Synopsis of the bee genus Coelioxys Latreille (Hymenoptera: Megachilidae) of Japan, with description of a new species
Abstract
Japanese species of the bee genus Coelioxys are reviewed, and 10 species in three subgenera are recognized: C. (Torridapis) fenestrata Smith, 1873, C. (T) ducalisSmith, 1853; C. (Allocoelioxys) formosicola Strand, 1913, C. (Boreocoelioxys) yanonis Matsumura, 1912, C. (B) hiroba Nagase, 2003, C. (B) hosoba Nagase, 2003, C. (B) inermisKirby, 1802, C. (B) alataFoerster, 1853, C. (B) rufescens Lepeletier & Serville, 1825, and C. (B) sakamotorum sp. nov. The lectotype of C. yanonis is designated. Coelioxys suisharyonis Strand, 1913, and C. hakonensisCockerell, 1919, are synonymized with C. yanonis, and C. taiwanensis Cockerell, 1927 is synonymized with C. formosicola. Supplementary descriptions are provided for C. yanonis, C. formosicola, C. hiroba and C. hosoba. Records of host bees are reviewed. A key to Japanese species is presented.
INTRODUCTION
Bees of the genus Coelioxys Latreille are cleptoparasites on other bees, principally bees of the genus Megachile. The genus, consisting of several hundreds of species, is distributed worldwide (Michener 2000), but its species-level taxonomy is still insufficiently studied, except for European and North American faunae.
In Japan, nine species have so far been recorded (Esaki et al. 1939; Hirashima 1989; Nagase 2003a; Maeta et al. 2004). The taxonomic identities of some species, however, are not certain yet (Nagase 2003a,b). The present study revises the Japanese fauna of Coelioxys based on much more material from many parts of Japan. Ten species, including one new species, are recognized.
MATERIALS AND METHODS
Specimens used in the present study were, in addition to those housed in my collection (abbreviated as HN), provided by many institutions and persons as follows: Entomological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka (ELKU); Systematic Entomology Laboratory, Faculty of Agriculture, Hokkaido University (SEHU); National Science Museum, Tokyo (NSMT); Museum of Nature and Human Activities, Sanda (MNHA); National Institute of Agro-Environmental Sciences, Tsukuba (NIAES); Y. Hirashima, Fukuoka (YH); Y. Maeta, Matsue (YM); K. Goukon, Tagajo (KG); R. Ishikawa, Tokyo (RI); S. Ikudome, Kagoshima (SI); E. Katayama, Otawara (EK); K. Nakamura, Utsunomiya (KN); T. Endo, Nishinomiya (TE); H. Suda, Sakura (HS); T. Nambu, Yorii (TN); H. Takahashi, Hachioji (HT); M. Kato, Tsuyama (MK); T. Tano, Fukui (TT); M. Yamada, Hirosaki (MY); H. Itami, Shibata (HI); and M. Schwarz, Ansfelden, Austria.
Definitions of the genus Coelioxys and its subgenera and of the host bee genus Megachile follow Michener (2000). Morphological terms are those of Mitchell (1973) but “genal concavity” is used instead of “hypostomal concavity.” Information on distribution records in the Palearctic region was extracted from Warncke (1992), Romankova (1995) and Proshchalykin (2004).
The following body parts were measured on pinned specimens by using an ocular micrometer under a stereoscopic microscope: wing length (from the outer margin of the tegula to the tip of the forewing), head width (the maximum distance between the outer eye margins), mesoscutal width (the distance between the inner margins of the tegulae). The body length is given as a mean together with the range, whereas the other measurements are given as the value for a median-sized (body length) specimen.
In the descriptions the following abbreviations are used: OOD, ocello-ocular distance (distance between posterior ocellus and inner eye margin at vertex); OCD, ocello-occipital distance (distance from posterior ocellus to the occipital margin in dorsal view); POD, postocellar distance (distance between posterior ocelli); MPD, maximum diameter of the posterior ocellus; IAD, interantennal distance (distance between inner margins of antennal sockets); and AOD, antenno-ocular distance (distance between outer margin of antennal socket and inner eye margin).
The shape of male metasomal tergum VI referred to as “inermis-type” (Fig. 10) in the present paper has the state in which the posterior margin is armed with six (two dorsal and four ventral) spines, all of which are more or less parallel and directed posteriorly; the space between the dorsal spines is deeply excavated; in dorsal view the apex of a dorsal spine is situated outside of the median ventral spine; and the dorsal spines are shorter than the ventral spines and often flattened dorso-ventrally.
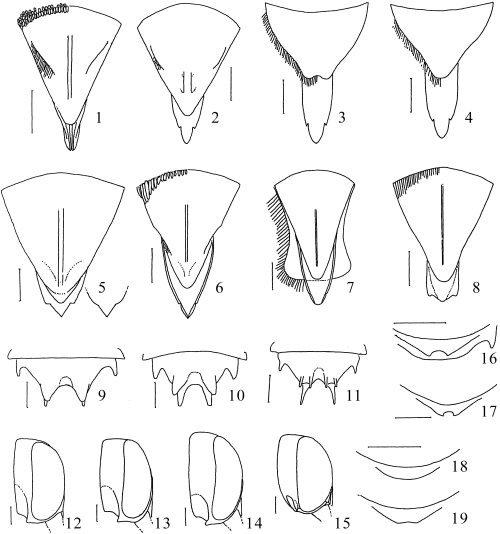
Characters of Japanese Coelioxys. 1,11,15C. formosicola; 2,3,10,14,18,19C. hiroba; 4,13C. hosoba; 5,9C. yanonis; 6,12,16C. sakamotorum sp. nov.; 7C. alata; 8,17. C. rufescens. 1–8 Terminal segments of female metasoma (1,2,5–8 dorsal view; 3,4 ventral view); 9–11 male tergum VI, dorsal view (10inermis-type); 12–15 head of male, lateral view, showing various genal concavities; 16–19 apices of male sternum IV, ventral view. Scale lines: 0.5 mm.
In the present study, conditions of appressed body hairs are represented by those of the apical hair bands of the tergum II. They can be categorized into three types:
- 1
Ordinary hairs (Fig. 20) are very elongate and feather-like, with the barbs along both sides of the shaft being much longer than the width of the shaft.
- 2
Broadened hairs (Figs 21,22) have a broadened shaft, and the barbs along the shaft are equal to or shorter than the width of the shaft; they are further subdivided into: (i) slightly broadened hairs with the shaft being subequal to the length of the barbs; and (ii) leaf-like hairs of which the shaft is much broader than the barbs.
- 3
Scale-like hairs (Fig. 23) usually have a highly broadened shaft, of which the surface is often polished or subpolished, and barbs along the margin of the shaft are lacking.
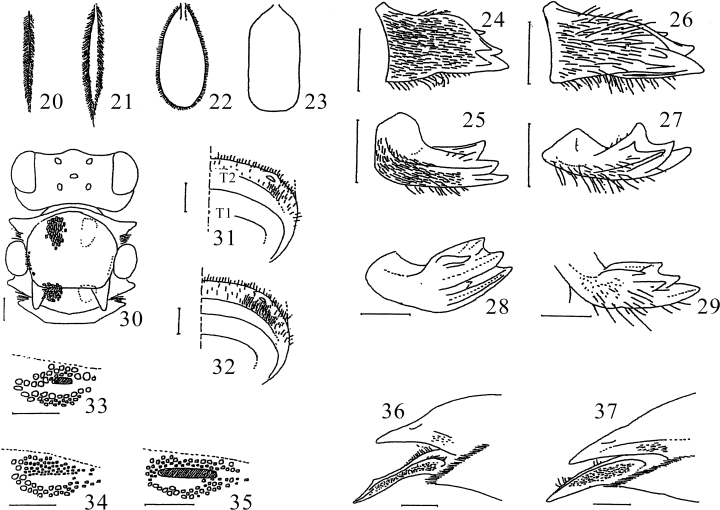
Characters of Japanese Coelioxys. 20,24,25,31,33,36C. hiroba; 21C. yanonis; 22,37C. sakamotorum sp. nov.; 23,30C. formosicola; 26,27,32C. hosoba; 28C. alata; 29,35C. inermis; 34C. rufescens. 20–23 Schematic Figures of median apical hairs of tergum II (20 ordinary hair; 21 slightly broadened hair; 22 leaf-like hair; 23 scale-like hair); 24–29 female right mandible (24,26 frontal view; 25,27–29 oblique view); 30 female head and thorax, dorsal view; 31,32 male tergum II, antero-dorsal view, showing hairs of gradular groove; 33–35 left sublateral fovea of male tergum II, hairs omitted; 36,37 terminal segments of female metasoma, lateral view. Scale lines: 0.5 mm.
