Survivorship and growth in the larvae of Luehdorfia japonica feeding on old leaves of Asarum megacalyx
Abstract
Although the papilionid butterfly Luehdorfia japonica, usually lays eggs on new leaves of the host plant (Asarum sp.; Aristolochiaceae), eggs of the butterfly were frequently found on old leaves of Asarum megacalyx in Suyama, Tokamachi, Niigata prefecture. Larvae hatched on new leaves and those hatched on old leaves did not show significant differences in their survival rate in the field. In laboratory breeding, about 90% of larvae that were fed old leaves survived and developed normally to the pupal stage. Their growth rate, however, was slightly lower than those that were fed new leaves. No nutritional differences were found between the old and new leaves. The reason why oviposition on the old leaves was so frequent and why larvae that hatched on old leaves could survive in the study area is discussed.
INTRODUCTION
A papilionid butterfly, Luehdorfia japonica Leech, is endemic to the central and western parts of Honshu, Japan. The butterfly is univoltine with adult emergence in early spring, and the females lay eggs in clusters on leaves of the larval host plant, which is any available species of the genus Asarum (Aristolochiaceae), and in most cases is an evergreen species. The hatched larvae feed on the leaf in groups during the early instar stages. After the leaves of original host plants are entirely eaten up, the larvae disperse in search of the next host plant, splitting up into continuously smaller groups, until they become solitary in the last (fifth) instar stage. In early summer, the larvae pupate near the ground, and the pupal stage lasts until the next spring.
It is known that when the host plant is an evergreen species, female butterflies usually choose new leaves (i.e. freshly growing current year leaves) for oviposition (Fuzisawa et al. 1964; Fukuda et al. 1982). However, in Suyama, Tokamachi, Niigata prefecture, we frequently found the eggs laid on old leaves (i.e. overwintered leaves produced in the previous spring) of Asarum megacalyx. Old leaves of plants are generally thought to be unsuitable as food for phytophagous insects because of higher physical and chemical defense levels in old leaves than in new leaves (McKey 1974; Rhoades & Cate 1976). Osada (1982, 1983) experimentally fed old leaves to isolated and grouped larvae of L. japonica and showed that the larvae accepted the old leaves as food, but the survival rates of the larvae in isolation and in small groups were lower than those in larger groups. Then, questions arise: can the larvae hatched on old leaves survive and normally develop in nature? If they can, is it because of beneficial effects of larval aggregation detected in this species (Osada 1978; Matsumoto 1989), and why do the female butterflies lay eggs on old leaves?
In the present study, we examined the frequency of oviposition on old leaves and on new leaves, and the survivorship pattern of the larvae hatched from eggs laid on old leaves in the field and compared it with that of the larvae hatched on new leaves. Then we conducted laboratory breeding experiments and evaluated the effects of leaf age and larval aggregation on the survival and growth rates from eggs to pupae.
MATERIALS AND METHODS
Field survey
Field observations were made in a secondary deciduous forest in 2004 and 2005 in Suyama, Matsunoyama, Tokamachi, Niigata (37°5′N, 138°36′E, altitude 300 m) where the beech Fagus crenata predominates, and there is a young (approximately 6 m tall) plantation of Japanese cedar, Cryptomeria japonica. Annual precipitation observed at the Matsunoyama branch office of Tokamachi City Hall (4 km from Suyama) is about 2800 mm. The larval host plant, A. megacalyx, occurs in several patches on the forest floor.
Survivorship data were collected in the following three plots. Plot 1 (12 m × 18 m) was set in a bushy concave area in the deciduous forest, plot 2 (12 m × 12 m) was set in the Japanese cedar forest, and plot 3 (12 m × 18 m) was set beside a trail in the deciduous forest. Each plot was subdivided into 1 m × 1 m quadrates, and all individual plants of A. megacalyx within the plots were marked with a small flag and distinguished from each other. Observations were made only in plot 1 and plot 2 in 2004 and in all three plots in 2005.
