Redefinition of Oligotrophus (Diptera: Cecidomyiidae) based on morphological and molecular attributes of species from galls on Juniperus (Cupressaceae) in Britain and Japan
Abstract
Morphological and molecular attributes of four species of Cecidomyiidae that induce galls on junipers, Juniperus spp., in Britain and Japan were used to redefine the genus Oligotrophus Latreille, which now includes Arceuthomyia Kieffer and Schmidtiella Rübsaamen (new synonyms). Illustrated morphological descriptions are provided for the four species Oligotrophus juniperinus (Linnaeus), Oligotrophus panteli Kieffer, Oligotrophus gemmarum (Rübsaamen) (new combination) and Oligotrophus nezu Kikuti (= Arceuthomyia nakaharai Inouye (new synonymy)) and phylogenies based on partial sequencing of the mitochondrial 12S rRNA gene are reported.
INTRODUCTION
Oligotrophus Latreille has been used to some extent in the past as a catch-all genus, but Gagné (1989) restricted its usage to species developing on junipers, Juniperus spp. (Cupressaceae), and provided a definition of the genus, based on the Nearctic species. That definition is revised here. Gagné (2004) included Oligotrophus in the tribe Oligotrophini within the supertribe Oligotrophidi, with three other nominal genera: ArceuthomyiaKieffer, 1913; SchmidtiellaRübsaamen, 1914; and WalshomyiaFelt, 1908; noting that the simple tarsal claws with long empodia are a possible synapomorphy for this group.
The genus Oligotrophus, as currently defined, contains eight nominal species that induce various conspicuous and inconspicuous bud galls on junipers in the Palearctic and Nearctic regions (Gagné 2004). Two additional genera, Schmidtiella Rübsaamen and Arceuthomyia Kieffer, include species that also induce galls on junipers and have been maintained until now as distinct genera on the basis of slight morphological differences. Recent collections of galls, larvae, and reared adults of four species, including the type-species of Oligotrophus, in Britain and in Japan were used to reassess the morphological characters of adults, larvae, and pupae, to determine their phylogeny based on molecular analysis and to redefine Oligotrophus. Schmidtiella and Arceuthomyia are now included as new generic synonyms. Lack of appropriate material has precluded the inclusion of other species of Oligotrophus, notably the European O. schmidti Rübsaamen and O. valerii (Tavares) and the North American O. betheli Felt, O. juniperi Felt, and O. pattersoni White.
MATERIALS AND METHODS
During the years 1995–2004, juniper galls containing live larvae and/or pupae were collected on a number of occasions in Scotland (by P. F. Entwistle), in England (by K. M. Harris and L. K. Ward) and in Japan (by S. Sato). Details are given in the “Material examined” section for each species studied. Some third instars and/or pupae were preserved in 99% isopropanol or ethanol for DNA analysis and the remaining material was used to rear adults for morphological study. Microscope slides of larvae, pupae, pupal exuviae, and adult gall midges were mounted in either Canada balsam or Hoyer’s medium, using standard procedures, and additional specimens were cleared in phenol and mounted temporarily in lactophenol for easier interpretation of 3D structures. Phase-contrast and bright-field illumination were used for all microscopic examinations and drawings were made using a plain mirror camera lucida. Gall structure was mainly recorded by scanning on a flat-bed scanner and reference specimens were preserved either dry or in 70% ethanol. Voucher specimens of gall midges and galls have been deposited in the Natural History Museum, London (NHM), in the Entomological Laboratory, Faculty of Agriculture, Kyushu University, Japan (ELKU), and in the Oxford University Museum of Natural History, UK. Accession numbers are given for slides deposited in the NHM.
