emerge before September may overwinter and become potential reproductives, but there are no data on what proportion of females undergo this process.
Stations
Ten study stations were established in sites of high nest density. The area of each study station was small (maximum of 390 m2), so we could find almost all nests established in each year. The field stations were distributed in three localities: two stations (SI and SII) in Naha City, two stations (UI and UII) in the southern part of Okinawa Hontô (main island of Okinawa) and six stations (SKI–SKVI) on Sesoko Islet near the northern part of Okinawa Hontô. Except for SII, which was located in a small field of sugarcane varieties, all stations were located in small grass fields in which several to several dozens of M. sinensis tussocks grew, surrounded by open space dominated by small gramineous weeds and a composite weed, Bidens frondosa L., which is the main nectar source of R. fasciata in Okinawa.
Survey method
Field surveys were carried out by the senior author from early April to November or December during the study years (1992–1998). Surveys were basically carried out once per month, but several surveys were performed during the founding stage, that is, during the period from early April to mid-May
At some stations (UI, SKI, SKIII and SK V), M. sinensis tussocks were partially cut by the property owners, and some became unsuitable for R. fasciata (due to excessive growth of M. sinensis or cover by trees) during the study period. To ensure that a sufficient number of survey stations was maintained, new stations were established during the study. Thus, some stations were studied over a long period of time (up to 6 years) but others over a shorter period of time (for details, see table 1 of Itô & Kasuya 2005).
Marking of nests and foundresses
As shown in Itô and Kasuya (2005), nests found at survey stations in spring were marked by fixing a numbered piece of red vinyl tape to the stem of the M. sinensis plant from which nests were hanging. Numbers of cells, eggs, larvae and pupae of R. fasciata, and cells showing evidence of parasitism by an ichneumon wasp, Arthula formosana (Uchida), were recorded during subsequent surveys. After the emergence of progeny, the number of emergents, which can be calculated by observing the windows at the bottom of cells to check for abandoned meconium, were recorded. However, this method produces an underestimate of the number of adults that have emerged, because many cells are used twice or more. The numbers of cells with eggs, larvae and/or those showing signs of adult emergence were sometimes not counted when the counting was risky due to the existence of many adults or a shortage of time.
Foundresses were taken from the nest using a plastic cylinder, transferred to a small glass vial using a sweep net, slightly anesthetized using carbon dioxide, and marked individually with four spots of five colors on the dorsal part of thorax, using a felt-tipped pen (Magic Opaque Color no. 551; Teranishi Chemical Industry, Osaka, Japan). Some newly emerged progeny females were marked using the same method.
Statistical methods
Although statistical comparisons were carried out using data for colonies established by different number of foundresses (different FGS), three group size categories are used in the tables to indicating general tendencies: 1 (single foundress), 2–5 and ≥6. Comparisons were also made between haplometrotic (founded by a single foundress) and pleometrotic (founded by a group of foundresses) colonies. The FGS of each colony was determined by marking and counting the foundresses found in each nest before emergence of the first progeny.
In some tables in the Results, only the total numbers or mean ratios calculated for each year are shown in the interests of brevity. In the statistical tests, however, the original data taken at each station in each year were also used for calculation.
We used the χ2-test (see Sokal & Rohlf 1973) and the U-test using yearly mean values, and also anova and logistic regression analysis using dummy variables. For comparison of data from haplometrotic and pleometrotic groups, the usual methods for two-way tables were also used (i.e. the G-test). Although some tables show ratios only, statistical tests were, as a rule, carried out using the original values and values calculated using these to avoid any effects arising from a difference in the original sample sizes (e.g. 1/2 and 50/100 both correspond to a decimal value of 0.5, but the two values have different levels of accuracy). For χ2-tests using values for different FGS and different years, data from two adjacent years were sometimes combined to reduce the proportion of values less than 5 (Ishii 1975).
RESULTS
Foundress group size
The frequency distribution of FGS over 6 years, compared with the expected probabilities of nests having i foundresses in 0-truncated Poisson and 0-truncated negative binomial distributions, is shown in Figure 1. Data were fitted using the methods developed by David and Johnson (1952) for the Poisson distribution, and by Sampford (1955) for the negative binomial distribution. The data did not fit the Poisson distribution, but rather fit the negative binomial distribution, with a large k-value (k = 0.127) indicating strong overcrowding. Although Figure 1 was based on combined values for nine stations and 6 years, distributions for each year and each station had basically similar trends.
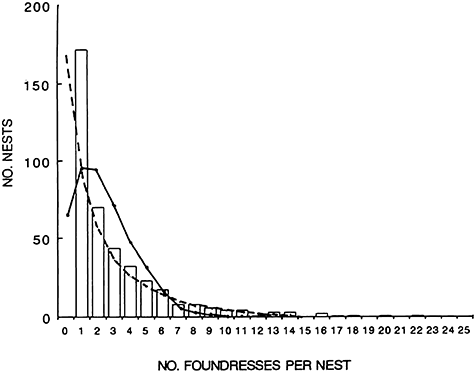
Frequency distribution of the number of foundresses per nest in Okinawan colonies of Ropalidia fasciata. The histogram shows the observed value, and solid and broken lines indicate the expected values given 0-truncated Poisson and 0-truncated negative binomial distributions, respectively.
Nearly half of the colonies (43 ± 4%) were established in spring by single foundresses (haplometrosis), but others were established by foundress groups (pleometrosis). The maximum FGS during the period between 1992 and 1996 was 22, somewhat smaller than the maximum value, 25 foundresses, observed since 1983.
It must be noted that, contrary to some other species, for example Polistes fuscatus, Polistes erythrocephalus (West-Eberhard 1969) and Belonogaster petiolata (Keeping & Crewe 1987), in which nests are usually initiated by a single female and other females join the nest later, foundresses of R. fasciata participate in nest foundation from the initial stage (Itôet al. 1985).