During the oviposition period, 7 May through 31 May, 2004, and 15 May through 10 June, 2005, we checked all leaves every third day to see whether eggs had been laid or not. When an egg cluster was found, we marked it by drawing a circle on the leaf surface around the eggs with a felt-tipped pen, and recorded the number of eggs and whether the leaf was old or new.
After hatching, the number of surviving larvae, their location and whether the leaves on which they occurred were new or old were recorded at 1 to 2 day intervals until fourth instar larvae appeared. Since the fourth and fifth instar larvae quickly consume whole leaves of an individual host plant and move to another plant (fourth instar larvae traveling up to 2 m per day and fifth instar, up to 4 m per day), we checked the host plants within 5 m from the edge of the plots every other day after the appearance of fourth instar larvae. Moreover, fourth and fifth instar larvae were marked with a unique pattern of small paint dots to enable individuals to be recognized. The survey was continued until the fifth instar larvae disappeared. Because of the intensive inspection, individual marking and rather low density of the larvae, we could follow the fate of all larvae hatched in the study plots without contamination, and survivorship curves were separately drawn for the larvae originating from old leaves and those from new leaves.
Laboratory experiments
Breeding experiments were conducted in the laboratory of Echigo-Matsunoyama Museum of Natural Science, Matsunoyama, Tokamachi city, Niigata prefecture, from 1 June to 15 July, 2005. Eggs and leaves of A. megacalyx used in the experiments were collected from a hilly outlying area of Ojiya city, which is known for heavy snowfall in winter, about 35 km distant from Suyama. The food leaf stock was kept in a plastic bag and preserved in a refrigerator set at approximately 5°C, then used within 1 week after collection. We did not collect extremely small or large leaves, and limited the size of the leaves to be used from 4.0 cm to 8.0 cm in midrib length to minimize unintended variation of breeding conditions.
Five series of treatments classified by leaf age and larval group size were prepared as follows:
-
Series 1: Larvae were kept singly and fed new leaves throughout the larval period (16 groups).
-
Series 2: Eight larvae were kept together in a group until the second molt, then the larvae were divided into four groups of two individuals, and they were kept singly after the third molt. All stages were fed new leaves (two groups).
-
Series 3: Larvae were kept singly and fed old leaves throughout the larval period (16 groups).
-
Series 4: Eight larvae were kept together in a group at first and the group size was reduced as in the same way as in Series 2. All stages were fed old leaves (two groups).
-
Series 5: Eight larvae were kept together in a group at first and the group size was reduced as in the same way as in Series 2. The larvae were fed new leaves until the second instar, but fed old leaves from the third instar onward (two groups).
The group size manipulation along with larval development in Series 2, 4 and 5 followed the change of larval group size in the field. Eight individuals per group were chosen as a group size of newly hatched larvae for Series 2, 4 and 5 because the average egg cluster size in the field was nine and seven in 2004 and 2005, respectively (Table 1), whereas the group size for the third instar was just tentatively chosen, because a representative group size in nature was rather difficult to determine due to variation caused by splitting up of original groups into smaller ones or individuals. Single breeding of the larvae in the fourth and fifth instar stages reflects the solitary nature of the larvae during these stages.
| 2004 | 2005 | |||||
|---|---|---|---|---|---|---|
| Eggs | Egg clusters | Cluster size | Eggs | Egg clusters | Cluster size | |
| New leaves | 242 | 27 | 9.0 ± 0.5 | 87 | 11 | 7.9 ± 0.7 |
| Old leaves | 45 | 5 | 9.0 ± 2.1 | 18 | 4 | 4.5 ± 1.7 |
| Total | 287 | 32 | 9.0 ± 0.5 | 105 | 15 | 7.0 ± 0.7 |
| Ratio of oviposition on old leaves | 16.1% | 26.7% | ||||
- Ratio of oviposition on old leaves is a percentage of egg clusters found on old leaves to the whole egg clusters.
Experimental eggs were carefully replaced from the original leaf to an experimental leaf just before hatching. The cultures were maintained in a plastic container (14 cm long, 12 cm wide and 7 cm deep) and kept in a thermostatic chamber set at 23 ± 1°C.
