Resurrection of Rabdophaga salicivora Shinji (Diptera: Cecidomyiidae), a Japanese gall midge formerly misidentified as a North American species, Rabdophaga rigidae (Osten Sacken), with observations on the phylogenetic relationships of its populations in Japan and the Russian Far East
Abstract
Intercontinental biotic connections between Eurasia and North America are common in many gall midge genera (Diptera: Cecidomyiidae), but only a few species have been recorded from both continents. In Japan, four gall midge species had been previously considered to be identical to North American species, but three of these cases have already been disproved. We examined the remaining species, Rabdophaga rigidae, which had been originally described from Japan as Rabdophaga salicivora in 1938, later recorded from the Russian Far East in 1967, and synonymized with a North American species, R. rigidae, in 1982. Morphological features and partial sequence data of the mtDNA cytochrome oxidase subunit I (COI) region suggested that the Japanese species is a distinct species and is identical to the species recorded from the Russian Far East. We therefore apply the original name, R. salicivora, to the Japanese and the Russian species. In addition, on the basis of a molecular phylogenetic analysis, we conclude that R. salicivora possibly came to the Japanese Archipelago through the Korean Peninsula and established itself first in the southern parts of Japan. Then, it expanded its distribution range to northern parts of Honshu, but could not reach Hokkaido, probably because of the Tsugaru Strait between Honshu and Hokkaido.
INTRODUCTION
Intercontinental biotic connections between Eurasia and North America are common in many genera of insects, but only a few species have been recorded from both continents (e.g. Cracraft 1975; Sanmartín et al. 2001). For example, this tendency can be confirmed when we compare a list of Japanese insects (Hirashima 1989/1990) with a list of North American insects, for example the Lepidoptera (Dyar 1902), Hymenoptera (Muesebeck et al. 1951), Diptera (Stone et al. 1965), and Heteroptera (Henry & Froeschner 1988). Similarly in gall midges (Diptera: Cecidomyiidae), many genera are common to the Japanese Archipelago and North America, but only a few species have been recorded from both sides of the Pacific Ocean, although some species have been accidentally introduced from one side to another by human activities, especially by plant importation. Examples of these species are Contarinia maculipennisFelt, 1933; Monarthropalpus flavus (von Schrank, 1776), and Obolodiplosis robiniae (Haldeman, 1847) (Gagné 1984, 2004).
In Japan, four gall midge species were previously identified as North American species, namely Asphondylia diervillaeFelt, 1907; Lasioptera impatientis (Osten Sacken, 1862), Schizomyia viticola (Osten Sacken, 1862), and Rabdophaga rigidae (Osten Sacken, 1862) (Yukawa & Masuda 1996). The first species induces leaf bud galls on Weigela species (Caprifoliaceae) during winter–spring in Japan and was identified as A. diervillae by Shinji (1938c). However, Uechi et al. (2004) established that it represents the winter–spring phase of a host-alternating species, Asphondylia baca Monzen, which induces fruit galls on Ampelopsis brevipedunculata (Maximowicz) Trautvetter var. heterophylla (Thunberg) Hara (Vitaceae) and Cayratia japonica (Thunberg) Gagnepain (Vitaceae) during summer–autumn. The second species induces stem galls on Impatiens noli-tangere Linnaeus (Balsaminaceae) in Japan and was identified as L. impatientis (Osten Sacken) by Shinji (1938e). However, that species is now known to belong in the genus Schizomyia and is known only from North America (Gagné 2004), so the Japanese species, which was correctly placed in Lasioptera by Shinji (1938e), is a new species that will be described in the near future (J. Yukawa et al., unpubl. data). The third Japanese species was also identified by Shinji (1938d). Because of the similarity of the conical leaf galls induced on Vitis coignetiae Pulliat ex. Planchon (Vitaceae), Shinji (1938d) thought it was the North American species Cecidomyia viticolaOsten Sacken, 1862, and later suggested that it was identical to Dasineura vitis Osten Sacken (error for viticola: Gagné 2004). We can ignore this species because viticola is now included in the genus Schizomyia (Gagné 2004), which is quite different from the genus Dasineura.
In the present paper, we examine the fourth species, which was originally described as Rabdophaga salicivora Shinji, 1938, based on adults reared from stem galls of Salix babylonica Linnaeus (Salicaceae), probably in Morioka City, Japan (Shinji 1938a), and later recorded from the Russian Far East (Kovalev 1967). However, Nijveldt and Yukawa (1982) synonymized R. salicivora with R. rigidae, although they noted a small difference between them in the shape of the tarsal claws. In order to judge whether the difference is clear enough to distinguish the two species, further details of morphological features and data of molecular analysis were compared among North American R. rigidae, the Japanese species that had been identified as R. rigidae, and the Russian Far East species that had been identified as R. salicivora. We also discuss the derivation of Japanese R. rigidae by comparing the sequencing data among individuals collected from several different localities in Japan and the Russian Far East.
The genus Rabdophaga is Holarctic and currently contains about 80 nominal species that, with a few exceptions, develop only on willows (Salix species). The interactions between species of Rabdophaga and species of Salix are complex and not fully understood. A comprehensive biosystematic revision of Rabdophaga is needed, but is most unlikely to be available in the foreseeable future. Our observations are therefore presented in this context.