Failure and reconstruction of nests
The relation between FGS and the nest failure rate up to the beginning of June, based on data for all colonies (taken in all years from all stations) is shown in Figure 2 (top). Here, nest failure is defined as abandonment or disappearance due to the nest falling. The proportion of failed nests decreased with an increase in FGS (r = −0.256, P < 0.01).
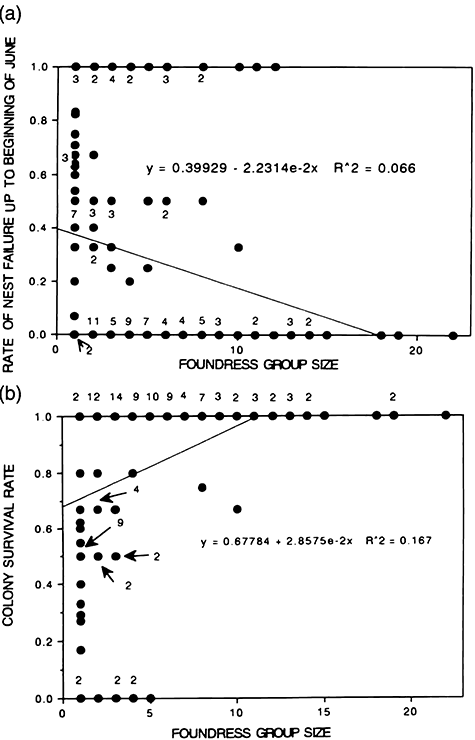
(a) Relationship between foundress group size (FGS) and rate of nest failure up to the beginning of June. (b) Relationship between FGS and colony survival rate up to the beginning of June. Numerals near circles indicate frequency. Each circle shows the nest failure rate of each study station in each year. For example, for FGS = 8, there were eight observations of stations made in 5 different years, of which two had a colony survival rate of 1, one a rate of 0.5, and five a rate of 0.
The rates of nest failure up to the beginning of June in relation to three categories of FGS are shown in Table 1. The G-test using mean values for all years and the χ2-test using values for each year (expected rates are calculated from two FGS categories in each year) were both applied. The failure rate of nests established by single foundress was significantly higher than the rate for nests established by 2–5 or ≥6 foundresses, as assessed using both the G-test and the χ2-test (P < 0.01 for both tests). For the χ2-test, the expected values can be calculated using the total failure rate or rates for particular years. Although the G-test revealed no significant difference among values obtained in different years, we usually used the rate for particular years.
| Year | FGS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2–5 | ≥6 | |||||||||||
| n | F | R | S | n | F | R | S | n | F | R | S | ||
| 1992 | Number | 11 | 6 | 1 | 6 | 12 | 5 | 3 | 10 | 4 | 0 | – | 4 |
| Rate | 0.55 | 0.17 | 0.55 | 0.42 | 0.60 | 0.83 | 0 | – | 1.00 | ||||
| 1993 | Number | 23 | 16 | 5 | 12 | 30 | 10 | 5 | 25 | 11 | 0 | – | 11 |
| Rate | 0.70 | 0.31 | 0.52 | 0.33 | 0.50 | 0.83 | 0 | – | 1.00 | ||||
| 1994 | Number | 35 | 19 | 5 | 21 | 40 | 8 | 2 | 34 | 14 | 4 | 3 | 13 |
| Rate | 0.54 | 0.26 | 0.60 | 0.20 | 0.25 | 0.85 | 0.29 | 0.75 | 0.93 | ||||
| 1995 | Number | 30 | 17 | 1 | 14 | 41 | 7 | 2 | 36 | 17 | 5 | 3 | 15 |
| Rate | 0.57 | 0.06 | 0.47 | 0.17 | 0.29 | 0.88 | 0.30 | 0.60 | 0.88 | ||||
| 1996 | Number | 29 | 12 | 3 | 20 | 23 | 6 | 4 | 21 | 13 | 3 | 3 | 13 |
| Rate | 0.41 | 0.25 | 0.69 | 0.26 | 0.67 | 0.91 | 0.23 | 1.00 | 1.00 | ||||
| Mean ± SD for all years | |||||||||||||
| Failure rate | 0.55 ± 0.10a | 0.28 ± 0.10b** | 0.16 ± 0.15b**c* | ||||||||||
| Reconstruction rate | 0.21 ± 0.10a | 0.51 ± 0.24b* | 0.78 ± 0.20b** | ||||||||||
| Colony survival rate | 0.57 ± 0.08a | 0.86 ± 0.03b** | 0.96 ± 0.06b** | ||||||||||
- n, Number of nests. a and b show that the difference between FGS = 1 and FGS = 2–5 is statistically significant at the 1% (**) or 5% (*) level. cshows that the difference between FGS = 2–5 and ≥6 is also significant at the 5% level. Values within the same row that have the same letter are not significantly different. Differences in the failure rate and colony survival rate between haplometrotic (F = 1) and pleometrotic (F = ≥2) nests were also significant.
The rates of reconstruction for failed pleometrotic nests were higher than those for haplometrotic nests (G-test and χ2-test, P < 0.05 for both tests). In colonies with large FGS (>10), no nest failed before June in 3 of the 5 years, and all failed nests were reconstructed.