TAXONOMIC NOTES AND DESCRIPTIONS
The diagnostic characters given in the key are not repeated except for the new species.
Coelioxys (Torridapis) fenestrata Smith
Coelioxys fenestrata Smith, 1873: 205–206 (female, Japan and China).
Specimens examined. Fifty-two females and 47 males, in addition to many specimens reared from the nests of Megachile sculpturalisSmith, 1853, in Hyogo Prefecture by Y. Hashimoto of the MNHA, were examined. Some of them are listed below.
Honshu: 1♀ (YM), Ikarigaseki, Aomori, 14.viii.1982, M. Yamada; 1♀ (YM), Jobojimachi, Iwate, 15.viii.1991, M. Yamada; 1♀ (YH), Rifucho, Miyagi, 23.ix.1979, K. Goukon; 1♀ (TT), Yashagaike, Fukui, 7.vi.1970, T. Tano; 1♀1♂ (NSMT), Meguro, Tokyo, 22.viii.1938, M. Yamanaka; 1♀ (HN), Kamakura, Kanagawa, 9.viii.1951, H. Nagase; 1♀ (NSMT), Ikeda, Osaka, 15.viiii.1946, K. Iwata; Kyushu: 2♀7♂ (HN), Satacho, Kagoshima, 5.viii.1979, H. Nagase; 3♂ (SI), Kashima, Koshikijima Is., 5.viii.1986, S. Ikudome. Korea: 1♂ (YH), Chojusan, 5.vii.1935, K. Kin.
Host record. Coelioxys fenestrata is well known as a parasite of Megachile sculpturalis (e.g. Iwata 1933), which would be the sole host of C. fenestrata in Japan.
Distribution. Japan (Honshu, Shikoku, Kyushu, Koshikijima Island, Tanegashima Island), Korea, Taiwan (Cockerell 1931), China (Smith 1873; Friese 1935).
Remarks. Coelioxys fenestrata has an univoltine life cycle and is a common bee in the southern areas of the main islands of Japan.
Coelioxys (Torridapis) ducalis Smith
Coelioxys ducalis Smith, 1853: 267 (female, India?).
Specimens examined. Yaeyama Islands: 1♀ (SI), Sonai, Iriomote Island, 9.ix.2001, T. Kusama. Taiwan: 1♀ (NSMT), Sungchi, near Taipei, 29.vi.1965, R. Ishikawa; 1♀ (NSMT), (no specific locality given), 1937, K. Iwata collection.
Distribution. Japan (Yaeyama Islands), Taiwan, China (Friese 1935; Wu 1993), South-east Asia.
Host record. Coelioxys ducalis is known to be a parasite of Megachile monticolaSmith, 1853 (Maeta et al. 2004).
Coelioxys (Allocoelioxys) formosicola Strand (1–19-38–60)
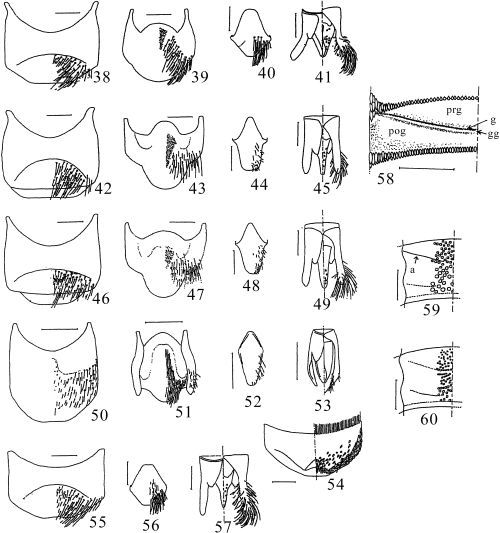
Characters of Japanese Coelioxys. 38–41C. yanonis; 42–45C. hiroba; 46–49C. hosoba; 50–54C. formosicola; 55–58C. sakamotorum sp. nov.; 59C. inermis; 60C. alata. 38,42,46,50,55 male sternum V; 39,43,47,51 male sternum VI; 40,44,48,52,56 male sternum VIII; 41,45,49,53,57 male genitalia; 54 male sternum IV; 58 left half of female tergum II, hairs and punctures omitted (g, gradulus; gg, gradular groove; prg, pregradular area; pog, postgradular area); 59,60 punctation of left median part of male tergum II, hairs omitted (a, anterior verge of gradular groove). Scale lines: 0.5 mm.
Coelioxys formosicola Strand, 1913: 64 (female, Taiwan).
Coelioxys taiwanensis Cockerell, 1927: 10 (male, Taiwan). Syn. nov.
Coelioxys brevis Eversmann, 1852: Yasumatsu (1956): 1489 (misidentification); Hirashima (1989) (ed.): 686 (listed).
Coelioxys sp.-A, Nagase (2003b): 4.
Although neither Strand’s (1913) original description of C. formosicola (female) nor Cockerell’s (1927) description of C. taiwanensis are detailed enough to allow me to decide to which species my specimens from Japan and Taiwan belong, M. Schwarz kindly compared Japanese specimens with the holotypes of C. formosicola and C. taiwanensis housed, respectively, in the Deutsches Entomologisches Institut and the American Museum of Natural History to confirm their identity. Consequently C. taiwanensis was revealed to be a junior synonym of C. formosicola. Below is detailed description of characters mainly based on Japanese specimens.
Female. Body length 9.5 mm (8.0–10.5 mm; n = 15); wing length 6.0 mm; head width 3.3 mm; mesoscutal width 2.5 mm.
Body black; antennae, legs and tegulae often slightly brownish; tarsi brown. Wings faintly smoky, darker along outer margin; nerves and stigma dark brown.
Hairs: Paraocular area and area below antennal socket covered with dense, rather long, decumbent hairs, mixed with sparse erect ones, almost completely obscuring surface sculpture; frons to vertex with sparse, suberect, brown to fuscous hairs, mixed with scattered, short, brownish-white, scale-like hairs; gena with dense, white, appressed hairs. Mesoscutum with a pair of elongate, triangular patches (margins are ill-defined) of brownish-white, scale-like hairs near anterior margin (Fig. 30); another pair of triangular patches of white, scale-like hairs covering posterolateral margin of mesoscutum, anterolateral corner of scutellum and inner anterior corner of axilla (Fig. 30); mesoscutum also with a small tuft of white hairs posterior to tegula, and a row of white hairs along lateral margin; median parts of mesoscutum and scutellum with very sparse, pale brown, suberect hairs, and very sparse, scattered, white, scale-like hairs;. lateral and ventral sides of mesosoma mostly covered with silky white, appressed hairs; posterior vertical surface of scutellum with a large patch of white, appressed hairs. Posterior margins of terga I–V with entire (not interrupted in the middle) hair bands, medially consisting of two or three rows of scale-like hairs and laterally widened to consist of six or more rows of hairs; horizontal part of tergum I anteriorly with an obscure band of narrow-triangular, apically pointed, scale-like hairs; tergum I laterally with large patches of dense, scale-like hairs, connecting anterior and posterior hair bands; tergal surface other than the apical bands nearly hairless; sternum I with a median patch of white, appressed hairs; sterna II–V with apical bands of white, appressed hairs, those of II–IV medially narrowed, and that of V nearly interrupted. Outer surfaces of all legs densely clothed with white, appressed hairs, mixed with short, erect hairs.
Flagellomeres I and II subequal in length; flagellomere X approximately 1.7-fold as long as its width. Transverse subocular carina (in the sense of Michener 2000) evident. Ocellar area weakly raised, front ocellus somewhat sunk; OOD longer than POD; POD approximately 2-fold as long as MPD; OCD about equal to or slightly longer than MPD; IAD approximately 1.5-fold as long as AOD. Punctures of vertex and mesoscutum large, very strong, with interspaces almost cariniform and microsculptured. Pronotal tubercle sharply carinate above, lamelliform, curving backward, with a tuft of long, white, hairs behind; median line of mesoscutum very fine. Parapsidal lines extremely faint. Posterior margin of dorsal surface of scutellum, in dorsal view, weakly rounded out and often notched in the middle. Posterior coxa weakly flattened and laterally ridged.