Larvae or pupae extracted from galls and then preserved in 99% isopropanol or ethanol were used for DNA analysis (Table 1). Asphondylia yushimai Yukawa & Uechi that had been collected from a pod gall on soybean, Glycine max (L) Merrill (Fabaceae), was used in the analysis as an outgroup taxon. For every individual specimen, total DNA was extracted from the whole body by using the DNeasy tissue kit (Qiagen, Tokyo, Japan) following the manufacturer’s instructions.
| Gall midge species | Host plant | Collection site (collector) | Collection date | Sample reference† | Accession no. |
|---|---|---|---|---|---|
| Oligotrophus gemmarum | Juniperus communis | UK, Porton Down (L. K. Ward) | 2 May 2004 | U453 | AB246368 |
| U455 | AB246369 | ||||
| U456 | AB246370 | ||||
| U457 | AB246371 | ||||
| U458 | AB246372 | ||||
| U459 | AB246373 | ||||
| Oligotrophus juniperinus | Juniperus communis | UK, Upper Teesdale (K. M. Harris) | 21 Sept. 2003 | S281 | AB246374 |
| S283 | AB246375 | ||||
| S285 | AB246376 | ||||
| Oligotrophus panteli | Juniperus communis | UK, Scotland, Sutherland (P. F. Entwistle) | 13 Apr. 2003 | S229 | AB246377 |
| Oligotrophus panteli from typical galls | Juniperus communis | UK, Upper Teesdale (K. M. Harris) | 16 Mar. 2004 | S359 | AB246378 |
| S360 | AB246379 | ||||
| S361 | AB246380 | ||||
| S362 | AB246381 | ||||
| S363 | AB246382 | ||||
| S364 | AB246383 | ||||
| Oligotrophus panteli from “gemmarum” galls | Juniperus communis | UK, Upper Teesdale (K. M. Harris) | 16 Mar. 2004 | U448 | AB246384 |
| 21 Mar. 2001 | U449 | AB246385 | |||
| U450 | AB246386 | ||||
| U451 | AB246387 | ||||
| Oligotrophus nezu | Juniperus rigida | Japan, Fukuoka Pref., Sasaguri Town (S. Sato) | S185 | AB246389 | |
| S186 | AB246390 | ||||
| Outgroup | |||||
| Asphondylia yushimai | Glycine max | Japan, Fukuoka Pref., Chikushino City (N. Uechi) | 24 July 2000 | SoyFuk15 | AB164447 |
- † Sample references correspond to the respective isolation names registered in DNA databases.
A region of the cytochrome oxidase subunit I (COI) gene of mtDNA has been analyzed on many occasions to examine the inter- and intraspecific variations of gall-inducing cecidomyiids (e.g. Yukawa et al. 2003; Uechi et al. 2004). However, the primer set that has been used for the COI region did not amplify the DNA of the Oligotrophus species from the UK or from Japan. Another primer set (COIS and COIA′), which has been used by Uechi et al. (2003) for Contarinia maculipennis Felt (Diptera: Cecidomyiidae), also did not amplify the DNA. Therefore, in the present study, a region of the 12S ribosomal mitochondrial gene was amplified and sequenced, following the methods described in Kambhampati and Smith (1995) and Dorchin et al. (2004). This region was effectively used for the analysis of intra- and intergeneric variations in the tribe Lasiopterini (Diptera: Cecidomyiidae) (Dorchin et al. 2004). The primers used for the amplification of the 12S region were as follows: forward, 5′-TACTATGTTACGACTTAT-3′ (SR-J-14199) and reverse, 5′-AAACTAGGATTAGATACCC-3′ (SR-N-14594) (Kambhampati & Smith 1995).
The nucleotide sequence data reported in this paper have been deposited in the DNA Data Bank of Japan (DDBJ), European Molecular Biology Laboratory (EMBL), and GenBank nucleotide sequence databases, with the accession numbers given in Table 1.
The sequence data were aligned using CLUSTAL X software (Thompson et al. 1997) and analyzed with the neighbor-joining method using the software package phylip version 3.573c (Felsenstein 1993). Evolutionary distances were computed using Kimura’s two-parameter distances (Kimura 1980). The resulting tree was subjected to the bootstrap test (Efron 1982; Felsenstein 1985) based on 1000 replications.
RESULTS
Morphological studies
The four species examined are morphologically very similar. Some differences in pupal characters are possibly diagnostic (see below) and there may be subtle differences between adults, but all four species are considered to be congeneric, sharing the characters given in the definition of Oligotrophus by Gagné (1989), which is restated and expanded below.