Survival rates of colonies
Colony survival rates (S in Table 1) up to the beginning of June increased with FGS (Fig. 2, bottom; r = 0.409, P < 0.01). χ2 values show that the survival rates of pleometrotic colonies were significantly higher than those of haplometrotic colonies (P < 0.01). A similar tendency was observed for colony survival rate up to the beginning of September (χ2-test; P < 0.01, Table 2). The number of stations used for the analysis was smaller than the number shown in Table 1, because stations at which M. sinensis tussocks were cut by the property owners during summer were excluded.
| Year | No. stations | FGS | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2–5 | ≥6 | |||||
| n | S | n | S | n | S | ||
| 1993 | 5 | 20 | 0.22 | 33 | 0.64 | 9 | 0.88 |
| 1994 | 4 | 25 | 0.23 | 32 | 0.41 | 11 | 0.73 |
| 1995 | 5 | 30 | 0.18 | 42 | 0.36 | 17 | 0.82 |
| 1996 | 4 | 28 | 0.25 | 22 | 0.49 | 15 | 0.67 |
| Mean ± SD | 103 | 0.22 ± 0.33a | 129 | 0.48 ± 0.12b** | 52 | 0.78 ± 0.09b**,c** | |
- n, Number of colonies. a,b,cDifference was highly significant (see footnote of Table 1). The difference between haplometrotic and pleometrotic nests was also significant.
Nests were often invaded by alien foundresses that had been marked on other nests. If an alien foundress was seen on a given day and the original foundresses who had initiated the nest disappeared over subsequent days, the nest was suspected to have been usurped, but if the original foundress was found to be coexisting with the alien foundress at the next observation time, the nest was considered to not have been usurped, and we deemed that the foundresses were coexisting. The percentage usurpation of haplometrotic nests was 86% of cases in which alien foundresses were seen at least once (Table 3). This value was significantly higher (Fisher's exact probability test, P = 0.001) than the value for pleometrotic nests (11%; Table 3). Six cases in the last column of Table 3 (“others”) were nests in which an alien foundress was seen once, but subsequent observation was not possible owing to disappearance of the nest. These cases were omitted from the exact probability test.
| Nest category | Successful usurpation | Coexistence | Other |
|---|---|---|---|
| Haplometrotic | – | 6 (0.86) | 1 (0.14) |
| Pleometrotic | 3 (0.11) | 19 (0.68) | 6 (0.21) |
- Rates are shown in parentheses. For category definition see the text.
Thus, pleometrotic colonies tend to survive up to the emergence of progeny adults better than haplometrotic colonies.
Survival rates of foundresses
Although the mean survival rate of marked foundresses up to early June in haplometrotic colonies was somewhat higher than that for foundresses in pleometrotic colonies (mean survival values of 0.36 and 0.20; Table 4), the difference was not significant. There was no significant difference in the survival rates up to late July between haplometrotic and pleometrotic colonies (Table 4). No marked foundress from a haplometrotic nest was recovered in early September, whereas 10 marked foundresses from pleometrotic nests were recovered, but it must be noted that the sample size (number of marked foundresses) for haplometrotic colonies was far smaller that for pleometrotic colonies (72:828; Table 4), because marking effort was directed toward colonies with larger FGS.
| Year | No. stations | Haplometrotic colonies | EarlySeptember | Pleometrotic colonies | EarlySeptember | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | EarlyJune | LateJuly‡ | n | EarlyJune | LateJuly | |||||
| 1993 | 8 | Number | 23 | 8 | 2 | 0 | 219 | 83 | 19 | 3 |
| Rate | 0.35 | 0.09 | 0 | 0.38 | 0.09 | 0.01 | ||||
| 1994 | 4 | Number | 15 | 1 | 0 | 0 | 185 | 13 | 10 | 3 |
| Rate | 0.07 | 0 | 0 | 0.07 | 0.05 | 0.23 | ||||
| 1995 | 5 | Number | 15 | 4 | 2 | 0 | 235 | 36 | 28 | 0 |
| Rate | 0.27 | 0.13 | 0 | 0.15 | 0.12 | 0 | ||||
| 1996 | 4 | Number | 19 | 6 | 1 | 0 | 189 | 34 | 22 | 4 |
| Rate | 0.32 | 0.05 | 0 | 0.18 | 0.12 | 0.02 | ||||
| Total | 72 | 19 | 5 | 0 | 828 | 166 | 79 | 10 | ||
| Mean rate ± SD | 0.36 ± 0.34 | 0.13 ± 0.27 | 0 | 0.20 ± 0.13 | 0.14 ± 0.13 | 0.02 ± 0.03 | ||||
- n, Number of foundresses marked. ‡Data from early August are included for some stations in some years.
Growth of nests and production of progeny
The ratio of the number of nests from which first adult emergence took place during May (May emergence) to all nests from which emergence took place during May and June seemed to increase with FGS (Table 5), suggesting that the nests with more foundresses develop earlier. Fisher's exact probability test showed that the difference between haplometrotic and pleometrotic colonies was not significant (but near significant; P = 0.063).
| Year | FGS | ||
|---|---|---|---|
| 1 | 2–5 | ≥6 | |
| 1992† | |||
| No. nests | 6 | 14 | 5 |
| Proportion May emergence | 0.33 | 0.86 | 0.80 |
| 1993 | |||
| First date of emergence | 4 June | 13 May | 11 May |
| No. nests | 6 | 13 | 5 |
| Proportion May emergence | 0 | 0.15 | 1.0 |
| 1994 | |||
| First date of emergence | 30 May | 23 May | 23 May |
| No. nests | 5 | 23 | 5 |
| Proportion May emergence | 0.60 | 0.96 | 0.60 |
| 1995 | |||
| First date of emergence | 23 May | 23 May | 23 May |
| No. nests | 15 | 2810 | 10 |
| Proportion May emergence | 0.40 | 0.32 | 0.40 |
| Proportion of nests for which first emergence took place in May relative to all nests from which emergence was detected during May and June | 0.33 ± 0.25 | 0.57 ± 0.40 | 0.70 ± 0.26 |
- † In this year, surveys were not performed in late April or early May.