The initial leaves supplied to newly hatched larvae were not replaced with new ones during the first to the second instar stage. We supplied one leaf to the third instar larvae in a culture every other day, two leaves to the fourth instar larvae every day, and three leaves to the fifth instar larvae every day, removing leftover leaves. There was no indication of food shortage throughout the experiments.
All cultures were checked every day and the number of surviving larvae in each instar was recorded. Any dead larvae were removed as soon as they were found. The body weight of third, fourth and fifth instar larvae was measured on the day after molting and pupae were also weighed 1 week after formation.
Nutritional contents of new and old leaves
We commissioned Japan Food Research Laboratories, Tokyo, with the analyses of the nutritional content of new and old leaves. New and old leaves were collected in Daigonji-Kogen, Matsunoyama, Tokamachi, about 10 km from Suyama, and sent to the laboratories. Methods of analyses followed the methods of analysis in feeds and feed additives by the Japan Scientific Reeds Association (2004), as follows: 3.5 ± 1.5 g of leaf samples were weighed, then dried at 135 ± 2°C for 2 h and the ratio of water was calculated. The rest of the leaf samples were dried at approximately 60°C then milled in advance of the subsequent analyses. Then 3.5 ± 1.5 g of leaf powder was burned at 575 ± 25°C for 2 h and the remaining ash was defined as minerals. Another 3.5 ± 1.5 g of leaf powder was boiled up with liquor acid, then with liquor alkalescent, and the residue was washed with ethanol, then with diethyl ether, and weighed. The value of crude fiber was obtained by subtracting the weight of ash in the quantification of minerals from that of the residue obtained by the above operation. Protein was quantified by the Kjeldahl method, and lipid was quantified by the Soxhlet extraction method. The concentration of sugar was obtained by subtracting the concentration of water, protein, lipid, fiber and minerals from 100. Bomb calories were measured by CA-4PJ (Shimazu, Kyoto, Japan).
RESULTS
Oviposition in the field