MATERIALS AND METHODS
Collection and preservation of specimens
Galls of Japanese R. rigidae on Salix spp., North American R. rigidae on Salix lasiolepis Bentham, Russian R. salicivora on Salix spp., Rabdophaga rosaria (Loew, 1850) on Salix purpurea Linnaeus, and Rabdophaga terminalis (Loew, 1850) on Salix fragilis Linnaeus were collected from various localities in Japan, Poland, Russia, UK, and the USA between 2000 and 2003 (Table 1). The collection sites of Russian R. salicivora and Japanese R. rigidae used for molecular analysis are shown in Figure 1.
| Gall midges | Host plant | Collection site (collector) | Collection date | n | Accession no. |
|---|---|---|---|---|---|
| North American R. rigidae | Salix lasiolepis | Flagstaff, Arizona, USA (P. W. Price) | 7–9 Apr. 2003 | 10 | AB244535–AB244544 |
| Russian R. salicivora | Salix rorida | Khanka Lake, Primorsk Kray, Russia (M. Tokuda) | 17 Sept. 2002 | 5 | AB244545–AB244549 |
| Russian R. salicivora | Salix sp. | Kommissaro vo, Primorsk Kray, Russia (M. Tokuda) | 17 Sept. 2002 | 9 | AB244550–AB244558 |
| Japanese R. rigidae | Salix sp. | Murakami City, Niigata Pref., Japan (S. Sato) | 1 Oct. 2002 | 5 | AB244559–AB244564 |
| Japanese R. rigidae | Salix eriocarpa | Toyota City, Aichi Pref., Japan (K. Yamagishi) | 28 Mar. 2003 | 3 | AB244565–AB244567 |
| Japanese R. rigidae | Salix eriocarpa | Kitagawa Town, Miyazaki Pref., Japan (J. Yukawa) | 23 Nov. 2002 | 10 | AB244568–AB244577 |
| Japanese R. rigidae | Salix eriocarpa | Kitagawa Town, Miyazaki Pref., Japan (J. Yukawa) | 15 Nov. 2001 | 3 | AB244578–AB244580 |
| Rabdophaga rosaria | Salix purpurea | Ojców National Park, Malopolskie Province, Poland (M. Skrzypczyńska) | Sept. 2002 | 1 | AB244581 |
| Rabdophaga terminalis | Salix fragilis | Wisley Botanical Garden, Woking, Surrey, UK (S. Sato) | 15 July 2003 | 2 | AB244582–AB244583 |
| Outgroup | |||||
| Asphondylia yushimai | Glycine max | Chikushino City, Fukuoka, Pref., Japan (N. Uechi) | 24 July 2000 | 1 | AB164447 |
- Pref., Prefecture.
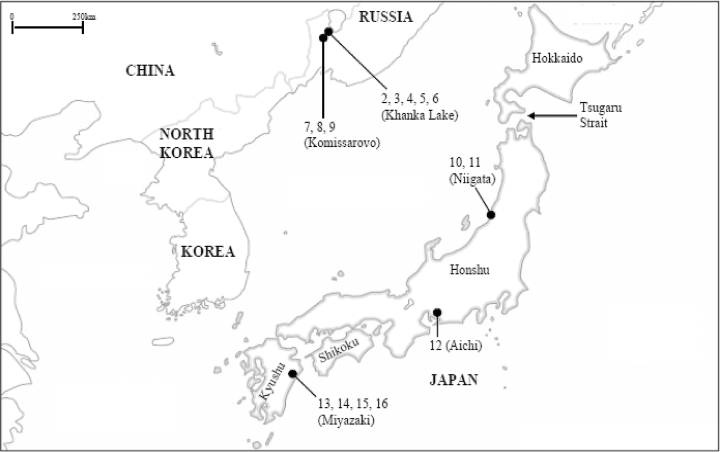
Collection sites of Russian R. salicivora and Japanese R. rigidae used for molecular analysis. The numerals on the map correspond to the numbers of haplotypes in Table 3.
Some of the galls were dissected under a stereoscopic microscope to obtain larval and pupal specimens. When some of the dissected galls contained mature larvae or pupae, the rest of the collected galls were kept in plastic cylinders (30 cm in diameter, 37 cm in height) to obtain adults and pupal exuviae. All specimens collected in the present study were preserved in 75% ethanol for morphological examination and 99.5% acetone for molecular phylogenetic analysis.
All specimens used in the present study are kept in the collection of the Entomological Laboratory, Faculty of Agriculture, Kyushu University, Japan.
Morphological studies and terminology
For microscopic study, some of the ethanol-preserved specimens were mounted on slides in Canada balsam using ethanol and xylene. Drawings were made with the aid of a drawing tube. Adult morphological terminology follows usage in Gagné (1981), except for thoracic setae and the mediobasal lobe of the male genitalia, for which terminology follows usage in Yukawa and Ohsaki (1988) and Nijveldt and Yukawa (1982), respectively. Morphological terminology of larvae generally follows usage in Möhn (1955; originally written in German) that was translated into English in Yukawa (1971), and the terminology for pupae follows that in Gagné (1994).