The number of new adults that emerged per colony up to early June seemed to increase with FGS, but the U-test revealed that only the difference between FGS values of 1 and ≥6 was significant (P < 0.05; N in Table 6; the χ2-test was not used here, because many values for FGS = 1 and 2–5 were smaller than 5). The difference between the numbers of adults produced per foundress up to early June on nests with different FGS was not significant (N/F in Table 6).
| Year | No. stations | FGS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1† | 2–5 | ≥6 | |||||||
| n | N | n | N | N/F | n | N | N/F | ||
| 1992 | 4 | 19 | 1.26 ± 1.36 | 24 | 3.38 ± 2.61 | 1.23 ± 1.00 | 5 | 12.20 ± 11.07 | 1.40 ± 1.43 |
| 1993 | 7 | 29 | 1.50 ± 1.91 | 34 | 2.97 ± 2.10 | 0.88 ± 0.68 | 12 | 8.48 ± 6.53 | 1.21 ± 0.58 |
| 1994 | 7 | 41 | 0.96 ± 1.01 | 43 | 3.16 ± 1.60 | 1.06 ± 0.55 | 11 | 7.17 ± 6.55 | 0.49 ± 0.40 |
| 1995 | 7 | 33 | 0.57 ± 0.59 | 30 | 3.16 ± 1.06 | 1.02 ± 0.39 | 16 | 5.69 ± 3.32 | 0.58 ± 0.48 |
| 1996 | 4 | 24 | 0.44 ± 1.33 | 22 | 0.80 ± 0.84 | 0.30 ± 0.27 | 14 | 4.66 ± 4.23 | 0.49 ± 0.37 |
| Mean ± SD | 0.94a ± 0.45 | 2.69a ± 1.07 | 0.90a ± 0.36 | 7.64b ± 2.93 | 0.83a ± 0.44 | ||||
- † In haplometrotic colonies (FGS = 1), N/F is the same as N. n, Number of colonies; aand b, the difference between FGS = 1 and ≥6 was significant at the 5% level. Values within the same row that have the same letter are not significantly different. Numbers per colony (N) and per foundress (N/F) are shown.
The number of adults produced per colony up to the end of July also seemed to increase with FGS (Table 7, adults; Fig. 3, top; r = 0.510, P < 0.01), but the difference was only significant when assessed using the U-test (P < 0.05), not the χ2-test, possibly due to the large variation between years. There was no significant difference among the number of adults produced per foundress up to the end of July (see Fig. 3, bottom).
| Year | No.stations | FGS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2–5 | ≥6 | ||||||||
| n | Cells | Adults† | n | Cells | Adults | n | Cells | Adults | ||
| 1993 | 4 | 15 | 3.96 ± 6.86 | 0.37 ± 0.64 | 24 | 54.5 ± 31.3 | 5.88 ± 3.33 | 6 | 160 ± 80.8 | (52.0 ± 49.0)† |
| 1994 | 4 | 40 | 13.7 ± 5.4 | 3.06 ± 0.85 | 45 | 51.1 ± 22.9 | 10.4 ± 6.4 | 16 | 90.6 ± 57.4 | 17.8 ± 11.6 |
| 1995 | 5 | 31 | 21.0 ± 8.1 | 3.11 ± 1.62 | 45 | 51.6 ± 21.5 | 8.36 ± 3.71 | 13 | 119.9 ± 71.9 | 21.6 ± 13.6 |
| 1996 | 4 | 29 | 14.5 ± 10.8 | 2.84 ± 3.02 | 24 | 37.8 ± 7.6 | 8.34 ± 1.33 | 9 | 104.7 ± 67.6 | 25.6 ± 19.4 |
| Mean ± SD‡ | 13.3 ± 7.0a | 2.35 ± 1.32 | 48.7 ± 7.4b | 8.23 ± 1.87 | 119.0 ± 26.3b,c | 29.3 ± 13.4 | ||||
- † The sample size for the number of adults that emerged is sometimes smaller than n, because the number was not counted for some nests; in such cases, values are shown in parentheses. ‡ SD is calculated using yearly means, including the values in parentheses. a,b and cindicate statistically significant differences at the 5% level. Values within the same row that have the same letter are not significantly different.
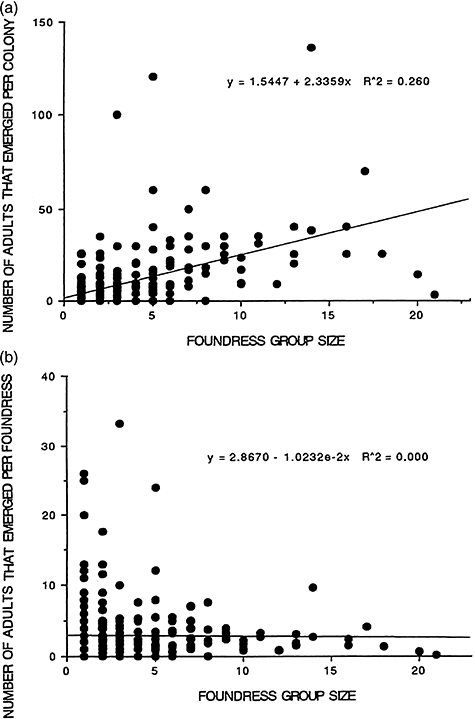
(a) Number of adults that emerged per colony up to the end of July. (b) Number of adults that emerged per foundress up to the end of July.