Oligotrophus Latreille, 1805
Oligotrophus Latreille (1805): 288. Type species: Tipula juniperinaLinnaeus (1758): 588.
Arceuthomyia Kieffer (1913): 49. Type species: Rhopalomyia valeriiTavares (1904). (new synonymy)
Schmidtiella Rübsaamen (1914): 89. Type species: Schmidtiella gemmarumRübsaamen (1914). (new synonymy)
Alassomyia Felt (1925): 147 [= AllomyiaFelt (1918): 380, preoccupied]. Type species: Allomyia juniperiFelt (1918).
Arceuthomyia and Schmidtiella are here synonymized, as they cannot be distinguished from Oligotrophus, as currently defined. In his description of Rhopalomyia valerii, Tavares (1904) stated that his species belonged in either Rhopalomyia or Oligotrophus and Kieffer (1913), in the briefest of diagnoses, made it type species of Arceuthomyia simply because the empodia are twice as long as the tarsal claws, a character that is difficult to quantify and insufficient to justify erection of a separate genus. Similarly, Schmidtiella gemmarum, the type species of Schmidtiella, conforms to our concept of Oligotrophus.
The genus Oligotrophus is defined here by the following combination of characters: relatively heavy-bodied oligotrophid midges with broad but flimsy wings up to 3 mm long (Fig. 2), slightly longer in males, and a general body vestiture of fine hair-like scales. The tergites and sternites are poorly differentiated but bear some setae; antennae have a variable number of 12–18 flagellomeres (Table 2), the male flagellomeres with long, narrow necks and female flagellomeres with short, indistinct necks; circumfila are simple in both sexes, as in most oligotrophids. Head shape is distinctive with the eye bridge narrow and broken medially so that there is a definite space between ommatidia; maxillary palpi very short, reduced to 1–3 segments, with a few short spiniform setae (Fig. 3); tarsal claws all simple and empodia conspicuously longer than claws (Fig. 4); ovipositor short and retracting into abdominal segment 8, tergite 8 completely or partly divided medially (Fig. 5); male genitalia (Fig. 6) with relatively large gonocoxites and small gonostyles, gonocoxites with large mediobasal lobes bearing numerous small papillae, aedeagus short, stout and slightly recurved, transparent and difficult to see in whole mounts of genitalia. Third instars are up to 3 mm long and relatively featureless, with a broad, short head capsule; no sternal spatula; only two pairs of terminal papillae (Fig. 7), all setae very short (approximately 10–15 µm) and with finely reticulate traction pads extending over most of the ventral surfaces of the abdominal segments.
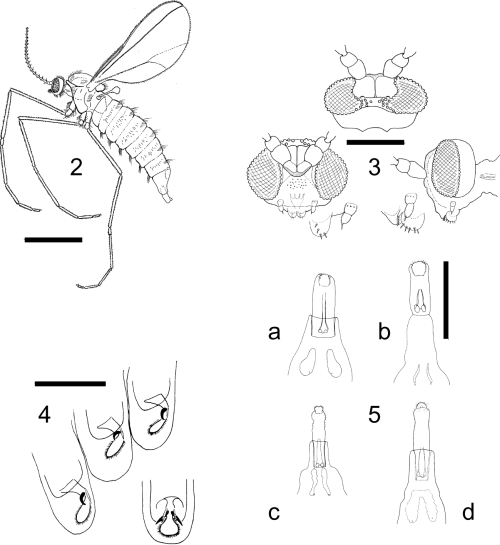
2 Oligotrophus panteli female (scale line: 1 mm); 3O. panteli head, dorsal, frontal and lateral views (scale line: 0.25 mm); 4O. juniperinus, tarsal claws and pulvilli in pupa, lateral view of legs 1, 2 and 3 with frontal view of leg 3 (scale line: 0.1 mm); 5 ovipositors and tergite 8 of (a) O. juniperinus, (b) O. panteli, (c) O. gemmarum and (d) O. nezu (scale line: 0.5 mm).