Tergum II with gradulus and gradular groove both absent, with a pair of lateral transverse impunctate areas. Punctures of terga rather evenly distributed, but laterally denser, becoming slightly smaller toward apical terga; space between punctures weakly microsculptured, not very shiny. Median ridge of tergum VI weak, basally obsolete; lateral sulcus of tergum VI (Fig. 1) distinct, with a tuft of long, white hairs. Sternum VI without lateral notch, but faintly constricted at midlength (Fig. 1); apex with a very minute incision, but sometimes obsolete, probably due to wear.
Male. Body measurements and color similar to female, but body slightly shorter.
Hairs: Facial hairs similar to that of female, but generally longer and slightly more yellowish. Body hairs also similar to that of female, except for terga IV and V with some scale-like hairs immediately posterior to graduli, and VI with abundant scale-like hairs basally, except in median area.
Genal concavity represented by an oval, ventrally faced, plate-like structure, with ventral surface slightly concave, glabrous and polished; length of longer axis of the oval plate about equal to length of two terminal flagellomeres combined; anterior part of the plate projecting from genal surface, in frontal view, visible below eye as a crescent-shaped projection. OOD longer than POD; POD approximately 2-fold as long as MPD; OCD much longer than in female, nearly 2-fold as long as MPD; IAD approximately 1.2-fold as long as AOD. Fore coxae without spines, but with oblique carinae. Front ocellar area, punctation of vertex and mesoscutum, pronotal tubercle as in female. Tergum II without sublateral fovea, gradulus or gradular grooves; III with gradular groove only faintly indicated laterally; IV and V with graduli more or less distinct but obsolete medially; tergum VI apically with eight (four dorsal and four ventral) spines; dorsal four spines short, divided into two pairs by median excavation (Fig. 11). Sterna I–III as in female; IV distinctly thickened toward apex, laterally forming small walls between ventral surface and lateral margin; the walls covered with dense, white, elongate, flattened hairs gradually changing to shorter leaf-like or scale-like hairs toward the medio-apical, round depression (Fig. 54); surface (not rim) of sternum IV apically with a distinct incision and scattered with very sparse, leaf-like hairs near apex; apex of rim of sternum IV exposed, with margin usually entire but often medially broadly and very shallowly emarginate. Genitalia and hidden sterna as in Figures 50–53.
Specimens examined. Forty-four females and 41 males were examined. Some of them are listed below. Honshu: 1♀1♂ (HN), Inamuragasaki, Kamakura, Kanagawa, 14.viii.1948, H. Nagase; 5♀ (RI), Tsujido, Fujisawa, Kanagawa, 13.viii.1953, R. Ishikawa; 1♀2♂ (HT), Nakajima Sand Dune, Hamamatsu, Shizuoka, 23.vii.1997, H. Takahashi; 1♀2♂ (TT), Terai, Ishikawa, 14.vii.1967, T. Tano; 3♀4♂ (YM), Sotozono, Taishacho, Shimane, 8.viii.2002, Y. Maeta; 5♀4♂ (TE), Hakoishi Coast, Kumihama, Kyoto, 26.vii.2002, T. Endo et al.; Kyushu: 1♀ (HS), Aoshima, Miyazaki, 27.ix.1965, H. Suda; 1♀1♂ (HN), Ichikicho, Kagoshima, 14.viii.1978, H. Nagase. Taiwan: 1♂ (NSMT), Shinka, 30.vii.1926, K. Sato; 1♂ (ELKU), Rokkiri, 1.ix.1927, J. Sonan and K. Shibata; 1♂ (ELKU), Anping, vii.1911, H. Sauter (labeled “Cotypus/Coel. afra sauteri Cockerell, Strand det.”).
Distribution. Japan (central and western Honshu, Kyushu, Tanegashima Island), Taiwan.
Host record. In Japan, C. formosicola has a bivoltine life cycle and a sand-nesting bee, Megachile kobensis Cockerell, 1918, is the only known host (Maeta et al. 1996; cited as C. brevis). As far as I observed, this species was always found near sandy places, sympatrically with M. kobensis. In Taiwan, where M. kobensis is not distributed, the host is unknown.
Remarks. This is the only Japanese Coelioxys that has four distinct white hair patches on the mesoscutum (Fig. 30).
Coelioxys formosicola has often been confused with an European species, C. brevis. They can be distinguished by the following characters: the gradular groove of tergum II in C. brevis is shallow but entire, associated with a nearly entire, transverse, impunctate band immediately posterior to the gradular groove, whereas in C. formosicola the gradular groove is lacking and only the transverse patches of impunctate areas are present laterally; the male sternum IV is not modified in C. brevis, whereas in C. formosicola it is apically distinctly thickened, apico-medially depressed and has the apex incised (Fig. 54); and the hairs of male tergum VI of C. brevis are rather bulky and long, different from the scale-like hairs of the preceding terga, whereas they are scale-like hairs similar to those of the preceding terga in C. formosicola.
Coelioxys (Boreocoelioxys) yanonis Matsumura (1–19-38–60)
Coelioxys yanonis Matsumura, 1912: 5 (female, Japan); Yano (1956): 1489; Hirashima (1989) (ed.): 686 (listed).
Coelioxys suisharyonis Strand, 1913: 62–63 (female, Taiwan). Syn. nov.
Coelioxys hakonensis Cockerell, 1919: 181 (female, Japan); Hirashima (1989) (ed.): 686 (listed). Syn. nov.
In the SEHU, there are two female specimens of C. yanonis that are certainly from the type series. One of them labeled “15.8.42 [probably year 42 of the Meiji Era, or 1909]/Kayatoge/From Mr Nakamura/Niigata” (where “/” denotes the beginning of a new line) is in poor condition, with both antennae and most of the tarsi missing. The other one does not have the date of collection, but is in good condition and the attached labels indicate that the specimen was undoubtedly used for the original description; they are: a white card paper of 5 mm × 11 mm in size, with “Japan/Matsumura” (in print), and on the back “Shibata/Hatakeyama” (in handwritten Japanese letters in black ink), of which “Shibata” is undoubtedly Shibata City in Niigata Prefecture and “Hatakeyama” is the name of the collector; a piece of paper of approximately 10 mm × 20 mm in size, on which is written “7” in Chinese characters, handwritten with red Japanese ink, indicating the number of the illustration in Matsumura (1912); a piece of paper 5 mm × 5 mm in size, with “2” (black, type-written); a piece of paper card 6 mm × 18 mm in size, with “Coelioxys yanonis/Matsumura ♀/K. Yasumatsu” (handwritten by Yasumatsu with black ink). The latter specimen is here designated as the lectotype, and I have added the label “Lectotype/Coelioxys yanonis/Matsumura, 1912/Desig. H. Nagase.”
I examined a specimen in the ELKU labeled “Cotypus/Coelioxys suisharyonis m. Strand det. ♀” and found that it is a dark-colored specimen of C. yanonis. After comparing Japanese specimens with the syntypes of C. suisharyonis in the Deutsches Entomologisches Institut, M. Schwarz came to the same conclusion. Furthermore, M. Mello of the US National Museum kindly compared the type specimen of C. hakonensis with my Japanese specimens and confirmed my proposed synonymy.
Detailed descriptions of specific characters follows.
Female. Body length 13.0 mm (11.0–15.0 mm, n = 20; lectotype 14.0 mm); wing length 9.5 mm (lectotype 9.5 mm); head width 4.0 mm (lectotype 4.0 mm); mesoscutal width 3.4 mm (lectotype 3.5 mm).
Body black; antennae and legs dark brown; tegulae and apical part of sternum I usually reddish brown, rarely dark brown; metasomal surface sometimes with faint bronzy sheen. Wings weakly infuscate, nearly transparent basally, darker in apical 0.4, with very slight purplish sheen; nerves usually reddish brown, occasionally darker.
Hairs: Face except vertex and clypeus covered with long, dense, mostly decumbent, usually brownish-yellow hairs. Clypeus at least medially without decumbent hairs, only with sparse and erect hairs, so that the rugosely reticulate surface is well visible; apical margin with dense, long hairs. Gena with rather sparse, grayish-white hairs, which are shorter near eyes and longer posteriorly. Vertex except for ocular area with few hairs. Mesoscutum with sparse, erect, pale grayish-brown hairs, not forming hair patches, except for tufts of hairs immediately anterior and posterior to tegula; hairs on other parts of mesosoma principally pale grayish brown, paler toward ventral side. Terga I–V with entire apical bands of yellowish-brown hairs; the bands medially consisting of one or two rows of slightly broadened hairs; tergum II band narrowest medially and widest laterally, with the maximum width less than 2-fold as wide as median width. Tergum I laterally often with some long, pale hairs, but lacking hair patches; surface of terga II–IV except for apical hair bands scanty of hairs; sterna II–V usually with entire apical hair bands of uniform width, but the bands sometimes interrupted medially possibly due to being worn off.