Production of new foundresses
The numbers of potential foundresses (1/2 of adults emerged during and after September) produced per colony (N) and per foundress (N/F) are shown in Table 8, using mean values for each station. In this table, colonies established in April that disappeared before September (failed and not reconstructed), and colonies that produced adults only before the end of August, are counted as zero production. Although values differed somewhat between stations (for example, SKI tended to produce large numbers) and between years (the productivity of 1993 was higher than in other years), N-values tended to increase with FGS (P < 0.05, anova). The difference between N-values for FGS values of 1 and ≥6 was significant (U-test, P < 0.05). However, there was no significant difference among the N/F-values of the three FGS categories.
| Year | Station | FGS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2–5 | ≥6 | |||||||
| n | N † | n | N | N/F | n | N | N/F | ||
| 1992 | SII | 5 | 0 | 4 | 0 | 0 | – | ||
| SKI | 8 | 0 | 5 | 10.0 | 3.34 | 4 | 18.8 | 1.70 | |
| Mean | 13 | 0 | 9 | 5.0 | 1.86 | 4 | 18.8 | 1.70 | |
| 1993 | SII | 9 | 1.44 | 3 | 8.33 | 3.17 | 1 | 123 | 20.5 |
| UI | 2 | 0 | 5 | 19.8 | 8.10 | 3 | 93.0 | 8.33 | |
| SKI | 5 | 21.8 | 19 | 29.5 | 9.66 | 2 | 19.0 | 0.90 | |
| SKII | 4 | 0 | 6 | 3.42 | 1.18 | 2 | 28.5 | 2.68 | |
| SKIV | 4 | 18.8 | 3 | 12.5 | 2.87 | – | |||
| Mean ± SD‡ | 24 | 8.41 ± 10.9 | 36 | 14.7 ± 10.2 | 5.00 ± 3.67 | 8 | 65.9 ± 50.3 | 8.10 ± 9.00 | |
| 1994 | UI | 21 | 9.40 | 9 | 14.1 | 4.33 | 3 | 9.50 | 1.37 |
| SKI | 13 | 10.9 | 20 | 29.5 | 8.94 | 12 | 26.5 | 3.61 | |
| SKII | 7 | 1.07 | 9 | 2.72 | 1.37 | – | |||
| SKIV | 8 | 1.13 | 10 | 7.60 | 2.65 | 1 | 55 | 9.2 | |
| SKV | 3 | 0 | 4 | 60.4 | 15.7 | – | |||
| Mean ± SD | 52 | 4.50 ± 5.20 | 52 | 22.9 ± 23.3 | 6.59 ± 5.84 | 16 | 30.4 ± 23.0 | 4.73 ± 4.03 | |
| 1995 | UI | 6 | 13.8 | 8 | 3.88 | 1.56 | 6 | 65.6 | 9.63 |
| SKI | 2 | 0 | 12 | 4.13 | 1.38 | 7 | 29.1 | 2.49 | |
| SKIV | 8 | 11.7 | 6 | 14.8 | 4.43 | 2 | 22.8 | 3.80 | |
| SKV | 12 | 8.08 | 4 | 5.00 | 2.50 | 1 | 95.0 | 4.75 | |
| SKVI | 5 | 0 | 17 | 25.2 | 11.7 | 1 | 32.5 | 3.30 | |
| Mean ± SD | 33 | 6.72 ± 6.47 | 47 | 10.6 ± 9.34 | 4.31 ± 4.30 | 17 | 49.0 ± 30.6 | 4.79 ± 2.89 | |
| 1996 | UI | 5 | 0 | 7 | 8.64 | 3.21 | 4 | 29.4 | 4.30 |
| SKIV | 5 | 18.2 | 7 | 9.29 | 4.24 | 3 | 64.5 | 8.50 | |
| SKV | 7 | 4.86 | 3 | 4.83 | 1.60 | 4 | 6.5 | 0.60 | |
| SKVI | 15 | 0.33 | 6 | 0.75 | 0.33 | 4 | 7.8 | 1.00 | |
| Mean ± SD | 32 | 5.85 ± 1.29 | 23 | 5.88 ± 3.94 | 2.35 ± 1.73 | 15 | 27.1 ± 27.1 | 3.60 ± 3.74 | |
| Mean ± SD§ | 5.10a,a ± 3.18 | 11.8a ± 7.32 | 4.02a ± 1.94 | 38.2b ± 19.0 | 4.58a ± 2.33 | ||||
- Half of the number of adults that emerged during and after September per colony (N) and per foundress (N/F) are shown. Colonies that disappeared or those that produced adults only before the end of August were counted as zero production. F, Number of foundresses found on nests in April; FGS, foundress group size; n, number of colonies. †In haplometrotic colonies (FGS = 1), N/F = N. ‡Means and standard deviations are calculated using station means, and standard deviations are smaller than those calculated using colony values. For example, the mean for FGS 2–5 in 1992 is (0 + 10)/2 = 5.0, but eight of nine colonies produced no adults and only one colony produced 50 adults. From these nine values, the mean and SD are 5.55 and 16.67, respectively. §Mean and SD are calculated from yearly means. For aand b, see the footnote of Table 7.
Contrary to Table 8, where N-values for colonies that disappeared before September were counted as zero, Table 9 shows the results of the calculation using values for all colonies except those that disappeared. The trends in N′ in relation to FGS were similar to those in Table 8. The values of N′ in colonies where FGS ≥ 6 were significantly larger than those in colonies where FGS = 1 (χ2-test, P < 0.01). N′/F-values for colonies where FGS ≥ 6 were smaller than N′/F of colonies where FGS = 1, but the difference was not significant.