More eggs were laid on new leaves than on old ones both in 2004 and 2005. The number of egg clusters on new leaves gradually increased, whereas on old leaves, oviposition occurred only at the beginning of the oviposition period and the eggs did not increase afterward (Fig. 1). When the first oviposition was observed, over 60% of A. megacalyx plants in each plot had started to put forth new leaves, but most of the new leaves were still folded and too small for oviposition. Eventually, 16.1% and 26.7% of the whole egg clusters were laid on old leaves in 2004 and 2005, respectively (Table 1).
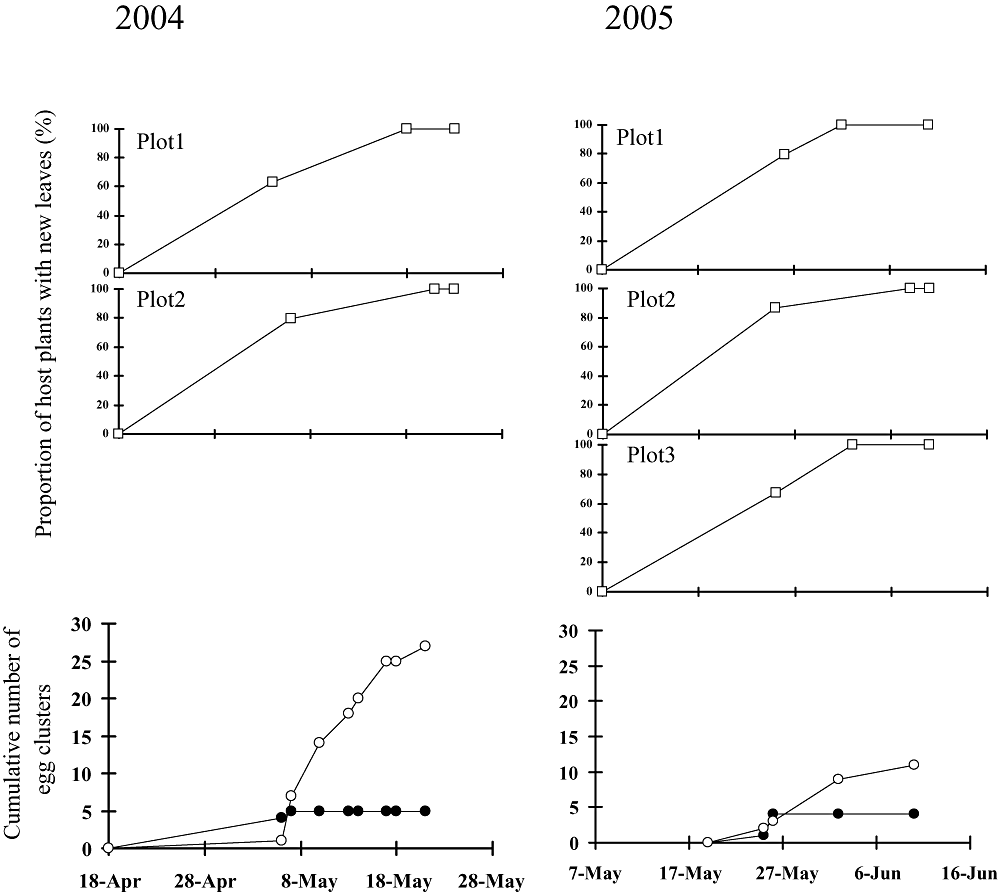
The proportion of Asarum megacalyx plants putting forth new leaves in (□) each plot and number of egg clusters laid on (●) old leaves and (○) new leaves in 2004 and 2005, respectively.
Survivorships of larvae in the field
After larvae had eaten up their original leaves and dispersed, they were mainly found on new leaves, but sometimes found on old leaves; 105 times on new leaves and 18 times on old leaves in 2004, and 145 times on new leaves and 8 times on old leaves in 2005, respectively. In fact, by the time the larvae started to disperse, most of old leaves had withered and few were still available.
The survivorship curves for the larvae hatched from eggs laid on new leaves and those from eggs on old leaves were variable; a higher survival rate was observed for the larvae hatched on the old leaves in 2004, whereas the opposite was true in 2005 (Fig. 2). There were no significant differences in the survival rate from eggs to fifth instar stage between the two cohorts both in 2004 (log–rank test, χ2 = 0.000457, P = 0.98) (Yanagii 2004) and 2005 (χ2 = 1.18, P = 0.28). Survival rate from eggs to second instar stage was not different in 2004 (χ2 = 2.55, P = 0.11) and in 2005 (χ2 = 3.50, P = 0.06), either.
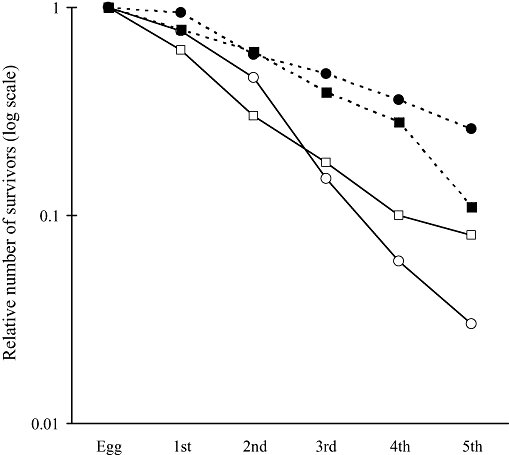
Survivorship curves up to the fifth instar larval stage for Luehdorfia japonica hatched from eggs laid on old leaves and new leaves of Asarum megacalyx in 2004 and 2005, respectively. (□) Hatched on old leaves in 2004, (○) hatched on new leaves in 2004, (▪) hatched on old leaves in 2005, and (●) hatched on new leaves in 2005.