In order to compare larvae, pupae, and adults of Japanese R. rigidae with those of North American R. rigidae, we borrowed slide-mounted specimens of 15 males, eight females, one pupa, and one larva of North American R. rigidae from the National Museum of Natural History, Smithsonian Institutions.
Molecular phylogenetic analysis
Acetone-preserved specimens of some Rabdophaga gall midges were used for molecular phylogenetic analysis (Table 1). Asphondylia yushimai Yukawa and Uechi 2003 was used as an outgroup species for the analysis. For every individual, total DNA was extracted from the whole body with the DNeasy tissue kit (Qiagen, Japan) following the manufacturer’s instructions. A region of the cytochrome oxidase subunit I (COI) gene of mtDNA was amplified, purified, sequenced, and electrophoresed following the methods described by Yukawa et al. (2003). The primers used for the amplification were as follows: forward, 5′-GGATCACCTGATATAGCAT TCCC-3′ (COIS) and reverse, 5′-CCCGGTAAAAT TAAAATATAAACTTC-3′ (COIA). This primer set has been effectively used for the analysis of intra- and interspecific variations in Cecidomyiidae (e.g. Uechi et al. 2003; Yukawa et al. 2003; Tokuda et al. 2004; Uechi et al. 2004). The nucleotide sequence data reported in this paper have been deposited in the DNA Data Bank of Japan (DDBJ), European Molecular Biology Laboratory (EMBL), and GenBank nucleotide sequence databases with the accession numbers shown in Table 1.
The sequence data were analyzed with the neighbor-joining method using the software package phylip Version 3.573c (Felsenstein 1993). Evolutionary distances were calculated using Kimura’s two-parameter method (Kimura 1980). The resulting trees were subject to bootstrap analysis (Efron 1982; Felsenstein 1985) with 1000 replications for the neighbor-joining cladogram.
RESULTS
Morphological studies
Morphological studies of North American R. rigidae and Japanese R. rigidae, together with molecular analysis of North American R. rigidae, Japanese R. rigidae, and Russian R. salicivora, revealed that the taxonomic position of Japanese R. rigidae should be revised to Rabdophaga salicivora Shinji, 1938 (Shinji 1938a), which is redescribed as follows, together with the designation of a neotype. Hereafter, we use the name, Japanese R. salicivora instead of Japanese R. rigidae.
Rabdophaga salicivora Shinji sp. rev.
Rhabdophaga salicivora Shinji (1938): Shinji (1938a).
Rhabdophaga salicivora Shinji: Shinji (1938b, 1939, 1944); Monzen (1955); Kovalev (1967); Yukawa (1971).
(= Rabdophaga rigidae (Osten Sacken): Nijveldt & Yukawa 1982, misidentification).
Male. Eye bridge five or six facets long medially; frontoclypeal setae dense, 23–60 in number. Palpus four-segmented; second palpal segment 0.9–2.1 times as long as first; third 0.9–1.3 times as long as second; fourth 1.2–2.1 times as long as third. Antenna: scape ventrolaterally with 6–23 setae; pedicel ventrally and dorsally with 8–29 setae; with 18–22 flagellomeres; basal enlargement of fifth flagellomere 0.9–1.4 times as long as wide; terminal flagellomere relatively small, rounded apically. Wing length 3.2–5.2 mm, 2.4–2.7 times as long as wide; R5 joining costa near or at wing apex. Legs densely covered with blackish brown hairs; tarsal claws of all legs toothed (Fig. 2); empodia as long as claws; length of respective segments as in Table 2. Abdominal tergites I–VI rectangular, wider than long, with caudal rows of setae and one pair of anterior trichoid sensilla; tergite VII narrower than preceding, the vestiture similarly arranged; tergite VIII not sclerotized, bare and with a pair of anterior trichoid sensilla. Abdominal sternites II–VII rectangular, wider than long, with caudal rows of setae, with scattered setae elsewhere; sternite VIII not sclerotized, with scattered setae elsewhere. Genitalia (Fig. 4) yellowish brown; cerci setose, each large, rounded apically; hypoproct nearly as long as and distinctly narrower than cerci, the apex concave; gonostylus weakly arched, tapering distally, with strong apical claw; inner angle of gonocoxite ventrally developed into a setose lobe provided with a few protuberances ventrodistally; aedeagus longer than hypoproct, cylindrical, distally narrower, apically truncated or slightly rounded. See Table 2 for detailed data of setal counts and measurements.