Apical margin of clypeus often slightly emarginate medially and with three to five minute tubercles, which are rarely obsolete. Flagellomere I distinctly shorter than II, II–IX approximately equal in length, X approximately 2-fold as long as its width. IAD slightly more than 2-fold as long as AOD. Ocellar area distinctly raised; POD somewhat variable, usually approximately equal to MPD and approximately 0.5-fold as long as OOD; OCD slightly longer than POD. Frons, gena and vertex grossly punctate.
Posterior margin of scutellum, in dorsal view, weakly arched and, in anterior view, medially obtusely angled; median line of mesoscutum represented by an obscure carina; parapsidal lines more distinct as carinae. Fore coxae anteriorly with small, angular projections.
Gradular grooves of terga II and III only weakly developed, very shallow even at lateral area and broadly interrupted medially; posterior to the grooves weakly swollen and very sparsely punctate transverse areas present. Punctures of terga II–VI gradually finer toward apical segments; space between punctures microscopically sculptured and only weakly shining. Tergum VI with strong median ridge, dorso-subapically with a pair of distinct depressions near median ridge, and with strong lateral ridges running very closely to and in parallel to lateral margins for more than half the length of the tergum; lateral sulcus between the ridge and the margin deeply grooved, with short hairs anteriorly. Sternum V bluntly pointed apically, basally with large punctures, and apically longitudinally rugoso-striate. Sternum VI longitudinally and finely punctate-striate in most part, with narrow punctate area basally; subapical margin with steps (not notches) differentiating the apex as a nearly equilateral triangular part (Fig. 5); shape of apex fairly stable, but very rarely somewhat modified (Fig. 5).
Male. Body length 11.0 mm (9.0–13.0 mm n = 20); wing length 9.0 mm; head width 3.8 mm; mesoscutal width 3.1 mm. Body color similar to female.
Hairs: Color paler than in female. Whole face covered with decumbent, long hairs. Hair condition on metasoma as in female, but apical hair band of tergum V nearly obsolete; terga V and VI with hair bands immediately posterior to graduli, the band of V usually narrowly interrupted medially.
Anterior margin of clypeus usually with three to five minute tubercles (sometimes obsolete). Lower gena with a large genal concavity, which reaches nearly middle of eye height at its posterior end, and has a distinct row of short, grayish-white hairs near anterior margin and longer hairs of the same color along posterior margin. Flagellomere I shorter (usually distinctly, but rarely very slightly so) than II; XI approximately 2-fold as long as its width. Ocellar area as in female. Vertex immediately posterior to posterior ocelli rather steeply sloping down toward occipital margin. IAD approximately 2-fold as long as AOD. Mesoscutum and scutellum as in female. Gradular grooves of tergum II broader and deeper than those of female; terga III–V with more or less distinct graduli. Sublateral fovea of terga II round to elliptic, variable in size, but generally small, sometimes as small as larger punctures nearby, with width of 2.125 (range 1.5–3.0; n = 20) when the head width is set as 80, 1- to 3-fold as wide as long; impunctate area around the fovea variable in size and shape, often more developed anteriorly to the fovea than posteriorly. Metasomal terga and sterna with much more developed posterior rims than in female; apical margin of posterior rim of sternum IV exposed, usually slightly emarginate in the middle, but sometimes entire (Figs 18,19). Posterolateral corners of tergum V with blunt spines, partly fused with posterior rim. Tergum VI inermis-type, but apices of two dorsal spines, in dorsal view, usually (with few exceptions) not situated beyond outer margins of two ventral median spines (Fig. 9). Genitalia and hidden sterna as in Figures 38–41.
Specimens examined. A total of 221 females and 197 males were examined. Some of them are listed below. Hokkaido: 1♀ (SEHU), Kosekiyama, Sapporo, 2.ix.1962, R. Nagase; 1♀ (SEHU), Tomari, Shakotan, 10.viii.1976, Sk. Yamane; 1♀ (HI), Hagiwaracho, Date City, 15.viii.2002, I. Kimura; Honshu: 1♀ (MY), Kudoki, Hirosaki, Aomori, 2.ix.2002, M. Yamada; 1♀ (YH), Kuriyagawa, Morioka, Iwate, 13.ix.1979, Y. Maeta; 1♀4♂ (KG), Aobayama, Sendai, Miyagi, 5.ix.1980, K. Goukon; 1♀3♂ (HI), Ijimino, Shibata, Niigata, 14.viii.1999, H. Itami; 4♂ (HN), Katashinamura, Gumma, 28.viii.2002, H. Nagase; 1♀2♂ (RI), Yoyogi Park, Shibuya, Tokyo, 25.vii.2001, R. Ishikawa; 2♀ (HN), Sanogawa, Fujinomachi, Kanagawa, 21.viii.2003, H. Nagase; 2♂ (HS), Daibosatsu Pass, Enzan, Yamanashi, 2.ix.1975, H. Suda; 2♀ (HN), Komoro, Nagano, 30.viii.1997, H. Nagase; 2♀ (TT), Yashagaike, Fukui, 20.viii.1978, T. Tano; 2♀ (RI), Minoo, Osaka, 30.viii.1954, S. Ueno and R. Ishikawa; 1♀ (MK), Kagaminomachi, Okayama, 16.viii.2003, M. Kato; Shikoku: 1♀ (SEHU), Matsuyama, Ehime, 18.ix.1958, K. Kamijo; 1♀ (SEHU), Kodokas (?), Kochi, 4.ix.1936, H. Okamoto; Kyushu: 2♀ (YH), Adachi Park, Kokura, Fukuoka, 1.ix.2001, Y. Hirashima; 14♀ (HN), Okuchi, Kagoshima, 1.ix.1980, H. Nagase. Korea: 1♂ (MNHA), Seoul, 11.vii.1974, I. Kudo; 1♂ (YM), Chunchon City, Seong Dong Ri, 31.vii.1998, Y. Maeta; 1♀ (YM), Hongcheon Gun, Someagok RI, Buk Bong, 19.viii.2001, Y. Maeta; 2♂ (NIAES), Mount Yogi, Suwon, 23.vii.1994, S. Yoshimatsu. Taiwan: 1♀ (ELKU), Suisharyo, x.1911, H. Sauter (labeled “Cotypus/Coelioxys suisharyonis m. Strand det. ♀”); 1♀ (ELKU), Arisan, 2–23.x.1918, J. Sonan and M. Yoshino (labeled “Coelioxys suisharyonis Strand, det. T. Shiraki”).
Distribution. Japan (Hokkaido, Honshu, Shikoku, Kyushu, Yakushima Island), Korea new record, Taiwan new record.
Host record. Megachile humilis Smith, 1879, is known to be the host (Maeta et al. 1996). Adults of C. yanonis appear from late summer to autumn, which coincides with the flying season of M. humilis. Judging from the size of the bees, it is likely that M. humilis is the main host of C. yanonis in Japan. Information on the hosts outside of Japan is unavailable.
Remarks. The postocellar distance of female C. yanonis is shorter than that of other Japanese species, such as C. hiroba and C. inermis, in which the postocellar distance is usually much longer than the maximum diameter of the posterior ocellus and only slightly shorter than the ocello-ocular distance.
The hair color in the Japanese specimens is quite variable; that is, hairs of the metasomal bands vary from nearly white to dark reddish brown, and the facial hairs from nearly white to deep brownish yellow or reddish brown. The specimens from Taiwan and Korea have fairly dark colored hairs, but some specimens from the southern parts of Japan have hairs just as dark as in Taiwanese and Korean ones. This species is the commonest Coelioxys in Japan, but it seems to be rare in Hokkaido.
Coelioxys (Boreocoelioxys) sakamotorum sp. nov. (1–19-38–60)
Coelioxys sp.-B, Nagase (2003b): 5.
Female. Body length 11.0 mm (9.0–13.0 mm, n = 20; holotype 9.5 mm); wing length 7.0 mm (holotype 6.5 mm); head width 3.5 mm (holotype 3.0 mm); mesoscutal width 2.5 mm (holotype 2.5 mm).
Body black; antenna and tegulae slightly brownish black; legs blackish to reddish brown; ventral side of tergum I and sternum I often reddish brown; sternum V and apex of tergum VI sometimes faintly reddish brown. Wings very weakly infuscate, much darker along outer margins; nerves dark reddish brown, stigma reddish brown.