| Species | Wing length (mm) | n | No. flagellomeres | n | |
|---|---|---|---|---|---|
| O. juniperinus | ♂ | 3.2–3.4 ± 0.2–3.6 | 4 | 17–17.5 ± 0.7–18 | 2 |
| ♀ | 2.4–2.8 ± 0.3–3.0 | 7 | 16–17.2 ± 0.8–18 | 7 | |
| O. nezu | ♂ | 2.7–2.8 ± 0.2–3.1 | 4 | 15–15.1 ± 0.4–16 | 7 |
| ♀ | 2.4–2.4 ± 0.1–2.5 | 4 | 4–15.3 ± 0.8–16 | 6 | |
| O. gemmarum | ♂ | 2.2–2.5 ± 0.2–2.8 | 9 | 12–12.9 ± 0.3–13 | 14 |
| ♀ | 2.2–2.4 ± 0.1–2.6 | 9 | 12–12.7 ± 0.3–13 | 15 | |
| O. panteli | ♂ | 2.0–2.3 ± 0.3–2.8 | 8 | 12–12.6 ± 0.5–13 | 15 |
| ♀ | 1.8–2.2 ± 0.2–2.6 | 17 | 12–13.0 ± 0.4–14 | 24 |
- n, Number of observations.
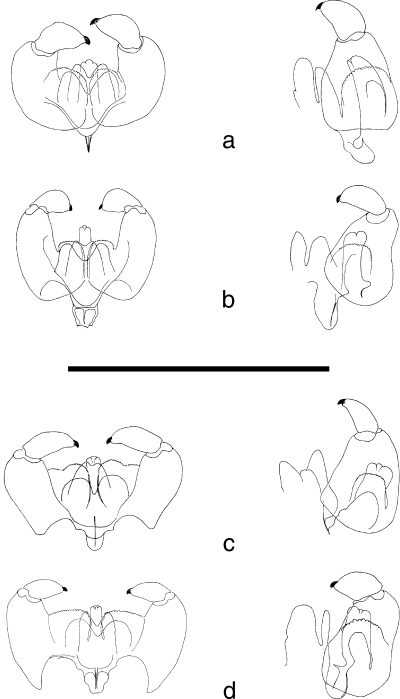
Male genitalia, dorsal and lateral views of (a) O. juniperinus, (b) O. panteli, (c) O. gemmarum and (d) O. nezu (scale line: 0.25 mm).
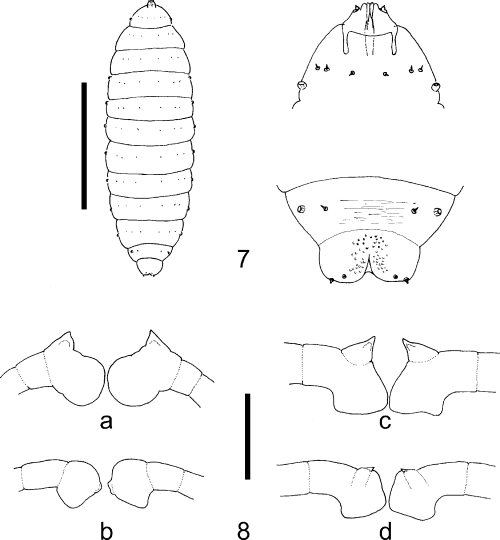
7 O. juniperinus third instar, dorsal, with anterior and posterior segments enlarged (scale line: 1.0 mm); 8 Antennal sheaths of pupal exuviae of (a) O. nezu, (b) O. gemmarum, (c) O. juniperinus and (d) O. panteli (scale line: 0.25 mm).
Larvae develop in inconspicuous or conspicuous bud galls on various species of Juniperus and pupate within the galls. Larvae do not make cocoons and therefore pupae lie naked within the galls, which open distally to facilitate adult emergence. Slight sclerotized cephalic armature is developed with modification of the antennal sheaths, which are most easily observed in pupal exuviae, and show some diagnostic differences between species (Fig. 8). There are also differences in wing and ovipositor length and in numbers of flagellomeres (Table 2), which may be diagnostic, but the four species dealt with here are morphologically very similar in most adult characters.