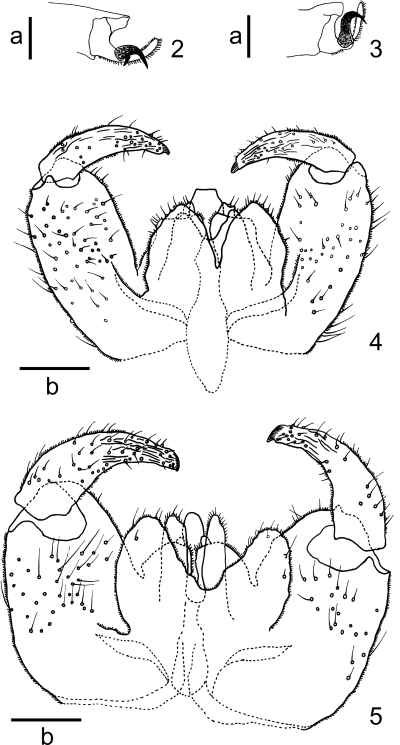
2,3 Tarsal claw. 2Rabdophaga salicivora Shinji; 3Rabdophaga rigidae (Osten Sacken). 4,5 Male genitalia, dorsal view. 4Rabdophaga salicivora Shinji; 5Rabdophaga rigidae (Osten Sacken). Scale lines: a, 0.05 mm; b, 0.1 mm.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | (Range) | n | Mean ± SD | (Range) | |
| Setae | ||||||
| Frontoclypeal | 5 | 43.2 ± 15.8 | (23–60) | 3 | 54.0 ± 18.5 | (40–75) |
| Mesepimeral | 7 | 51.0 ± 13.1 | (32–69) | 6 | 76.2 ± 11.2 | (63–93) |
| Wing | ||||||
| Length (mm) | 7 | 4.4 ± 0.6 | (3.2–5.0) | 8 | 4.9 ± 0.2 | (4.7–5.2) |
| Width (mm) | 8 | 1.8 ± 0.2 | (1.4–2.1) | 8 | 1.9 ± 0.2 | (1.8–2.4) |
| Length/width | 7 | 2.5 ± 0.1 | (2.4–2.7) | 8 | 2.6 ± 0.2 | (2.1–2.8) |
| Palpal segment length (µm) | ||||||
| First | 7 | 65.7 ± 17.6 | (34.2–83.1) | 6 | 66.0 ± 14.1 | (39.1–78.1) |
| Second | 7 | 69.9 ± 8.8 | (53.8–78.2) | 7 | 69.2 ± 7.7 | (63.6–83.1) |
| Third | 5 | 78.2 ± 12.9 | (63.6–97.8) | 5 | 84.1 ± 14.1 | (73.4–102.7) |
| Fourth | 5 | 106.6 ± 25.7 | (88.0–151.6) | 5 | 106.6 ± 13.6 | (88.0–122.3) |
| Fifth flagellomere | ||||||
| Length of basal enlargement (µm) | 8 | 80.7 ± 8.3 | (73.4–97.8) | 8 | 81.3 ± 5.2 | (73.4–88.0) |
| Width of basal enlargement (µm) | 8 | 71.5 ± 8.2 | (58.7–83.1) | 8 | 60.5 ± 6.4 | (48.9–68.5) |
| Length of stem (µm) | 8 | 58.7 ± 5.2 | (48.9–63.6) | 8 | 8.0 ± 2.5 | (4.9–9.8) |
| Foreleg (mm) | ||||||
| Fe | 8 | 1.6 ± 0.2 | (1.3–1.8) | 7 | 1.6 ± 0.2 | (1.4–1.8) |
| Ti | 8 | 1.6 ± 0.2 | (1.1–1.7) | 7 | 1.6 ± 0.1 | (1.4–1.7) |
| T2 | 8 | 1.6 ± 0.2 | (1.2–1.8) | 6 | 1.5 ± 0.1 | (1.4–1.6) |
| T3 | 8 | 0.7 ± 0.1 | (0.5–0.7) | 6 | 0.6 ± 0.1 | (0.5–0.6) |
| T4 | 8 | 0.4 ± 0.1 | (0.3–0.5) | 5 | 0.4 ± 0.0 | (0.3–0.4) |
| T5 | 7 | 0.2 ± 0.0 | (0.2–0.3) | 5 | 0.2 ± 0.0 | (0.2–0.2) |
| Midleg (mm) | ||||||
| Fe | 8 | 1.7 ± 0.2 | (1.2–1.8) | 6 | 1.7 ± 0.1 | (1.5–1.8) |
| Ti | 8 | 1.6 ± 0.2 | (1.2–1.8) | 7 | 1.6 ± 0.2 | (1.4–1.8) |
| T2 | 8 | 1.6 ± 0.2 | (1.1–1.7) | 6 | 1.4 ± 0.1 | (1.3–1.5) |
| T3 | 8 | 0.7 ± 0.1 | (0.4–0.7) | 6 | 0.6 ± 0.0 | (0.5–0.6) |
| T4 | 8 | 0.4 ± 0.1 | (0.3–0.5) | 6 | 0.4 ± 0.0 | (0.3–0.4) |
| T5 | 8 | 0.2 ± 0.0 | (0.1–0.3) | 6 | 0.2 ± 0.0 | (0.2–0.3) |
| Hindleg (mm) | ||||||
| Fe | 8 | 1.8 ± 0.2 | (1.5–2.0) | 7 | 1.9 ± 0.1 | (1.8–2.1) |
| Ti | 8 | 1.9 ± 0.3 | (1.4–2.2) | 7 | 2.0 ± 0.1 | (1.8–2.1) |
| T2 | 5 | 2.1 ± 0.2 | (1.8–2.3) | 7 | 1.9 ± 0.2 | (1.7–2.2) |
| T3 | 5 | 1.0 ± 0.1 | (0.9–1.0) | 7 | 0.8 ± 0.1 | (0.7–0.9) |
| T4 | 5 | 0.6 ± 0.1 | (0.5–0.6) | 7 | 0.5 ± 0.1 | (0.5–0.6) |
| T5 | 5 | 0.3 ± 0.0 | (0.3–0.3) | 7 | 0.2 ± 0.0 | (0.2–0.3) |
Female. Eye bridge and palpal segments as in male. Antenna with 20–24 flagellomeres; basal enlargement of fifth flagellomere 1.1–1.8 times as long as wide. Abdominal tergites I–VI rectangular, wider than long, with caudal rows of setae and a pair of anterior trichoid sensilla; tergite VII narrower than preceding, the vestiture similarly arranged; tergite VIII not sclerotized, with caudal rows of setae and one pair of anterior trichoid sensilla. Abdominal sternites II–VI rectangular, wider than long, with rows of caudal setae, with scattered setae elsewhere; sternite VII rectangular, narrower than preceding, with scattered setae elsewhere and one pair of anterior trichoid sensilla; sternite VIII not sclerotized, bare. Ovipositor yellowish brown, elongate, 419.3–516.8 µm long, not sclerotized; cerci elongated, entire (Fig. 6). See Table 2 for detailed data of setal counts and measurements.