Survivorships of larvae in breeding experiment
In the laboratory experiments, three larvae supplied with old leaves died, that is, two first instar larvae did not eat old leaves at all and starved in Series 3, and one larva died at the end of fourth instar in Series 4 (Table 2). All the other larvae grew normally to the pupal stage. Survival rates of larvae were not significantly different among the five treatments (log–rank test, χ2 = 0.215, P = 0.99).
| Series | Leaves supplied as food | Initial number of larvae/culture | Larval instar | Pupa | ||||
|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||||
| 1 | New leaves | 1 | 16 | 16 | 16 | 16 | 16 | 16 |
| 2 | New leaves | 8 | 16 | 16 | 16 | 16 | 16 | 16 |
| 3 | Old leaves | 1 | 16 | 14 | 14 | 14 | 14 | 14 |
| 4 | Old leaves | 8 | 16 | 16 | 16 | 16 | 15 | 15 |
| 5 | New leaves (first to second instar) and old leaves (third to fifth instar) | 8 | 16 | 16 | 16 | 16 | 16 | 16 |
Developmental period
The larvae grew faster in breeding with new leaves (taking approximately 23 days in Series 1 and 2) than in breeding with old leaves (approximately 28 days in Series 3 and 4) (Table 3). The larval duration was intermediate (approximately 25 days) between the two in Series 5 where the food was changed from new leaves to old leaves on the way. The durations of larval stage were significantly different among the five treatments (Kruskal–Wallis test, H = 21.0, P < 0.001 (Sokal & Rohlf 1995), Table 4). There were significant differences in durations taken to complete each instar among the five treatments (Kruskal–Wallis test, H = 37.1, P < 0.0001 in the first instar stage, H = 15.1, P < 0.01 in the second instar stage, H = 31.6, P < 0.0001 in the third instar stage, H = 17.1, P < 0.01 in the fourth instar stage and H = 46.1, P < 0.0001 in the fifth instar stage; Table 4).
| Series | Duration of each larval stage (day) | |||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | Total | |
| 1 | 3.2 ± 0.1 | 2.8 ± 0.1 | 3.3 ± 0.2 | 4.3 ± 0.2 | 9.5 ± 0.1 | 23.1 ± 0.2 |
| 2 | 3.0 ± 0.0 | 3.0 ± 0.0 | 2.9 ± 0.2 | 4.6 ± 0.2 | 9.5 ± 0.1 | 22.9 ± 0.2 |
| 3 | 3.6 ± 0.1 | 3.5 ± 0.2 | 4.4 ± 0.3 | 5.1 ± 0.2 | 11.4 ± 0.2 | 27.9 ± 0.6 |
| 4 | 4.5 ± 0.1 | 3.5 ± 0.1 | 4.6 ± 0.1 | 5.3 ± 0.1 | 11.1 ± 0.3 | 29.1 ± 0.5 |
| 5 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.8 ± 0.2 | 5.1 ± 0.1 | 10.7 ± 0.3 | 25.6 ± 0.5 |
| Pair of treatments | Duration of each larval stage (day) | |||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | Total | |
| 1 and 3 | −2.14 | −3.09* | −2.98* | −2.52 | −4.82* | −4.60* |
| 2 and 4 | 5.19* | −3.34* | −4.55* | −3.25* | −4.45* | −4.80* |
| 2 and 5 | – | – | −3.20* | −2.56 | −3.91* | −4.44* |
| 1 and 2 | 1.79 | −1.79 | 1.17 | −1.16 | 0 | 0.54 |
| 3 and 4 | −3.54* | 0.35 | −0.50 | −0.77 | 1.01 | −1.90 |
- * P < 0.05. The upper three rows show the differences between old leaves and new leaves, and the lower two rows show the differences in the number of larvae per culture.