Hairs: Pale yellowish brown on face, grayish white elsewhere. Hairs of clypeus and supraclypeal area short and decumbent, mixed with sparse erect ones, obscuring rugose surface sculpture; paraocular area with much longer hairs, which are mostly decumbent and mixed with sparse erect hairs; gena with sparser hairs; vertex with much sparser erect hairs. Mesoscutum and scutellum with very sparse, erect hairs; dorsal half of mesopleuron with abundant, long, white hairs. Terga I–V with entire apical band of white, leaf-like hairs arranged in single rows in the middle (Fig. 58); each hair band widened laterally, on tergum I the band laterally extending anteriorly to form distinct, white, triangular hair patches; on terga II and III, hair bands more than 2-fold wider at lateral side than at median narrowest part. Sternum I with a median patch of grayish-white hairs; sterna II–V with entire, apical white hair bands; except for hair bands, surfaces with very few hairs on terga II–V, and short, sparse, suberect hairs on sterna II–V.
Ocellar area distinctly raised. Flagellomere I shorter than II. POD approximately equal to OCD, shorter than OOD, and much longer than MPD.
Median line and parapsidal lines of mesoscutum faint. Mesoscutum, axillae and scutellum coarsely and densely punctate; interspaces between punctures almost linear. Axillae flat, free from scutellar margin in approximately posterior 0.3, with apices pointed and slightly curved inward. Scutellum not keeled; in dorsal view posterior margin weakly and bluntly angled, sometimes with median incision, slightly exceeding level of axillal apex. Metanotum transverse and nearly vertical. Propodeum densely punctate; propodeal triangle without hair, mat and usually with basal sculpture. Forecoxa anteroventrally with a distinct keel, of which median part projects forward rectangularly.
Gradular grooves of terga II and III narrow but distinct (Fig. 58), medially very shallow, on III often obsolete; bottom of gradular groove with a row of punctures; posterior to the gradular groove impunctate or very sparsely punctate transverse areas developed; terga IV and V also with sparsely punctate transverse areas, which are much smaller than those of terga II and III. Lateral carina of tergum VI rather strong, longer than 0.3 of exposed portion of the tergum (Fig. 37), running somewhat obliquely and thus making outline of the tergum in dorsal view weakly concave laterally (Fig. 6); lateral sulcus beneath the carina distinct, with a tuft of white hairs; median ridge of tergum VI distinct at least in its apical 0.6; tergum VI subapically with a pair of longitudinal depressions between median ridge and lateral carina. Sternum VI rather broad, broader than tergum VI in apical half of the tergum (Fig. 6), with small notches and sharp spines subapically (Fig. 6), with apical triangular part elongate and weakly convex laterally; downward curvature of sternum VI relatively weak (Fig. 37).
Male. Measurements similar to female, except for slightly shorter body length.
Body black; antennae, mandibles, tegulae and legs dark reddish brown; ventral part of tergum I, most of sternum I, and sometimes basal part of sternum II reddish brown.
Hairs: Facial hairs long, mostly decumbent, and mixed with sparse erect ones; hairs on clypeus, supraclypeal and paraocular areas pale yellowish brown, much longer than in female; hairs on gena and vertex similar to those of female. Terga I–V with entire apical bands (but usually interrupted medially on V) of leaf-like, white hairs, arranged in single rows in median part; hair bands of terga I–IV widened laterally as in female; terga V and VI with entire hair bands immediately posterior to graduli, which are usually half hidden under preceding terga; tergum IV also with weak hair patches posterior to graduli. Sternum I medioapically with a round, white hair patch; sterna II–IV with white apical hair bands, which are nearly the same width throughout.
Ocellar area prominently and roundly raised. Genal concavity large, in lateral view attaining about half of height of eye, with a row of white hairs close to the anterior margin and abundant, long, white hairs along the posterior margin. OOD longer than POD, approximately as long as OCD and much longer than MPD. Vertex behind posterior ocelli sloping down toward occipital margin. Mesosoma and wings as in female.
Gradular grooves of terga II and III narrow but entire, with a row of punctures in the bottom of each; posterior to gradular grooves sparsely punctate transverse areas weakly developed; terga IV and V with distinct graduli, without posterior transverse impunctate areas; sublateral fovea of tergum II elliptic to elongate, 2- to 3-fold as wide as long, with width of 4.2 (range 3.5–5; n = 9) when the head width is set as 80. Tergum V with a pair of blunt spines apico-laterally, partly fused with apical rim. Tergum VI inermis-type. Apical rim of sternum IV with a small but distinct emargination in the middle (Fig. 16). Genitalia and hidden sterna as in Figures 55–57.
Holotype. ♀ labeled “Usui/Sakura City/Chiba Pref./22.x.1975/H. Suda leg.” and “Holotype/Coelioxys/sakamotorum sp. nov./det. H. Nagase 2006,” deposited in the Entomological Laboratory, Faculty of Agriculture, Kyushu University.
Paratypes. Honshu: 1♀ (KG), Tomiyacho, Miyagi, 21.ix.1989, K. Goukon; 1♀1♂ (NSMT), Kiyohara, Utsunomiya, Tochigi, 22.viii.1949, M. Yamanaka; 1♂ (YM), Hitachi-Omiya, Ibaraki, 29.ix.1973, Y. Maeta; 1♀ (TN), Kouya, Ogawa, Saitama, 10.ix.1994, T. Nambu; 1♂ (ELKU), Ranzan, Saitama, 12.ix.1998, K. Hara; 1♀ (ELKU), Ojamachi, Sakura, Chiba, 16.viii.1971, H. Suda; 1♀ (HN), Iida, Sakura, Chiba, 30.ix.1971, H. Suda; 1♀ (HS), Tennodai, Choshi, Chiba, 14.ix.1987, H. Suda; 1♀ (HN), Yamanashi, Yotsukaido, Tennodai, 4.ix.1996, H. Suda; 1♂ (HN), Shisui, Sasaimachi, Chiba, 8.v.1975, H. Suda; 1♂ (ELKU), Takadacho, Chiba, 5.viii.1995, H. Suda; 1♂ (HN), Murakami, Yachiyo, Chiba, 2.vi.2001, H. Suda; 1♀ (ELKU), Mikunitoge, Yamakita, Kanagawa, 3.vii.2001, H. Nagase; 1♀ (HN), Funako, Atsugi, Kanagawa, 5.ix.2001, T. Ishizaki; 1♀ (HN), Sarugashima, Atsugi, Kanagawa, 4.v.2004, H. Nagase; 1♀ (RI), Kitagomura, Shizuoka, 8.vii.1950, R. Ishikawa; 1♀ (MNHA), Ichikawacho, Hyogo, 23.vi.1996, T. Sasai; 1♀ (YM), Yumigahama, Tottori, 5.ix.1993, Y. Maeta; Kyushu: 1♀ (HN), Satacho, Kagoshima, 8.vii.1978, H. Nagase; 1♀ (SI), Kanoya, Kagoshima, 15.ix.1981, S. Ikudome.
Other specimens examined. Honshu: 2♀ (TN), Oshikiri, Konan, Saitama, 27.vi.1993, T. Nambu; 1♂ (KN), Kami-Mikawamachi, Tochigi, 14.viii.2000, T. Murakami; 1♂ (NSMT), Kinuta, Tokyo, 26.v.1932, S. Asahina; 1♀ (NIAES), Ogomachi, Gumma, 23.ix.1974, H. Kawano; 1♂ (MNHA), Sumoto, Awajishima, Hyogo, 3.vi.1990, T. Ikeda; 1♀ (KG), Rifucho, Miyagi. K. Goukon (Nest and cell: 79 Mg-I A-1).
Distribution. Japan (Honshu, Kyushu).
Host record. K. Goukon, Tagajo, Miyagi Prefecture reared a female of C. sakamotorum from a nest cell of Megachile humilis Smith, 1879, which was dug out from an underground nest in Rifucho, Miyagi Prefecture, on 18.ix.1979. The adult emerged in 1980, but the date of emergence was not recorded (K. Goukon, unpubl. data, 1980; the last listed specimen under the “Other specimens examined” heading).
Etymology. “Sakamoto” is the family name of my wife, to whom this species is dedicated.