| Year | Station | FGS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2–5 | ≥6 | |||||||
| n | N′ † | n | N′ | N′/F | n | N′ | N′/F | ||
| 1992 | SKI | – | 1 | 50.0 | 16.7 | 1 | 7.5 | 6.8 | |
| 1993 | SII | 2 | 6.5 | 2 | 12.5 | 4.8 | 1 | 123 | 20.5 |
| UI | – | 3 | 30.0 | 13.5 | 3 | 93.0 | 8.3 | ||
| SKI | 2 | 54.5 | 12 | 46.7 | 11.1 | 2 | 38.0 | 2.7 | |
| SKII | 1 | 0 | 5 | 5.1 | 1.8 | 1 | 6.0 | 0.8 | |
| SKIV | 1 | 7.5 | 2 | 18.8 | 4.3 | – | |||
| Mean ± SD‡ | 4 | 17.1 ± 25.1 | 5 | 22.6 ± 16.3 | 7.1 ± 5.0 | 4 | 65.0 ± 52.8 | 8.1 ± 8.9 | |
| 1994 | UI | 4 | 49.4 | 4 | 31.8 | 9.8 | 1 | 28.5 | 4.1 |
| SKI | 3 | 47.2 | 10 | 59.1 | 17.7 | 9 | 35.3 | 4.4 | |
| SKII | 2 | 12.3 | 4 | 6.1 | 3.1 | – | |||
| SKIV | 1 | 9.0 | 4 | 19.0 | 6.6 | 1 | 55.0 | 9.2 | |
| SKV | – | 4 | 10.4 | 15.7 | – | ||||
| Mean ± SD | 4 | 29.5 ± 21.8 | 5 | 25.3 ± 21.3 | 10.6 ± 6.1 | 3 | 39.6 ± 13.8 | 5.9 ± 2.9 | |
| 1995 | UI | 2 | 83.0 | 3 | 10.3 | 4.2 | 6 | 65.6 | 9.6 |
| SKI | – | 2 | 24.8 | 8.3 | 5 | 40.8 | 3.5 | ||
| SKIV | 2 | 46.8 | 3 | 29.7 | 8.9 | 2 | 22.8 | 3.8 | |
| SKV | 2 | 48.5 | 1 | 20.0 | 10.0 | 1 | 95.0 | 4.8 | |
| SKVI | 1 | 0 | 8 | 53.6 | 24.8 | 1 | 32.5 | 3.3 | |
| Mean ± SD | 4 | 44.6 ± 34.0 | 5 | 27.7 ± 16.2 | 11.3 ± 7.9 | 5 | 51.3 ± 20.9 | 5.0 ± 3.3 | |
| 1996 | UI | – | 3 | 20.2 | 7.5 | 3 | 39.2 | 5.8 | |
| SKIV | 3 | 30.3 | 3 | 21.7 | 9.9 | 3 | 64.5 | 8.5 | |
| SKV | 2 | 17.0 | 1 | 14.5 | 4.8 | 3 | 8.7 | 0.3 | |
| SKVI | 4 | 1.3 | 3 | 1.5 | 0.7 | 3 | 10.3 | 1.3 | |
| Mean ± SD | 3 | 16.2 ± 14.5 | 4 | 14.5 ± 9.2 | 5.7 ± 4.0 | 4 | 30.7 ± 26.6 | 4.0 ± 3.9 | |
| Mean ± SD§ | 4 | 26.9a,a ± 13.3 | 5 | 28.0a ± 13.3 | 10.3a ± 4.3 | 4 | 38.8b ± 21.7 | 6.0a ± 3.1 | |
- The number that emerged per colony (N ′) and per foundress (N ′/F) were calculated using values for colonies excluding those that completely failed (failed but not reconstructed), in contrast to Table 8, in which completely failed colonies were counted as zero production. FGS, Foundress group size; n, number of colonies. †In haplometrotic colonies, N ′/F = N ′. ‡See the footnote of Table 8. §For definitions for a and b, see the footnote of Table 6. As N ′/F = N ′in FGS = 1, a,afor this column indicates a difference between N ′ and N ′/F values of different group sizes.
These results suggest that colonies established with larger FGS produced larger numbers of new foundresses per colony, but that the productivity per foundress did not differ among the three FGS categories.
Effects of natural enemies
An ichneumon parasitoid, Arthula formosana, is an important natural enemy of R. fasciata in Okinawa. Foundresses may be often absent on a haplometrotic nest because of foraging, but on a pleometrotic nest some foundresses always sit on their nest. Thus, nests with a larger number of foundresses may be defended better against oviposition by parasitoids. Percentage parasitism by A. formosana on nests established by different number of foundresses during May and June is shown in Table 10. Values for FGS ≥ 6 were significantly lower than for FGS = 1 and 2–5. Although anova showed that differences in percentage parasitism between years and stations were significant (e.g. percentage parasitism at UI and SKIII was, in all FGS categories, large) at 6 of 7 stations, percentage parasitism was lowest in nests with more than five foundresses and, at 5 of the 7 stations, highest in haplometrotic nests (Table 10).
| Station | No. years | FGS | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2–5 | 6 | |||||
| n | % | n | % | n | % | ||
| UI | 4 | 1 | 20.0 | 1 | 53.0 | ||
| 9 | 31.3 | 5 | 27.6 | 1 | 19.0 | ||
| 2 | 25.0 | 3 | 45.0 | 6 | 24.3 | ||
| 1 | 50.0 | 5 | 32.8 | 3 | 39.8 | ||
| Mean ± SD | 35.4 ± 13.0 | 31.4 ± 10.5 | 34.0 ± 15.4 | ||||
| SKI | 4 | 1 | 4.0 | 7 | 2.6 | 3 | 13.0 |
| 3 | 13.6 | 13 | 22.7 | 1 | 10.0 | ||
| 1 | 33.0 | 6 | 18.5 | 5 | 8.2 | ||
| 2 | 23.5 | 2 | 15.0 | 5 | 10.8 | ||
| Mean ± SD | 18.5 ± 12.5 | 14.7 ± 8.7 | 11.1 ± 1.4 | ||||
| SKII | 2 | 3 | 35.7 | 62 | 20.517.0 | 2 | 2.0 |
| Mean | 35.7 | 18.8 | 2.0 | ||||
| SKIII | 1 | 1 | 47.0 | 3 | 38.0 | 1 | 12.0 |
| SKIV | 4 | 3 | 48.3 | 2 | 48.0 | ||
| 1 | 13.0 | 7 | 22.8 | ||||
| 3 | 21.7 | 4 | 38.8 | ||||
| 3 | 11.7 | 3 | 7.7 | 3 | 1.0 | ||
| Mean ± SD | 23.7 ± 17.0 | 29.3 ± 17.8 | 1.0 | ||||
| SKV | 2 | 3 | 31.7 | 21 | 34.00 | 13 | 15.00 |
| Mean | 31.7 | 17.0 | 7.5 | ||||
| SKVI | 2 | 3 | 31.7 | 8 | 28.9 | 1 | 21 |
| 2 | 8.5 | 1 | 0 | 2 | 0 | ||
| Mean | 20.1 | 14.5 | 10.5 | ||||
| Mean ± SD | 30.3 ± 10.2 | 23.4 ± 9.4 | 11.2 ± 11.0 | ||||
- n, Number of colonies.