Oligotrophus juniperinus (L., 1758) (2–5-9)
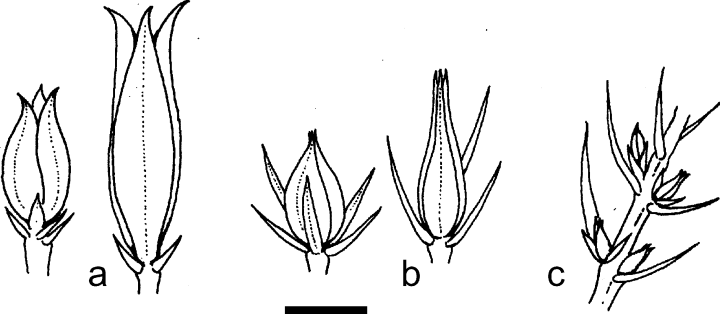
Galls of (a) O. juniperinus, (b) O. panteli and (c) O. gemmarum (scale line: 5 mm).
Tipula juniperina Linnaeus (1758): 588.
This is one of only two Linnaean species in the family Cecidomyiidae, and the species was originally described from Sweden. Larvae induce conspicuous and distinctive galls on terminal and lateral buds of Juniperus communis L. (Fig. 9a). The galls are up to approximately 15 mm long, vase-shaped and constructed of two whorls, each of three modified leaves, with the inner whorl enclosing a single larva or pupa. Leaves of the outer whorl are characteristically widened and thickened throughout their length, strongly tapered terminally and with the tips curving outwards. Fully developed galls are quite easily identified but at earlier stages of development may resemble those of other species of Oligotrophus. The species occurs widely throughout Europe, especially at higher latitudes and/or altitudes, and is especially abundant in Scandinavia. In Britain, it is restricted to Scotland and northern England (especially the Lake District and Teesdale), with the southernmost confirmed record from latitude 54°N. In continental Europe, it is present at much lower latitudes (though usually at higher altitudes) in France, Italy and the Balkan peninsula. The species has not been recorded east of the Ural Mountains (60°E; Skuhravá & Skuhravý 1993). A detailed description of this species was published by Winnertz (1854) and it was studied more recently by Sylvén (1975) and Sylvén and Tastas-Duque (1993) in the context of their comparative morphological studies of the Palearctic Oligotrophidi (as Oligotrophini).
Although this species is well known and widely recorded in Europe, knowledge of its biology is minimal. Skuhravá and Skuhravý (1998) summarized it briefly, recording that the species is univoltine, with adults emerging from the end of April to the end of May. Females lay eggs on buds and larvae feed during the summer, overwintering as fully developed larvae within the galls. Larvae pupate in the following April. Recent observations by P. F. Entwistle in Scotland suggest a 24-month life-cycle, with oviposition in June/July of year 1, most larval development in year 2 and adult emergence in year 3 (P.F. Entwistle, unpubl. data).
Specimens examined. All from galls on Juniperus communis. Scotland: Cairngorms, Loch Etchanachan, vi.1969 (L. K. Ward), 1 pupa, 1 larva on slide 20401; Edderton, Dornoch Firth, 12.vi.1995 (P. F. Entwistle), 2♀ on slides 19909/19916 and 6 pupal exuviae on slides 19919 and 19927, 14.vi.1995 (P. F. Entwistle), 1♂ on slide 19908. England: Cumbria, Coniston, Church Beck Crags, 11.v.1999 (K. M. Harris), 12 larvae on slides 20062 and 20397–20399; Cumbria, Patterdale, Silver How Point, 12.v.1999 (K. M. Harris), 3 larvae on slide 20400; County Durham, Upper Teesdale, High Force, 16.iii.2004 (K. M. Harris), 2 pupae on slides 20278–20279.
Oligotrophus panteli Kieffer, 1898 (2–5-9)
Oligotrophus panteli Kieffer (1898): 18.
This species was also described from Juniperus communis in Europe, originally from France, and is now known to occur throughout the western Palearctic. Fully developed typical galls (Fig. 9b) are approximately the same size or a little smaller than those of O. juniperinus, up to approximately 10 mm long, approximately twice as long as those of O. gemmarum, and are easily distinguished by the shape of the outer whorl of modified needles, which taper strongly towards the finely pointed tips, which are not recurved. In the UK the distribution of this species overlaps that of O. juniperinus and galls of the two species may occur together on the same branches of a single host plant. Atypical galls, found in Upper Teesdale, are indistinguishable from those of O. gemmarum (see below).