Remarks. This species is similar to Coelioxys alfkeni Popov, 1946 (replacement name for C. mongolica Alfken, 1936; non Friese, 1935), described from Mongolia. I did not examine the type specimens of C. alfkeni, but based on the examination of a female specimen compared with the types of C. alfkeni by M. Schwarz and a photograph of a syntype male of C. alfkeni, they can be separated by the combination of the following characters: the metasomal hair bands, in both sexes, consist of leaf-like hairs (shaft of a hair much wider than the length of barbs) in C. sakamotorum, whereas there are slightly broadened hairs (width of shaft subequal to the length of barbs) in C. alfkeni; body hairs of both sexes more abundant in C. sakamotorum, especially on the dorsolateral parts of the tergum I and the mesopleuron, whereas they are rather sparse on these parts in C. alfkeni; in C. sakamotorum the female vertex behind the posterior ocelli has a narrow space only weakly sloping down before the steep slope, whereas in C. alfkeni it slopes steeply down immediately behind the posterior ocelli, and consequently the ocello-occipital distance is distinctly longer than the maximum diameter of the posterior ocellus in C. sakamotorum and nearly equals the latter in C. alfkeni; the sublateral fovea of the male tergum II is distinct in C. sakamotorum, whereas it is obsolete to weak and shallow in C. alfkeni (Alfken 1936); the original description states that the male has no fovea, but the photograph of a syntype shows that it has small fovea, the length of which is approximately 3–3.5 when the head width is set as 80.
Coelioxys (Boreocoeioxys) hiroba Nagase (1–19-38–60)
Coelioxys sp.-C, Nagase (2003b): 5.
Coelioxys hiroba Nagase, 2003a: 426 (female, male, Japan).
Some characters added to the original description are as follows.
Female. Body length 11.5 mm (10.5–13.0 mm; n = 20); wing length 8.0 mm; head width 3.4 mm; mesoscutal width 3.0 mm; IAD approximately 1.7-fold as long as AOD. Vertex in anterior view weakly arched; ocellar area weakly raised; upper margin of mandible ecarinate, but with blunt ridge apically continuous to the carina of middle tooth (Fig. 24). Except for the apical bands, terga II–VI with very few hairs, and sterna II–IV with sparse, suberect, short but conspicuous, brownish-white hairs.
Male. Body length 10.0 mm (8.0–12.0 mm; n = 20); wing length 7.5 mm; head width 3.3 mm; mesoscutal width 2.8 mm. Vertex behind posterior ocelli rather flat, not distinctly sloping posteriorly as in female; IAD approximately 1.7-fold as long as AOD. Sublateral fovea of tergum II 2.0- to 3.5-fold as wide as long, with width of 2–6 (average 4.32; n = 20) when the head width is set as 80; punctures in pregradular areas of terga II–IV finer and more uniformly distributed than those in postgradular areas. Tergum II, except for apical hair band, generally with sparse hairs but hairless in pregradular area; gradular groove with sparse (occasionally no) hairs, which cover from the lateral area toward the middle but not exceeding the inner margin of the foveal area (lateral fovea and surrounding sparsely punctate area) (Fig. 30). Terga V and VI with weak transverse patches of white hairs immediately posterior to graduli (instead of “with weak basal hair bands” of the original description); tergum VI inermis-type (Fig. 10).
Specimens examined. Including types, 304 females and 312 males were examined. Some of them, other than types, are listed below. Hokkaido: 1♀ (ELKU), Sapporo, 20.viii.1927, K. Sato; 1♂ (ELKU), Aizankei, Ishikari, 27.vii.1952, T. Shirozu; Honshu: 2♂ (MY), Hiraka, Nurukawa, Aomori, 4.ix.1996, M. Yamada; 1♂ (HN), Ogunimachi, Yamagata, 17.ix.2003, H. Itami; 1♂ (HN), Kamiishikawa, Shibata, Niigata, 1.ix.2001, H. Itami; 1♀ (RI), Karuizawa, Nagano, 8.vii.1959, R. Ishikawa; 4♀ (HN), Foot of Mount Tanigawa, Gunma, 29.vii.1961, H. Nagase; 2♂ (TT), Taniyama, Fukui, 8.vi.1975, T. Tano; 1♂ (YM), Mount Makuragi, Shimane, 22.ix.1992; 1♀ (TE), Nishinomiya, Hyogo, 27.vi.2001, H. Taketani and N. Yamamoto; 1♀ (HN), Kagaminomachi, Okayama, 8.vi.2003, M. Kato; 1♂ (MNHA), Flower Town, Sanda, Hyogo (Trap Nest 1995-54-33 R-1); Shikoku: 1♂ (SI), Monobemura, Kochi, 23.ix.1976, S. Ikudome; Kyushu: 1♀ (SI), Kujumachi, Oita, 16.viii.1991, Sk. Yamane; 6♂ (HN), Satacho, Kagoshima, 8.vii.1978, H. Nagase. Korea: 1♀ (NIAES), Odesan National Park, 8.vi.1994, T. Matsumura.
Distribution. Japan, Korea new record.
Host record. Maeta et al. (1996) presumed Megachile tsurugensis Cockerell, 1924, as a host of C. hiroba (reported as C. inermis), which was confirmed by the adult male of C. hiroba having emerged together with adults of M. tsurugensis from a trap nest set at Sanda, Hyogo Prefecture (Y. Hashimoto, unpubl. data, 1996; adult emerged in 1996, but the date was not recorded; the last specimen under “Honshu”).
Remarks. The condition of hairs in the gradular groove of the tergum II is best observed when the specimen is viewed antero-dorsally with light source from the same direction. This species is common in Honshu and Kyushu, but apparently rare in Hokkaido. Judging from the collection records, adult females appear in April through October, which suggests that the species has more than one generation per year.
Coelioxys (Boreocoelioxys) hosoba Nagase (1–19-38–60)
Coelioxys sp.-D, Nagase (2003b): 6–7.
Coelioxys hosoba Nagase, 2003a: 427–428 (female, male, Japan).
Examination of the additional specimens has revealed that the state of the genal concavity used in the original description (Nagase 2003a) to distinguish the male of C. hosoba from that of C. hiroba is not always stable, that is, the posterior margin of the concavity is not always clearly indicated, and some specimens of C. hiroba have a longer excavation. On the other hand, hairiness of the gradular groove of the tergum II is a more reliable character to separate them. Consequently, the description of the male is revised as follows.
Male. Very similar to male of C. hiroba, but distinguished from the latter primarily by having abundant hairs in the gradular groove of tergum II, with the hairy area ranging from the lateral area toward the middle beyond the inner margin of foveal area (Fig. 32). Genal concavity in lateral view attaining approximately 0.4 of eye height.
Specimens examined. A total (including types) of 97 females and 87 males were examined. Some of them, other than types, are listed below. Hokkaido: 3♂ (RI), Mount Petegari, 24.vii.1970, R. Ishikawa; 1♀ (SI), Hokkaido University Campus, Sapporo, 1.ix.1977, S. Ikudome; 2♀ (EK), Otofukemachi, Katogun, emerged mid-late vi.2000, E. Katayama; Honshu: 1♂ (MY), Amagamori, Misawa, Aomori, 13.viii.1987, M. Yamada; 2♂ (RI), Karuizawa, Nagano, 24.viii.1955, R. Ishikawa; 1♀1♂ (HN), Nikaido, Kamakura, Kanagawa, 3.v.1957, H. Nagase; 2♂ (RI), Subashiri, Shizuoka, 10.vii.1950, R. Ishikawa; 1♀ (NSMT), Sumiyoshi, Osaka, 19.vi.1931, H. Katayama; 2♂ (YH), Tamachi, Tsuyama, Okayama, 8.v.1966, T. Ono; 1♂ (TT), Terai, Ishikawa, 12.ix.1967, T. Tano; 1♀ (YM), Nishikawazu, Matsue, Shimane, 21.iv.1994, Y. Okajima. Korea: 1♂ (YM), Kyonggido, Kwangnung, 2.viii.1998, Y. Maeta; 1♂ (YM), Hongcheon Gun, Seong Dong RI, Buk Bang Myon, 19.viii.2001, Y. Maeta.
Distribution. Japan, Korea new record.
Host record. Katayama (2001) recorded Megachile willughbiella munakatai Hirashima and Maeta, 1974, as the host of C. hosoba (reported as C. inermis) and that the nest of M. w. munakatai collected in Otofukemachi, Hokkaido, in July 1999 produced adults of both M. w. munakatai and C. hosoba in June 2000. Maeta (1979) reared a male of C. hosoba (reported as Coelioxys sp.) from an underground nest of Megachile japonica Alfken, 1903, collected in Asagishi, Iwate Prefecture, in June 1975; the adult parasite emerged in June 1976 synchronously with the host. I have examined the voucher specimens of the above records. I also examined five males out of nine males and one female of Coelioxys sp. collected on 22 April 2004 by H. Takahashi near a nesting site of M. japonica in Musashi-murayama, Tokyo, and they are C. hosoba. All these observations and the adult collection data (collected in May through September) suggest that C. hosoba may have a multivoltine life cycle but could be univoltine when hosts, such as M. w. munakatai and M. japonica, have a univoltine life cycle.