The foundress group size was known for ten nests that were known to have been destroyed by ants. These were: five nests with a single foundress, three nests with two foundresses, one nest with three foundresses, and one nest with ten foundresses. Thus 50% of the nests destroyed by ants were haplometrotic ones. Nests established by one or two foundresses represented 80% of the nests destroyed by ants. From the original data shown in Figure 1, the ratios of haplometrotic nests and nests with one or two foundresses to all nests were 43% and 60%, respectively. Although the sample size was small, the above-mentioned values seem to suggest a high rate of ant predation on nests established by small foundress groups.
DISCUSSION
Foundress group size
Eusocial polistine wasps can be divided into independent-founding species, in which new nests are established by one or more foundresses, some of which become queens, after emergence of the progeny, and swarm-founding species, in which nests are established by groups of queen(s) and workers. Colonies of the former species are founded either by a solitary queen (haplometrosis) or by multiple queens (pleometrosis). Pleometrosis raises the question of whether the reproduction is limited to one or a few foundresses, or shared by many foundresses (communal breeding or polygyny), and the former situation raises the question of why some females join in the founding of a pleometrotic nest rather than founding their own nest. Not only behavioral and genetic factors, but also ecological, including demographic, factors may contribute to FGS and number of effective queens (Yanega 1997; see also Brockmann 1997).
In the first part of this study, it was shown that the mean life span of foundresses and workers of R. fasciata was 40–50 days and 10–20 days, respectively (Itô & Kasuya 2005). These values are similar to or somewhat shorter than corresponding values for other independent founding wasps studied to date. In contrast, the mean and maximum life spans of colonies are approximately 100 and 240 days, respectively, far longer than those of other temperate polistine wasps.
Figure 1 shows the distribution of the number of nests established by different numbers of foundresses. Nearly half (43%) of Okinawan R. fasciata nests were established by a single foundress, but 15% of nests were established by more than five foundresses. The maximum number of foundresses was 22. The distribution pattern of the frequency of FGS did not fit a random (0-truncated Poisson) distribution, but rather fit a 0-truncated negative binomial distribution with overcrowding. This result basically supports Itô and Iwahashi's (1987) earlier findings that none of FGS distributions of R. fasciata observed at seven localities could be fitted by the truncated Poisson distribution, but that six of seven distributions fitted the truncated negative binomial distribution well.
In contrast, the distribution pattern of FGS for R. plebeiana in Australia for new nests (this species frequently uses old nests to initiate a new colony cycle) did not differ from the Poisson distribution (Itôet al. 1988). FGS distributions in two ant species, Solenopsis invicta (Tschinkel & Howard 1983) and Messor pergandei (Pfennig 1995), significantly departed from the truncated Poisson distribution due to overcrowding. These authors considered that nest preference or nest site preference by founding queens may have been the reason for overcrowding (see also Bernasconi & Strassmann 1999).
Differing from these ants, which found nests with unrelated individuals after a nuptial flight, foundresses of R. fasciata tend to overwinter on their natal nests and to found new nests with foundresses that have emerged from the same nests (Itô & Kasuya 2005). Foundresses that have been isolated from their natal nests, for example by strong wind or their natal nest falling, may find it difficult to find their relatives and may prefer to initiate nests solitarily. This may be the reason for the high frequency of single foundress nests. The possible existence of “pioneers” that find new habitats, especially when foundress density is high, as suggested by Itô and Iwahashi (1987), could be another reason, but further studies are necessary to understand the effects of foundress density on the proportion of haplometrotic colonies.
Effects of FGS on nest and colony survival rates
In Okinawan R. fasciata, the rate of nest failure of pleometrotic colonies was significantly lower than that of haplometrotic colonies (Fig. 2 and Table 1), whereas the rate of reconstruction of failed nests was higher for pleometrotic colonies than for haplometrotic ones (Table 1). This result supports earlier findings for this species (Itô 1987; Kojima 1989). A similar trend was found for Polistes fuscatus in Ontario (Gibo 1978); that is, in exposed sites the survival and reconstruction rates for haplometrotic nests were lower than those for pleometrotic nests (0.067 vs 0.167 for survival rate and 0 vs 0.385 for reconstruction rate up to the first emergence of reproductive offspring). For Mischocyttarus labiatus, Litte (1981) also reported a higher reconstruction rate of failed nests for pleometrotic than for haplometrotic colonies.
The fact that the survival rate up to September of haplometrotic colonies was lower than the survival rates of pleometrotic colonies in all the years studied (Table 2) suggests a positive effect of FGS on the colony survival rate of R. fasciata.
The higher survival rate of pleometrotic colonies relative to haplometrotic ones is known in many other polistine species. Itô (1987) determined the survival rates of haplometrotic and pleometrotic nests of four Panamanian independent-founding wasp species: Mischocyttarus angulatus, M. basimacula, Polistes versicolor and P. canadensis. Although his sample size was small, all the haplometrotic nests failed during his study, but the failure rate of the pleometrotic nests ranged from 0 to 50%. The colony survival rates up to emergence of the first progeny in haplometrotic and pleometrotic colonies were 0.62 and 0.78 in Mischocyttarus mexicanus (Litte 1979), 0.07 and 0.17 in P. fuscatus (Gibo 1978; exposed sites), and 0.20 and 0.80 in P. annularis (Strassmann 1989).