The biology and ecology of this species have not been studied in any detail and, following the finding in Upper Teesdale of small bud galls that may be of a 1-year cycle, merit further study. In Upper Teesdale in 2004, pupae were present in galls in early April and adult emergence probably peaked in May. Further north, in Scotland, adult emergence may be up to 1 month later.
Specimens examined. All from galls on Juniperus communis. Scotland: Highland Region, Beinn Eighe, vi.1969 (L. K. Ward), 2♀ and pupal exuviae on slide 20401; Grampian Region, Slocht, 1996 (P. F. Entwistle), 1♂ on slide 19896, 1♀ on slide 20053; Bridge of Brown, 1996 (P. F. Entwistle), 2♂ on slides 19890/19891, 5♀ on slides 19893, 19894, 20054, 20056 and 20057; South Lecht, 1996 (P. F. Entwistle), 1♀ on slide 19895; Lecht Mine, 1996 (P. F. Entwistle), 1♀ on slide 19901, Deeside, near Corgarff, 1996 (P. F. Entwistle), 2♂ on slides 20050/20051 and 1♀ on slide 20055; iv.1997 (P. F. Entwistle), 3♂ on slides 19897–19899, 8♀ on slides 19902–19907 and 20060/20061; Sutherland, Spinningdale, 13.iv.2003 (P. F. Entwistle), 4♂ on slides 20359/20360 and 20391/20392, 1♀ on slide 20361, 2 pupal exuviae on slide 20362, 1 larva on slide 20402 and 2 pupae on slides 20403/20404; Aberdeenshire, near Balmoral, 16.x.1994 (P. F. Entwistle), larvae on slide 19910; near Braemar, 24.vi.1999 (P. F. Entwistle), pupa on slide 20406; Deeside, v.1997 (P. F. Entwistle), 2♀ on slides 20389/20390. England: County Durham, Upper Teesdale, High Force, 05.iv.2004 (K. M. Harris), 2 pupae on slide 20405 and 7 in vial.
Oligotrophus gemmarum (Rübsaamen, 1914), new combination (2–5-9)
Schmidtiella gemmarum Rübsaamen (1914): 89.
The gall of this species, also on Juniperus communis, is very small and easily overlooked. It is a simple bud gall in either terminal or lateral buds (Fig. 9c). Each fully developed gall is approximately 3–4 mm long and contains a single larva. Some larger galls resemble miniature O. juniperinus galls and may have resulted in false records of this species in southern England. In the UK it is restricted to southern England, but identical galls, now known to be induced by Oligotrophus panteli, have been found at one northern locality, in Upper Teesdale. In continental Europe there are records from the Netherlands, France, Germany, Poland and the Czech Republic, based on the galls, but the similarity to some galls of O. panteli reported here suggests that identification of the two species on galls alone is not accurate. Ward (2004) gave details of the biology of this species in southern England and recorded that one colony, at Riddlesdown, Surrey, has declined to extinction since 1969 as a result of habitat change. She noted that galls, which may be on terminal or lateral buds (including male cone buds), are most conspicuous from April onwards; pupae are present during May and adults emerge in late May and early June. Flight is weak and adults live for only a few days, during which time females lay eggs around the developing buds of next season’s growth. Larvae hatch in June and enter buds, but many die at this stage and there is early intraspecific competition so that by August there is only one larva per bud. Larvae feed until the autumn and then overwinter before completing development in the following spring.