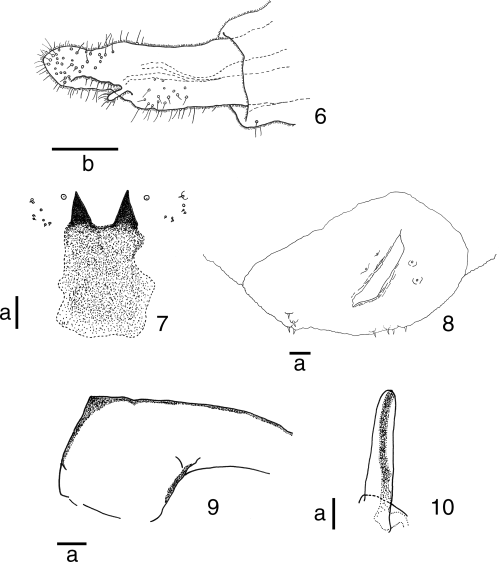
Rabdophaga salicivora Shinji. 6 Ovipositor, lateral view; 7 sternal spatula and adjacent papillae of mature larva, ventral view; 8 terminal segment of mature larva, ventral view; 9 basal portion of antennal sheath of pupa; 10 prothoracic spiracle of pupa. Scale lines: a, 0.05 mm; b, 0.1 mm.
Mature larva. Reddish brown; second antennal segment approximately 19.6 µm long; cervical papillae without seta; six dorsal papillae present, each with seta, 14.7–24.5 µm long; two dorsal papillae of eighth abdominal segment, each with approximately 14.7 µm long seta; two pleural papillae, each with a 9.8–19.6 µm long seta; stigma normal in number and position; four pairs of terminal papillae each with seta that is approximately 14.7 µm long (Fig. 8); sternal spatula 195.6–269.0 µm in length, 1.6–2.0 times as long as maximum width, distally incised by U-shaped emargination, forming a pair of narrow triangular lobes (Fig. 7); width between tips of two lobes 68.5–107.6 µm; basal part of sternal spatula roughly rectangular in shape, not strongly sclerotized (Fig. 7); six lateral papillae in two clusters of three each on each side of midline; two papillae in each cluster with minute seta (Fig. 7); two sternal papillae present on each of all thoracic segments, each without seta; inner pleural papillae with seta on all thoracic segments; on first to seventh abdominal segments, four anterior ventral papillae without seta and a pair of posterior ventral papillae each with seta; on eighth abdominal segment, four ventral papillae without setae; four anal papillae without setae.
Pupa. Base of antenna slightly developed, forming an obtuse angle anteriorly (Fig. 9); cephalic pair of setae elongate, 97.8–146.7 µm in length; frons smooth; one of two pairs of lower facial papillae each with seta that are 9.8–29.3 µm long; one of three pairs of lateral facial papillae each with 9.8–14.7 µm long seta; prothoracic spiracle 115.0–180.5 µm in length, rounded apically (Fig. 10); abdominal spiracles short, rounded apically, present on second to seventh abdominal segments; each abdominal segment except terminal one with many short spines dorsally and ventrally; terminal abdominal segment with many short spines only dorsally.
Host plants in Japan. Salix babylonica Linnaeus “Shidare-yanagi,”S. bakko Kimura “Bakko-yanagi,”S. gilgiana Seemen “Kawa-yanagi,”S. jessoensis Seemen “Shiro-yanagi,”S. kinuyanagi Kimura “Kinu-yanagi,”S. koriyanagi Kimura “Kori-yanagi,”S. sp. nr. korrensis Andersson “Kôrai-yanagi” (Nijveldt & Yukawa 1982); Salix eriocarpa Franchet and Savatier “Ja-yanagi” (new host record in this paper).