Remarks. In the female, C. hosoba can be distinguished from C. hiroba by the shape and the hair condition of the mandibles. Although the female mandibles of C. hosoba (Figs 26,27) show some variation, the mandibular hairs are always distinctly longer and more erect in C. hosoba than in C. hiroba.
Coelioxys (Boreocoelioxys) inermis (Kirby) (20–37, 38–60)
Apis inermis Kirby, 1802: 229 (female, male, England).
Synonymy: see Warncke (1992).
Following are some morphometric and diagnostic characters of the species based on Japanese specimens.
Female. Body length 10.5 mm (9.5–13.0 mm; n = 10); wing length 8.0 mm; head width 3.5 mm; mesoscutal width 3.1 mm; male: body length 10.0 mm (8.5–11.0 m; n = 10); wing length 8.0 mm; head width 3.5 mm; mesoscutal width 2.9 mm.
Hair bands on female terga II–IV narrowed (often interrupted) medially, where it is less than 0.3-fold as wide as at the lateral widest part. Male tergum VI inermis-type; hair band on tergum I interrupted medially; sublateral fovea of tergum II (Fig. 35) large, 3.5–6 (mostly 4–5.5)-fold as wide as long, width of 8–11 when the head width is set as 80; posterior end of genal concavity attaining approximately 0.4 of eye height.
Specimens examined. Hokkaido: 1♀ (YH), Otaru, viii.1926, Tamanuki; 2♂ (ELKU), Tokachi, Nishiashoro, 9.viii.1953, Y. Hirashima; 2♂ (ELKU), Tokachi, Kawakami (near Honbetsu), 17–27.vii.1953, Y. Hirashima; 1♂ (ELKU), Shikotsuko, Morappu, 11–13.vii.1953, Y. Hirashima; 1♂ (YM), Sapporo, 6.viii.1959, Y. Maeta; 1♀ (RI), Tokachi, Hirouchi, 12.vii.1960, R. Ishikawa, 1♀ (YM), Sapporo, emerged from host nest on 6.v.1973, Y. Maeta (attached label “host Meg. ainu”); 1♀ (SI), Jozankei, Sapporo, 30.viii.1977, S. Ikudome; 1♀ (SI), Sapporo, 1.ix.1977, S. Ikudome; 1♀ (SI), Sapporo, 25.vi.1979, S. Sakagami; 3♀3♂ (NIAES), Sapporo, M. Toda (♀: 10.vii.1979; 10.ix.1979; 1.viii.1989; ♂: 30.vii.1979; 5.vi.1989; 12.vi.1989); 1♀ (ELKU), Mount Daisetsu, 15.viii.1984, O. Tadauchi; 1♀ (ELKU), Wakkanai, 16.viii.1984, O. Tadauchi; 1♂ (ELKU), Oniwaki, Risiri Island, 17.vii.1984, O. Tadauchi; 1♂ (ELKU), Kussharo-Lake, Teshikaga, 21.vii.1984, O. Tadauchi; 1♀ (ELKU), Tokachimitsumata, Kamishihoro, 11.ix.1984, O. Tadauchi; 1♀ (HN), Furencho, Kamikawa, 7.vii.2001, M. Kato.
Distribution. Japan (Hokkaido, Rishiri Island), Eastern Russia, Sakhalin, Europe, North Africa.
Host record. Megachile ainu Hirashima and Maeta, 1974, has been reported as a host of C. inermis in Hokkaido (Maeta et al. 1996; see also the list of specimens examined). The voucher specimen was examined.
Coelioxys (Boreocoelioxys) alata Foester (1–19-38–60)
Coelioxys alata Foerster, 1853: 296 (female, Germany).
Synonymy: see Warncke (1992).
This is the first record of C. alata from Japan and some morphometric and important characters are provided based on Japanese specimens.
Female. Body length 12 mm; wing length 9.0 mm; head width 3.6 mm; mesoscutal width 3.1 mm.
Body hairs mostly white, but hairs on vertex as well as long, sparse, erect hairs on mesoscutum fuscous; facial hairs sparse, pale gray. Clypeal hairs distinctly longer than those of C. inermis, C. hiroba or C. hosoba. OOD approximately 1.5-fold as long as POD; POD approximately equal to OCD, and approximately 1.5-fold as long as MPD; IAD approximately 1.5-fold as long as AOD. Apical half of mandible broadened, spoon shaped (Fig. 28).
Tergum I without apical hair band, but with lateral patches of white hairs; apical hair bands on terga II–IV narrowed medially; tergum V with very weak hair band. Sternum V broadly expanded apically, fan-shaped, with apical margin rounded or very obtusely angled, furnished with very long, reddish-brown hairs along posterior and lateral margins (Fig. 7); apex of sternum VI rounded, with very small, subapical notches.
Male. Body length 10.5 mm; wing length 8.5 mm; head width 3.5 mm; mesoscutal width 3.1 mm; sublateral fovea of tergum II transverse, approximately 7-fold as wide as long, very large, with width of 12 when the head width is set as 80.
Specimens examined. Hokkaido: 1♀ (ELKU), Kussharoko, Teshikaga, 12.ix.1984, O. Tadauchi; 1♀ (HN), Furencho, Kamikawa, 18.vii.2001, M. Kato; 1♂ (ELKU), Kawakami, near Honbetu, Tokachi, 17–27.vii.1953, Y. Hirashima.
Distribution. Japan (Hokkaido), eastern Russia including Primorskij Kraj, north and eastern Europe.
Remarks. In addition to the characters mentioned in the key, the following character may help to separate C. alata male from C. inermis male: punctures of terga III–V in C. alata are smaller and much more evenly distributed than in C. inermis.
Coelioxys (Boreocoelioxys) rufescens Lepeletier and Serville (1–19, 20–37)
Coelioxys rufescens Lepeletier and Serville, in Latreille et al. (1825): 109 (female, male, France).
Synonymy: see Warncke (1992).
Some morphometric and important characters for Japanese specimens are as follows.
Female. Body length 11.0 mm; wing length 8.0 mm; head width 3.7 mm; mesoscutal width 2.7 mm.
Body black; antennae, legs, tegulae and extreme lateral margins of terga reddish brown. Body hairs, including facial hairs and metasomal apical hair bands, white to yellowish white, but those on frons to vertex and mesoscutum slightly grayish white. Clypeal hairs long, as long as or only slightly shorter than paraocular hairs. OCD slightly longer than POD; OOD much longer than OCD. Posterior margin of scutellum medially obtusely angled. Tergum I without apical hair band, but with distinct lateral hair patches; terga II–V with entire apical hair bands, which are not strongly narrowed in the middle; gradular groove of tergum II narrow, medially almost obsolete; posterior transverse impunctate or sparsely punctate areas on terga II and III only weakly indicated. Sternum I with a median hair patch, II–V with broad apical hair bands. Lateral sulcus of tergum VI obsolete; sternum VI with subapical notches obtuse, apical triangular part short, and rectangularly or obtusely angled apically (Fig. 8); downward curvature of sternum VI hardly discernible, only apex bent downward.
Male. Body length 10.5 mm; wing length 8.5 mm; head width 3.5 mm; mesoscutal width 3.1 mm.
Body black; antennae, tegulae, forelegs and all tarsi reddish brown, femora and tibiae of mid- and hindlegs dark brown. Body hairs white, except those of vertex and mesoscutum fuscous. OOD approximately 1.4-fold as long as POD and approximately equal to OCD; POD approximately 2-fold as long as PMD. Tergum I without apical hair band, but with a pair of white lateral hair patches; II–IV with entire apical bands of ordinary hairs; V with very poor hair band. Gradular grooves medially obsolete, distinct on terga II and III, faint on IV and V. Sublateral fovea of tergum II completely filled with very finely reticulate structure (Fig. 34). Median apical rim of sternum IV with a small but distinct emargination (Fig. 17).
Specimens examined. Hokkaido: 1♀ (YM), Ashoro, Tokachi, 13.viii.1959, Y. Maeta; Honshu: 1♂ (TT), Shimouchinami, Fukui, 18.viii.1974, T. Tano.
Distribution. Japan (Hokkaido, Honshu), eastern Russia including Primorskij Kraj, South Kurile, China (Friese 1935), Europe, North Africa.