Specimens examined. All from galls on Juniperus communis. England: from Wiltshire, Porton Down, collected 18.v.1981 (L. K. Ward), 1♂ on slide 19874; collected 02.v.2004 (L. K. Ward), 5♂ on slides 20276, 20364–20366 and 20408, 5♀ on slides 20352/20353 and 20368–20370, 1 pupa on slide 20277 and 8 pupal exuviae on slides 20367 and 20387; Surrey, v/ix.1968 (L. K. Ward), 1♂, 1♀, 5 larvae; Riddlesdown, vi/ix.1968 (L. K. Ward), 1♂, 1♀, 5 larvae; Box Hill, vi/viii.1968 (L. K. Ward), 3♀, 3 larvae; Betchworth, 08.iv.1968 (L. K. Ward), 7 larvae; Hackhurst, 18.vi.1968 (L. K. Ward), 1♂, 1♀ Oxfordshire, Aston Rowant, vii.1967 (L. K. Ward), 19 larvae; Ewelme, 1969 (L. K. Ward), 1 larva.
Oligotrophus nezu Kikuti, 1940 (2–5-7,8)
Oligotrophus nezu Kikuti (1940): 35.
Arceuthomyia nakaharai Inouye (1959): 5. (new synonymy)
This univoltine species induces galls on Juniperus rigida and, so far as is known, is restricted to Japan. Adults emerge from pupae in galls in late April to early May and lay eggs on young needles. Larvae hatching during the summer enter buds and overwinter as first instars, which complete development in the following spring and pupate in galls. Fully developed galls on leaf buds are approximately 7–11 mm long and are similar to those induced by O. juniperinus. A detailed description of adult males and females is given in Yukawa (1971), as A. nakaharai, and a photograph of the gall was published as Plate(B)-013 in Yukawa and Masuda (1996).
Specimens examined. All from galls on Juniperus rigida. Japan: Forest Research Institute, Kyushu University, Sasaguri Town, Fukuoka Prefecture, 22.iii.2001 (S. Sato), 1♀ with exuvium on slide 20354 and 7 pupal exuviae, collected from galls in the field, on slides 20385/20386; Sasaguri Town, Fukuoka Prefecture, 20.iv.2000 (S. Sato), 4 larvae on slide 20063; Saheki Town, Okayama Prefecture, collected 24.iv.2004 (E. Inoue), emerged 02/04.v.2004 (S. Sato), 2♂ on slides 20273/20274, 1♀ on slide 20275; Kumenan Town, Okayama Prefecture, collected 29.iv.2004, emerged 06/07.v.2004 (S. Sato), 2♂ on slides 20355/20357, 2♀ on slides 20356/20358 and 4 pupal exuviae on slide 20407.
Molecular analysis
The length of the sequenced DNA fragment varied from 385 to 388 bp between species. This region corresponds to bases 14200–14593 of the Drosophila yakuba genome (Clary & Wolstenholme 1985).
There were no sequential differences between two individuals of O. nezu and three individuals of O. juniperinus, respectively, and thus their monophyly was supported by 100% bootstrap values in the neighbor-joining tree (Fig. 1). All individuals of O. gemmarum from Porton Down were included in one clade, although two different types of gall, a mini-juniperinus gall and a typical gemmarum gall, were recognized. Sequence data of the suspected O. gemmarum individuals from High Force, Teesdale coincided completely with those of the individuals of O. panteli from the same locality, indicating that at this location, and possibly at other locations in northern England and Scotland, O. panteli induces two different types of gall, one of which is indistinguishable from the galls of O. gemmarum. The monophyly of the O. panteli individuals from Teesdale and one individual of O. panteli from Scotland was supported by a 100% bootstrap value.
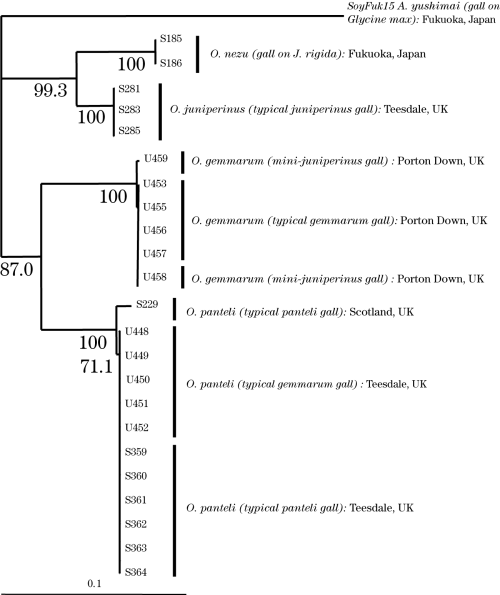
Neighbor-joining tree for Oligotrophus species, based on the mitochondrial 12S gene (including gaps) and Kimura’s two-parameter distances. Bootstrap values are indicated for nodes gaining more than 70% support (1000 replications). Host plant and locality data are given in Table 1 and typical galls of O. juniperinus, O. panteli and O. gemmarum are illustrated in Figure 9.