Host plants in the Russian Far East. Salix dolichostyla Seemen (Kovalev 1967); Salix rorida Lackschewitz (new host record in this paper).
Specimens examined. Neotype, male (on slide, Cecid. no. A3026) emerged on 28 April 1975 from a gall on S. jessoensis collected by K. Yamagishi on 20 April 1975 from Tsuchikawa, Ojiya City, Niigata Prefecture. Shinji’s original type series is lost: it has not been found either in Iwate University (previously Morioka Kôtô Nôrin Gakkô), Morioka, Japan, where he worked in 1938, or elsewhere. Other specimens examined: Japan: four males (on slide, Cecid. no. A3001-04), four females (on slide, Cecid. no. A3005-08) and one pupa (on slide, Cecid. no. A3009), Higashihamatani, Sasayama City, Hyogo Prefecture, 22 April 1965, adults emerged from 28 April to 8 May (A. Nakanishi); one male (on slide, Cecid. no. A3013), two females (on slide, Cecid. no. A3014-15) and two pupae (on slide, Cecid. no. A3016-17), Motonakagô, Ojiya City, Niigata Prefecture, 3 January 1974, adults emerged on 21 February 1974 (K. Yamagishi); six mature larvae (on slide, Cecid. no. A3018-23), Tsuchikawa, Ojiya City, Niigata Prefecture, 4 October 1974 (K. Yamagishi); two mature larvae (on slide, Cecid. no. A3041-42), Shizukuishi River, Morioka City, Iwate Prefecture, 21 March 1975 (K. Yamagishi); two mature larvae (on slide, Cecid. no. A3024-25), Inoseto, Beppu City, Ôita Prefecture, 22 March 1975 (K. Setoya); two males (on slide, Cecid. no. A3027-28), two females (on slide, Cecid. no. A3031-32) and four pupae (on slide, Cecid. no. A3035-38), Tsuchikawa, Ojiya City, Niigata Prefecture, 20 April 1975, adults emerged from 28 April and 3 May 1975 (K. Yamagishi).
Gall. Subglobular or spindle-shaped, woody swelling on the twig (Fig. 11); color green, shading to brown; length 8–20 mm, diameter 5–18 mm; monothalmous (with single larval chamber). Japanese name of the gall: “Yanagi-zuifushi” (Monzen 1929; Monzen 1955; Yukawa 1976), “Yanagi-eda-tamafushi” (Shinji 1944), “Yanagi-eda-maruzuifushi” (Yukawa and Masuda 1996).

Stem gall of Rabdophaga salicivora Shinji on Salix jessoensis.
Parasitoid in Japan. Platygaster urnicola Yamagishi, 1980 (Platygastridae).
Distribution. Japan (Honshu, Kyushu), Russian Far East, Korea (Muramatsu 1916; Kovalev 1967; Nijveldt & Yukawa 1982; Yukawa & Masuda 1996).
Life history. Muramatsu (1916) and Masaki (1932a,b,c) studied the general biology of R. salicivora under the name Rabdophaga salicis Schrank, which was a misidentification of the species (Yukawa 1971). According to their observations, R. salicivora is univoltine and adults emerge from April to May. Females lay their eggs on the host leaf buds that are about to open or in the vicinity of the buds. The egg stage lasts 7 to 9 days, and milky-white first instars crawl into the buds and settle at a place surrounded by woody tissue. Galls become conspicuous in mid-July and are fully developed by mid-September. In the galls, the larvae mature in autumn and are covered with a whitish membrane that they produce. Full-grown larvae overwinter in the galls on the shoots and pupate in the galls from March to April.
Molecular phylogenetic analyses
The amplified mtCOI gene fragment was 439 bp long. This region corresponded to bases 1752–2190 of the genome of Drosophila yakuba Burla (Diptera: Drosophilidae) (Clary & Wolstenholme 1985). The monophyly of the clade including Russian R. salicivora from Khanka Lake and Komissarovo, Russia and Japanese R. rigidae from Niigata, Aichi, and Miyazaki Prefectures, Japan (East Asian clade hereafter) was supported by a 90.9% bootstrap value (Fig. 12). The clade including North American R. rigidae individuals (North American clade hereafter) was more distant from the East Asian clade than from the clade including R. rosaria and R. terminalis individuals from Europe (Fig. 12). There were 51 (12.9% of 439 bp) to 71 bp (18.7%) differences (Table 3) and 8–13 differences in the 146 deduced amino acid residues between the East Asian and North American clades.