Nest usurpation
Table 3 shows what occurred after the establishment of alien foundresses on nests. In six of the seven haplometrotic nests (86%) that accepted alien foundresses, only alien foundresses were seen in subsequent observations and the original foundresses disappeared (usurpation). However, in 19 of the 22 pleometrotic nests that accepted alien foundresses, the original foundresses generally coexisted with the alien foundresses, with only three nests (14%) losing their original foundresses. Naturally, haplometrotic colonies may lose their original foundress not only because of attack by a newcomer but also because of natural death. However, the large difference between haplometrotic and pleometrotic nests suggests that pleometrotic colonies have better defense capabilities against usurpation. A similar tendency has been observed in other Polistes species, including P. metricus (Gamboa 1980) and P. fuscatus (Klahn 1988).
Natural enemies
Table 10 suggests that colonies with many foundresses have better defense capability against parasitization by Arthula formosana, the most important parasitoid of R. fasciata in Okinawa (Kojima 1989). If so, the situation is different from that in P. metricus, for which no evidence of a relation between FGS and parasitism has been found (Gamboa 1978), and P. exclamans, for which larger colonies, possibly produced by larger foundress groups, are more heavily attacked by parasitoids (Strassmann 1981). In addition, R. fasciata nests established by many foundresses are less likely to be destroyed by ants than haplometrotic or two-foundress nests. Although the sample size is small, this observation is important, because ants are the most important natural enemy of R. fasciata in Okinawa (Itô & Kasuya 2005), where bird predation has not yet been reported.
Survival rate of foundresses
There was no significant difference in the survival rates of foundresses between haplometrotic and pleometrotic colonies (Table 4). In contrast, in M. mexicanus (Litte 1977), M. labiatus (Litte 1981) and P. fuscatus (Noonan 1981), the survival rates of queens (sole ovipositor) or dominant foundresses on pleometrotic nests were significantly higher than queens on haplometrotic nests.
Colony productivity and FGS
Colonies established by larger foundress groups tended to produce workers earlier than those established by one or a small number of foundresses (Table 5). This fact, however, may be not directly due to immatures on nests with many foundresses having a faster growth rate, because pleometrotic nests are usually founded earlier than haplometrotic ones.
Colonies with a larger FGS constructed larger nests and produced a significantly larger number of progeny adults up to both early June and the end of July (Tables 6,7) relative to colonies with a smaller FGS. In addition, larger FGS colonies produced more potential foundresses for the next year than did smaller FGS colonies (Table 8).
However, the larger productivity of pleometrotic colonies relative to haplometrotic ones does not mean that joining nest foundation is a better strategy for each joiner, even when most foundresses can reproduce (this might be a rare occurrence, we know that this is true only for R. rufoplagiata; Sinha et al. 1993). Production of potential foundresses per foundress in R. fasciata did not vary significantly with FGS (Tables 8,9). Gibo (1978) found a similar tendency in P. fuscatus: although pleometrotic colonies in exposed sites produced far more reproductives than haplometrotic colonies (12.33 vs 2.53), the difference in productivity per foundress was small (4.44 vs 2.53). Using the information provided by West-Eberhard (1969) for P. fuscatus and P. erythrocephalus, the per capita cell productivity of 2–5 foundress nests and 6–10 foundress nests can be calculated to be 75–85% and 55–60%, respectively, of the corresponding values in haplometrotic nests.
Based on the fact that the per capita productivity of cells up to late July in 6–10 foundress colonies was more than twice that of single foundress colonies (although the difference was not significant owing to large variances), and that more than one foundresses laid eggs on many nests and that many of these eggs were not eaten, Itô (1993) considered that Okinawan R. fasciata is truly polygynous, providing support to the communal aggregation hypothesis (Lin & Michener 1972; Itô 1993) of early evolution of eusociality. However, the results presented here seem to contradict this idea. When there is no difference between colonies with different FGS with respect to the per capita productivity of potential foundresses, independent founding is the better strategy for future subordinate foundresses, even when relatedness between co-foundresses is near 0.75.
Possible role of partial bivoltinism
Itô and Kasuya (2005) showed that the life span of R. fasciata foundresses in Okinawa was 40–50 days after nest foundation, which is similar to, or somewhat shorter than, the life span of other temperate polistine wasps. On the other hand, the mean life span of colonies was approximately 100 days and the maximum life span was 240 days, which is far longer than the maximum colony life spans of other temperate species (140–150 days). These facts suggest partial bivoltinism in this species in Okinawa, which would necessitate serial queen replacement over the colony lifetime. Production of gynes after death of all the foundresses, as suggested in this study, supports this idea.
Hunt and Amdam (2005) proposed an explanation for the evolution of eusociality in Polistes. They considered that the bivoltine life cycle of the solitary ancestors of Polistes might have developed into a combination of worker-producing and gyne-producing periods. In their view, “the bivoltine ground plan hypothesis of caste evolution in Polistes inaugurates a changed perspective on the evolution and maintenance of sociality in insects.” Although this is a very hypothetical view, and it is unclear whether this could apply to eusocial groups other than Polistes, their emphasis on the role of bivoltinism is important. Queen replacement is known in many primitively eusocial wasps, and the roles of relatedness, dominance and other factors have been intensively investigated (e.g. Polistes annuralis, Peeters et al. 1995 and Ropalidia marginata,Arathi & Gadagkar 1998; see Gadagkar 2001). Investigation of queen replacement in the Okinawan population of R. fasciata, including queen-removal experiments, is an important topic for future study.
Demographic studies conducted over a longer period and experimental manipulations of colony structure are necessary to develop a better understanding of the social and demographic structure of R. fasciata. Detailed data on demography are indispensable for accurate estimation of the inclusive fitness of any eusocial species.
ACKNOWLEDGMENTS
We are grateful to Eiiti Kasuya for providing many suggestions regarding the statistical methods, and to Shun'ichi Makino, Shinya Miyano, Koji Tsuchida and Kazuki Tsuji for their helpful suggestions.