Body weight of larvae and pupae
In all of the stages, larvae fed on new leaves were heavier than those fed on old leaves (Table 5). There were significant differences in weight of larvae of all measured instars and pupae among the five treatments (Kruskal–Wallis test, H = 44.2, P < 0.0001 in third instar larvae, H = 53.4, P < 0.0001 in fourth instar larvae, H = 55.4, P < 0.0001 in fifth instar larvae and H = 39.5, P < 0.0001 in pupae; Table 6). The differences in pupae, however, were smaller than those in larvae.
| Series | Larval instar stage | Pupa | ||
|---|---|---|---|---|
| Third | Fourth | Fifth | ||
| 1 | 0.065 ± 0.003 | 0.249 ± 0.012 | 0.740 ± 0.027 | 0.558 ± 0.014 |
| 2 | 0.070 ± 0.006 | 0.220 ± 0.015 | 0.832 ± 0.026 | 0.580 ± 0.014 |
| 3 | 0.042 ± 0.005 | 0.140 ± 0.008 | 0.494 ± 0.024 | 0.488 ± 0.016 |
| 4 | 0.026 ± 0.002 | 0.117 ± 0.005 | 0.446 ± 0.013 | 0.435 ± 0.010 |
| 5 | 0.050 ± 0.003 | 0.164 ± 0.007 | 0.551 ± 0.018 | 0.491 ± 0.012 |
| Pair of treatments | Larval instar stage | Pupa | ||
|---|---|---|---|---|
| Third | Fourth | Fifth | ||
| 1 and 3 | 3.26* | 4.35* | 4.07* | 2.66 |
| 2 and 4 | 4.75* | 4.63* | 4.74* | 4.62* |
| 2 and 5 | 2.34 | 3.06* | 4.75* | 3.79* |
| 1 and 2 | −0.32 | 1.38 | −2.21 | −1.04 |
| 3 and 4 | 3.10* | 2.34 | 1.55 | 2.51 |
- * P < 0.05. The upper three rows show the differences between old leaves and new leaves, and the lower two rows show the differences in the number of larvae per culture.
The effects of the number of larvae in a container on the growth of the larvae were not clear (Table 5); in the case of feeding on new leaves, larvae that bred in groups were heavier than that bred alone, but the reverse was observed when larvae were fed with old leaves. Multiple comparisons by the Steel-Dwass test did not show a difference in the weight of larvae and pupae between the cultures bred in isolation and those bred in groups, either (Table 6).
Differences in the composition of nutrients
The concentrations of water, sugar, protein, lipid, fiber and mineral were slightly larger in old leaves (Table 7), and consequently bomb calories were also higher in old leaves. These results do not indicate a lower quality of the old leaves as food.
| Composition of nutrient (%) | Bomb calories (kcal/100 g) | ||||||
|---|---|---|---|---|---|---|---|
| Water | Sugar | Protein | Lipid | Fiber | Mineral | ||
| New leaves | 85.6 | 7.7 | 2.3 | 1.1 | 2.0 | 1.3 | 67 |
| Old leaves | 81.8 | 10.1 | 2.8 | 1.6 | 2.2 | 1.5 | 86 |
DISCUSSION
So far the oviposition on old leaves by L. japonica has been thought to be exceptional (e.g. Fuzisawa et al. 1964; Fukuda et al. 1982; Watanabe et al. 1996 etc.), but field data recording whether the leaves on which oviposition took place were new or old are limited. Tabuchi (1974) described an egg cluster on an old leaf of Asarum takaoi (evergreen species) only once during his 28 years of observation in Hakuba, Nagano prefecture. Nanba et al. (1989) reported that oviposition on old leaves of A. takaoi was found only once out of 18 times of observation from 1986 to 1987 in Okayama prefecture. Moreover, the second author of the present paper (Matsumoto 1990) observed 148 egg clusters laid on A. takaoi in a hilly suburb of Kanazawa city, Ishikawa prefecture, which has mild snowfall in winter, and none of them were laid on old leaves. Unfortunately, comparative results on the oviposition on other host plants have never been reported, but the above mentioned cases indicate that oviposition on old leaves rarely occurs at least for A. takaoi. In contrast to the above previous observations, the frequency of oviposition on old leaves in the study area (Table 1) was rather high, though they occurred only at the beginning of the oviposition period (Fig. 1). We suppose that the extraordinarily heavy snow in the study area affects the frequent oviposition on old leaves. In a heavy snow area, timing of the snow thaw varies from place to place depending on topography and vegetation, and emergence of the butterfly and leaf flush of the host plant follow disappearance of the covering snow on the spot. Although L. japonica emerges soon after the snow has thawed, production of new leaves by A. megacalyx starts at least 1 week later (A. Hatada, unpubl. data, 2005). Under these circumstances, available new leaves should be limited for females that emerge early, and the females may choose old leaves to oviposit as a compromise. The observation by Tabuchi (1974) supports the hypothesis that heavy snowfall affects the oviposition on old leaves; he found an egg cluster on an old leaf in the spring after a winter with unusually heavy snowfall. Another observation of the oviposition on Asarum nipponicum var. echizen by the first author of the present paper was also consistent with the hypothesis; the observation was done in Minami-echizen town, Fukui prefecture, after a heavy snowfall for the first time in 20 years, and the percentage of the oviposition on old leaves to that both on old and new leaves was 20.8% (A. Hatada, unpubl. data, 2006).