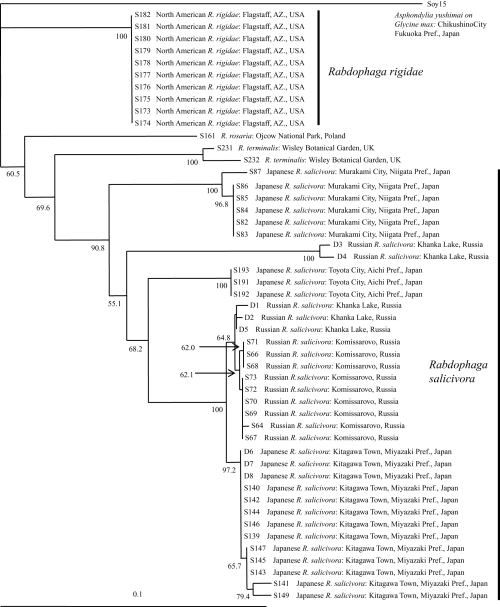
Neighbor-joining tree for Rabdophaga rigidae and its allied species based on 439 bp of the mitochondrial cytochrome oxidase subunit I (COI) gene and Kimura’s two-parameter distance. Bootstrap values are indicated for nodes gaining >50% support (1000 replications). Detailed data of the specimens are given in Table 1 and Figure 1.
| Gall midge* | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. (S173-182) | – | 54 | 56 | 68 | 71 | 55 | 56 | 55 | 54 | 51 | 51 | 54 | 55 | 60 | 56 | 59 | 51 | 49 | 52 | 76 |
| 2. (D1) | 14.1 | – | 3 | 48 | 48 | 2 | 4 | 3 | 4 | 41 | 42 | 28 | 6 | 11 | 7 | 11 | 59 | 52 | 55 | 88 |
| 3. (D2) | 14.4 | 0.7 | – | 49 | 49 | 1 | 3 | 2 | 3 | 42 | 43 | 27 | 5 | 10 | 6 | 10 | 60 | 53 | 56 | 90 |
| 4. (D3) | 18.1 | 11.9 | 12.2 | – | 4 | 48 | 50 | 49 | 50 | 50 | 52 | 49 | 48 | 53 | 49 | 52 | 67 | 67 | 71 | 89 |
| 5. (D4) | 18.7 | 11.9 | 12.2 | 0.9 | – | 48 | 50 | 49 | 50 | 50 | 52 | 47 | 48 | 53 | 49 | 52 | 69 | 70 | 74 | 91 |
| 6. (D5) | 14.1 | 0.5 | 0.2 | 11.9 | 11.9 | – | 2 | 1 | 2 | 41 | 42 | 28 | 4 | 9 | 5 | 9 | 59 | 52 | 55 | 89 |
| 7. (S64) | 14.4 | 0.9 | 0.7 | 12.5 | 12.5 | 0.5 | – | 1 | 2 | 41 | 43 | 29 | 6 | 11 | 7 | 11 | 61 | 53 | 56 | 90 |
| 8. (S67, 69, 70, 72–73) | 14.1 | 0.7 | 0.5 | 12.2 | 12.2 | 0.2 | – | 1 | 41 | 42 | 28 | 5 | 10 | 6 | 10 | 60 | 52 | 55 | 89 | |
| 9. (S66, 68, 71) | 13.8 | 0.9 | 0.7 | 12.4 | 12.4 | 0.5 | 0.5 | 0.2 | – | 40 | 41 | 27 | 5 | 10 | 6 | 10 | 60 | 51 | 56 | 87 |
| 10. (S82-86) | 12.9 | 10.0 | 10.3 | 12.5 | 12.5 | 10.0 | 10.3 | 10.0 | 9.8 | – | 6 | 32 | 39 | 44 | 40 | 43 | 54 | 53 | 56 | 86 |
| 11. (S87) | 13.2 | 10.3 | 10.5 | 13.0 | 13.0 | 10.3 | 10.6 | 10.3 | 10.0 | 1.4 | – | 35 | 42 | 47 | 43 | 46 | 57 | 54 | 57 | 84 |
| 12. (S191-193) | 13.8 | 6.7 | 6.4 | 12.2 | 11.6 | 6.7 | 6.9 | 6.7 | 6.4 | 7.7 | 8.4 | – | 28 | 33 | 29 | 33 | 58 | 55 | 60 | 87 |
| 13. (S139-140, 142, 144, 146, D6-8) | 14.1 | 1.4 | 1.2 | 11.9 | 11.9 | 0.9 | 1.4 | 1.2 | 1.2 | 9.5 | 10.3 | 6.7 | – | 5 | 1 | 5 | 55 | 49 | 53 | 89 |
| 14. (S141) | 15.6 | 2.6 | 2.3 | 13.3 | 13.3 | 2.1 | 2.6 | 2.3 | 2.3 | 10.9 | 11.7 | 8.0 | 1.2 | – | 4 | 6 | 60 | 54 | 58 | 93 |
| 15. (S143, 145, 147) | 14.4 | 1.6 | 1.4 | 12.2 | 12.2 | 1.2 | 1.6 | 1.4 | 1.4 | 9.8 | 10.6 | 6.9 | 0.2 | 0.9 | – | 4 | 56 | 50 | 54 | 90 |
| 16. (S149) | 15.3 | 2.6 | 2.3 | 13.0 | 13.0 | 2.1 | 2.6 | 2.3 | 2.3 | 10.6 | 11.4 | 8.0 | 1.2 | 1.4 | 0.9 | – | 59 | 54 | 58 | 93 |
| 17. R. rosaria (S161) | 12.8 | 15.2 | 15.4 | 17.5 | 18.1 | 15.2 | 15.8 | 15.5 | 15.5 | 13.7 | 14.6 | 14.8 | 14.0 | 15.4 | 14.3 | 15.2 | – | 53 | 57 | 82 |
| 18. R. terminalis (S231) | 12.4 | 13.2 | 13.5 | 17.6 | 18.5 | 13.2 | 13.5 | 13.2 | 13.0 | 13.6 | 13.9 | 14.1 | 12.4 | 13.8 | 12.7 | 13.8 | 13.5 | – | 8 | 86 |
| 19. R. terminalis (S232) | 13.2 | 14.1 | 14.3 | 18.8 | 19.6 | 14.1 | 14.4 | 14.1 | 14.4 | 14.4 | 14.7 | 15.4 | 13.5 | 14.9 | 13.8 | 14.9 | 14.6 | 1.8 | – | 92 |
| 20. A. yushimai (Soy15) | 20.8 | 24.8 | 25.4 | 25.1 | 25.8 | 25.1 | 25.4 | 25.1 | 24.7 | 24.2 | 23.5 | 24.6 | 25.2 | 26.5 | 25.5 | 26.6 | 22.8 | 24.1 | 26.0 | – |
- * Key to entries: 1. North American R. rigidae; 2–9. Russian R. salicivora; 10–16. Japanese R. salicivora (= Japanese R. rigidae); 17. R. rosaria; 18–19. R. terminalis; 20. A. yushimai. Numbers in parentheses correspond to individual numbers inFigure 12.