DISCUSSION
Our morphological and molecular data for the four species studied indicate that they should all be assigned to Oligotrophus, as currently defined. In addition, Arceuthomyia, which was erected by Kieffer (1913) with a minimal generic diagnosis simply stating that the genus differed from Rhopalomyia especially because the empodia are twice as long as the tarsal claws, is also synonymized although authentic material of the type species has not been available to us for study. A morphological description of O. betheli (as O. apicis) by Appleby and Neiswander (1965) indicates that that species also conforms to the definition of Oligotrophus given here, with the notable exception that the female cerci in that species are separate rather than fused.
In addition to establishing new generic and specific synonymies, our study raises new questions about the status of described and undescribed species that have been recognized in the past solely on supposed differences in gall structure. Bühr (1964, 1965), dealing with the plant galls of central and northern Europe, provided keys for the identification of 10 different galls on junipers, most of which are simple bud galls. There is no doubt that fully developed galls of O. juniperinus and of O. panteli are distinct. However, the much smaller and inconspicuous bud galls that are characteristic of O. gemmarum may be indistinguishable from galls of other species and, as indicated by the DNA data for O. panteli from Upper Teesdale, may represent the 1-year phase of a 1 or 2-year cycle, comparable to that reported in Taxomyia taxi (Inchbald) by Redfern (1975). Until proven otherwise, it seems that within the UK, O. gemmarum is the only species of Oligotrophus in southern England, and that from Teesdale northwards any galls that correspond to those of O. gemmarum cannot be assigned with certainty to any one species. Also, because of the slight morphological difference between species and the difficulties in collecting and rearing adults, it seems that identities can only be confirmed by DNA sequencing of larvae. In continental Europe, the situation is more complicated, because more alleged species of Oligotrophus have been recorded on Juniperus communis and also on other species of juniper, notably J. sabina, J. oxycedrus and J. macrocarpa (Dauphin & Aniotsbehere 1993). Further research is also needed to establish the position of the genus Oligotrophus within the Oligotrophidi.
In Japan, a gall midge that transforms the terminal buds of J. rigida into slender, hexangular or occasionally pentangular pyramid-shaped galls was known as Rhopalomyia uetsukii Inouye (Inouye 1959). However, during the course of the present study, we reared adults from the galls and confirmed by the wing venation, palpal segments, and ovipositor that the gall midge is neither a species of Rhopalomyia nor Oligotrophus, but a species of Lasioptera. We concluded that this gall midge is a gall maker because we found its larvae and pupae in the closed galls. They did not live in vacated galls that may be used by successor gall midges, as has been noted for Lasioptera yadokariae Yukawa and Haitsuka (Yukawa & Haitsuka 1994). Therefore, this species will be redescribed elsewhere in the future.
ACKNOWLEDGMENTS
We thank Prof Osamu Tadauchi (ELKU) and Dr Keiji Yasuda (Okinawa Prefectural Agricultural Research Center) for use of facilities for DNA analysis and Mr Etsuho Inoue (Okayama Prefecture) for collecting the galls of O. nezu. We gratefully acknowledge assistance that we received from Dr Raymond J. Gagné (Systematic Entomology Laboratory, US Department of Agriculture), and Dr Netta Dorchin (Bucknell University), who both reviewed an early draft of this paper. The provision of specimens, information, and comments by Mr Philip Entwistle (whose work in Scotland instigated this study) and of Dr Lena Ward, who has a long-standing interest in invertebrates associated with junipers in Britain, is also acknowledged. This study was partly supported by a Research Fellowship of the Japanese Society for the Promotion of Sciences for Young Scientists (to N. U.).