Remarks. This is the first record of C. rufescens in Japan. This species is widespread in the Palearctic region, but seems to be rare in Japan.
Key to Japanese species of the genus Coelioxys
Female
- 1
Eyes not hairy. Distal half of forewings strongly darkened. Large species; body length 17 mm or more .........................2. Subgenus Torridapis
- –
Eyes densely hairy. Distal half of forewings weakly infuscate. Smaller species; body length 15 mm or less .................................3
- 2
Head including vertex and genae covered with dense, bright red or yellowish-red hairs. Apical spine of mid-tibia as long as or longer than width of mid-tarsomere I ...........................C. ducalis Smith
- –
Vertex and genae with sparse, short, dark (occasionally yellowish-brown) hairs. Apical spine of mid-tibia much shorter than width of mid-tarsomere I ..........................C. fenestrata Smith
- 3
Mesoscutum with four conspicuous patches of scale-like, brownish-white hairs (Fig. 30) .................Subgenus Allocoelioxys C. formosicola Strand
- –
Mesoscutum without conspicuous hair patches .........................4. Subgenus Boreocoelioxys
- 4
Apex of sternum V laterally broadly expanded, fan-shaped (Fig. 7) ..................C. alata Foerster
- –
Apex of sternum V not laterally expanded ......5
- 5
Sternum VI with apical triangular part nearly equilateral, lateral margin subapically not notched, but only stepped or angled (Fig. 5). Tergum I without conspicuous lateral patches of light-colored hairs .........................C. yanonis Matsumura
- –
Sternum VI with apical triangular part obtuse or elongate triangle (Figs 2–4,6,8), lateral margin notched. Tergum I with distinct lateral patches of light-colored hairs or with antero-lateral expansions of apical hair band ........................6
- 6
Sternum VI with apical triangular part rectangularly or obtusely pointed (Fig. 8), and subapical notch obtusely pointed. .....................................C. rufescens Lepeletier and Serville
- –
Apical triangular part of sternum VI elongate, with a pair of small but sharp spines at the base (Figs 2–4) .............................7
- 7
At least median hairs of apical bands of tergum II–IV broadened and leaf-like (20–37, 38–60) .....................................C. sakamotorum sp. nov.
- –
All hairs of apical bands of tergum II–IV ordinary (Fig. 20) ...............................8
- 8
Median part of dorsal margin of mandible expanded upward, so that dorsal and ventral margins of basal half of mandible are subparallel (Fig. 24); basal 0.6 of outer surface of mandible covered with short, appressed, brownish-yellow hairs of mostly uniform length, often mixed with sparse erect hairs, which are shorter than width of antennal pedicel. Apex of sternum V broad, usually slightly emarginate, but sometimes truncate .....................C. hiroba Nagase
- –
Dorsal margin of mandible not expanded, so that mandible more or less evenly tapering from base toward apex; hairs on mandible irregular in length, with many erect hairs, which are equal to or longer than width of antennal pedicel. Apex of sternum V bluntly pointed ...............................9
- 9
Tergum I with entire apical hair band. Outer surface of mandible more or less flat .............................C. hosoba Nagase
- –
Tergum I only with a pair of lateral, triangular, white hair patches. Outer surface of mandible medially somewhat swollen and the mandible weakly bent inward at the swelling, so that the swelling appears weakly bulbous or elbow-like, and outer surface of mandible is not flat ..........................C. inermis (Kirby)
Male
- 1
Eyes not hairy. Distal half of forewings strongly darkened. Larger species; body length usually 15 mm or more ............................2
- –
Eyes densely hairy. Distal half of forewings at most weakly clouded. Smaller species; body length 14 mm or less ............................3
- 2
Head including vertex and genae covered with dense, bright red or yellowish-red hairs. Apical spine of mid-tibia as long as or longer than width of mid-tarsomere I ........................C. ducalis Smith
- –
Vertex and genae with only sparse, short, dark (sometimes yellowish-brown) hairs. Apical spine of mid-tibia much shorter than width of mid-tarsomere I ...............................C. fenestrata Smith
- 3
Mesoscutum with four conspicuous patches of scale-like, brownish-white hairs (Fig. 30). Apex of tergum VI with eight spines ....................C. formosicola Strand
- –
Mesoscutum without conspicuous hair patches. Apex of tergum VI with six spines ....................4
- 4
Apices of dorsal two spines of tergum VI not situated outside of outer margins of ventral median two spines (Fig. 9). Tergum I without distinct, lateral, pale hair patches ..........................C. yanonis Matsumura
- –
Ventral median two spines of tergum VI situated rather close together, so that apices of the dorsal two spines are always situated outside of the outer margins of the ventral median two spines (Fig. 10; inermis-type). Tergum I with more or less conspicuous, triangular, lateral hair patches ..................5
- 5
Hairs along medioapical margins of terga II–V broadened and leaf-like .......................C. sakamotorum sp. nov.
- –
Hairs along medioapical margins of terga II–V ordinary........................6
- 6
Sublateral fovea of tergum II without [glandular?] opening, entirely filled with very finely reticulate structure (Fig. 34). Apical margin of posterior rim of S4 usually weakly projected and medially with a small but distinct emargination (Fig. 17) ......................................C. rufescens Lepeletier and Serville
- –
Sublateral fovea of tergum II with a conspicuous opening .........................7
- 7
Tergum II with large, transverse, sublateral fovea; the fovea at least 5-fold as wide as long, with width of 8 or more when the head width is set as 80. Tergum I without entire apical hair band but with conspicuous, triangular, white hair patches laterally ........8
- –
Fovea of tergum II not large, elliptic to transverse, at most 4-fold (usually 2- to 3.5-fold) as wide as long, with width of at most 6.5 when the head width is set as 80. Tergum I with entire apical hair band ................................9
- 8
Fovea of tergum II very large, approximately 7-fold as wide as long, with width of 12 when the head width is set as 80. Lateral, white hair patch of tergum I not very well developed. Punctures in pregradular and postgradular areas not much different in size and distribution (Fig. 60). Apical hair band of tergum I narrowly interrupted in the middle. Hairs in gradular groove of tergum II mainly simple ..............................C. alata Foerster
- –
Fovea of tergum II approximately 3.5- to 6-fold as wide as long, with width of 8–11 when head width is set as 80. Lateral, triangular, white hair patches of tergum I very well developed. Punctures of postgradular area usually distinctly larger and more irregular in distribution than those of pregradular area. Apical hair band of tergum I medially widely interrupted. Hairs of gradular groove of tergum II mainly with short barbs. (Gradular groove of tergum II densely hairy, which in some light appears dark colored. (Fig. 59)) .......................C. inermis (Kirby)
- 9
Hairs in gradular groove of tergum II variable, generally sparse but occasionally absent. Hairs in the groove ranging from lateral area toward middle but not exceeding the inner margin of foveal areas (fovea and surrounding sparsely punctate area) (Fig. 31; this character is best observed in antero-dorsal view with a light source from the same direction). Genal concavity usually short; in lateral view, posterior end attaining at most 0.4 of height of eye (Fig. 14) ..............................C. hiroba Nagase
- –
Gradular groove of tergum II densely hairy, hairy area ranging from lateral area toward middle beyond inner margin of foveal area (Fig. 32). Genal concavity usually attaining 0.4 of height of eye ......................C. hosoba Nagase
Notes on distribution of Japanese Coelioxys species
Although the specimens for this study were gathered from as many areas of Japan as possible, no specimens of Coelioxys from the Ryukyu Islands (from the Amami Islands to the Yaeyama Islands) were available, except for a few records of C. ducalis from the Yaeyama Islands. The Ryukyu Islands are inhabited by nine Megachile species (Ikudome 2000), and at least three Coelioxys species are distributed both in Taiwan and the main islands of Japan. The scarcity of Coelioxys species in the Ryukyu Islands is presumably due to some paleogeographic reasons.
ACKNOWLEDGMENTS
I would like to express my thanks to: M. Schwarz for confirming some synonymies, comparison of my specimens with the type materials and valuable advice on the Palearctic species; M. Mello for examining the type specimen of C. hakonensis; the late D. B. Baker for providing me with his personal notes on the Old World Coelioxys; O. Tadauchi (ELKU) and A. Shinohara (NSMT) for supplying material and literature; and Y. Hirashima for his valuable comments on an earlier draft of the manuscript. I also thank K. Yoshizawa (SEHU), A. Nakanishi and Y. Hashimoto (MNHA), and K. Yasuda (NIAES) for arranging the loans of specimens in the respective collections, and persons mentioned in the Materials and Methods section for offering me valuable specimens.