The present study indicates that old leaves of A. megacalyx allow the survival of L. japonica larvae in both the field and laboratory breeding. It is understandable that the survivorship patterns in the field do not clearly differ between the larvae born on old leaves and those born on new leaves for middle and late instar larvae, because they can move to new leaves after consuming the original leaf. However, it is notable that survival rates up to the second instar stage were not different between the larvae hatched on old leaves and those on new leaves in the field. In laboratory breeding, most larvae fed old leaves also could grow up to the pupal stage, suggesting that larvae can accept old leaves as their food even in their early instar stage.
As for the growth rates of larvae in early instar stages, on the other hand, the old leaves are certainly not as suitable as the new leaves; larvae fed on old leaves took longer to develop and attained a lighter body weight than those fed on new leaves in laboratory breeding. Hiura (1978) speculated that there were two disadvantages for the larvae of L. japonica to feed on old leaves of evergreen Asarum species: poor nutritional value and toughness of the tissue. Since the concentration of nutrients in the old leaves is nearly the same or even a little higher than that in the new leaves as mentioned above, Hiura's (1978) first speculation cannot apply to the old leaves of A. megacalyx. The toughness of the tissue, however, could be a burden for larval growth, especially in their early instar stage. Although we did not evaluate the toughness of the leaves, the old leaves of A. megacalyx are clearly much tougher than the young leaves, as is generally the case in the evergreen broad leaf plants (Abe & Matsuda 2000; Cizek 2005). The leaf toughness in old leaves probably requires more labor for mastication, resulting in higher energy consumption and slower intake of nutrition than that in new leaves. The differences in growth rates measured by the body weight between treatments supported the hypothesis that old leaves are inefficient as a food resource. The most extreme contrast was that the average body weight of newly molted third instar larvae (representing the growth during the first and second instar stages) in Series 4 (fed old leaves) was only 37% of the weight of larvae at the same stage in Series 2 (fed new leaves).
On the contrary, the leaf toughness seems not to be a serious problem in later larval stages. The much smaller difference in the body weights of the pupae among treatments than that of the larvae just after the fourth molt suggests that fifth instar larvae were large enough to cope with tough leaf tissue and could efficiently digest old leaves. Actually, larvae were sometimes found on old leaves in the field, and the old leaves were used as a food resource for the older larvae. Moreover, the amount of leaves eaten by larvae geometrically increases with their growth. Leaf consumption during the fifth instar stage accounts for about two-thirds of the total leaf consumption of the entire larval stage (Fuzisawa et al. 1964), and thus undergrown individuals can catch up in growth during this stage.
In contrast to our results, Osada (1982, 1983), based on materials from Fukui prefecture, reported that larvae of L. japonica in isolation and in small groups showed very low survival rates when fed on old leaves of the local host plant, A. nipponicum var. echizen. It would be fascinating to investigate whether the difference between the two localities is due to different ability of L. japonica larvae to feed on old leaves or different edibility of old leaves of the host plants or both.
ACKNOWLEDGMENTS
We are grateful to Dr Masahiro Nagano and Dr Takuo Sawahata, Echigo-Matsunoyama Museum of Natural Science, for their useful comments on our study plans. We also thank Mr Kousuke Takahashi for his help in the field surveys.