Several haplotypes were included in the East Asian clade. Individuals obtained from one locality formed one clade, except for those collected from Khanka Lake, which were distantly divided into two clades (Fig. 12). As for the Japanese individuals, those from Miyazaki Prefecture were closer to Russian R. salicivora individuals from Komissarovo or some of the Russian R. salicivora individuals from Khanka Lake than to Japanese R. salicivora from Aichi or Niigata Prefecture (Fig. 12).
DISCUSSION
Differences between North American R. rigidae and Japanese R. salicivora
We found two morphological differences between North American R. rigidae and Japanese R. salicivora with respect to the shape of the tarsal claws and the aedeagus of the male genitalia. North American R. rigidae has untoothed tarsal claws on all legs (Fig. 3) and an apically rounded aedeagus (Fig. 5), whereas Japanese R. salicivora has toothed tarsal claws on all legs (Fig. 2) and apically truncated or slightly rounded aedeagus (Fig. 4). The gall shapes of North American R. rigidae and Japanese R. salicivora are somewhat different. The former transforms the buds into subglobular to tapered galls (Gagné 1989), and the latter into smooth subglobular galls (Fig. 11). Molecular analysis indicated that North American R. rigidae and Japanese R. salicivora are included in two different clades: the North American and the East Asian clades (Fig. 12). In addition, three individuals of R. rosaria and R. terminalis collected from Europe were located between the North American and East Asian clades (Fig. 12). Based on these results, we conclude that the two species are quite distinct.
As a result, no known intercontinental biotic connections exist between North America and the Japanese Archipelago at the species level in gall-inducing cecidomyiids, except for the following species, which have been accidentally introduced into Japan: Contarinia maculipennis Felt and Obolodiplosis robiniae (Haldeman) (Gagné 1984, 2004; Tokuda et al. 2002; Duso & Skuhravá 2003; Kodoi et al. 2003; Uechi et al. 2005).
Derivation of Japanese R. salicivora
Molecular analysis indicates that Japanese and Russian R. salicivora are identical because the monophyly of the East Asian clade was supported by a high bootstrap value (90.9%; Fig. 12). Within the East Asian clade, Japanese R. salicivora individuals collected from Miyazaki Prefecture, southern Japan were closer to Russian R. salicivora individuals than those from Niigata and Aichi Prefectures, central Japan. In addition, Salix stem galls that are quite similar to those induced by R. salicivora have been recorded from the Korean Peninsula (Saitô 1932; Paik et al. 2004). These observations indicate that Japanese R. salicivora possibly came to the Japanese Archipelago through the Korean Peninsula when both areas were connected, and that it become established first in the southern parts of Japan. Then, it expanded its distribution range to the northern parts of Honshu, but did not reach Hokkaido (Yukawa & Masuda 1996), probably because of the existence of the Tsugaru Strait between Honshu and Hokkaido.
ACKNOWLEDGMENTS
We would like to express our thanks to Dr K. M. Harris (former Director of the International Institute of Entomology, UK) for his critical reading of an early draft. We are grateful to Dr R. J. Gagné (Systematic Entomology Laboratory, Plant Science Institute, US Department of Agriculture, USA) for arranging a loan of North American Rabdophaga rigidae specimens kept in the National Museum of Natural History, Smithsonian Institutions, Washington, DC, USA. Thanks are also due to Prof. P. W. Price (Northern Arizona University, USA), Prof. Małgorzata Skrzypczyńska (Agricultural University of Krakow, Poland), Prof. Kenzo Yamagishi (Meijo University, Japan), and Dr Makoto Tokuda (National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan) for their kindness in providing materials.




