Mechlorethamine, procarbazine and prednisone for the treatment of resistant lymphoma in dogs
Abstract
Forty-one dogs with resistant lymphoma were treated with a modified MOPP (mechlorethamine, vincristine, procarbazine and prednisone) protocol (MPP [mechlorethamine, procarbazine and prednisone] administered on a 21-day cycle, shortened from the 28-day MOPP cycle). The overall response rate to MPP was 34% for a median of 56 days (95% confidence interval 30–238). Seventeen percent of dogs had a complete response for a median duration of 238 days, 17% had a partial response for a median of 56 days and 32% had stable disease for a median of 24 days. Histological grade or cell morphology on cytology was associated with response. Minimal toxicity was observed with the MPP protocol, suggesting that further dose intensification or addition of another chemotherapeutic agent would be possible.
Introduction
MOPP (mechlorethamine [Mustargen; Ovation Pharmaceuticals, Deerfield, IL, USA], vincristine [Vincristine; Mayne Pharma (USA), Paramus, NJ, USA], procarbazine [Matulane; Sigma-Tau, Gaithersburg, MD, USA] and prednisone [Prednisone; Roxane Laboratories, Columbus, OH, USA]) (Table 1) is an effective rescue protocol for canine lymphoma.1 A retrospective study of 117 dogs treated with MOPP for resistant lymphoma reported a complete remission rate of 31% for a median duration of 63 days and a partial remission rate of 34% for a median duration of 47 days.1 The MOPP protocol was generally well tolerated; however, 28% of dogs experienced gastrointestinal side-effects. These adverse effects were severe enough to require hospitalization in 13% of treated dogs, and three dogs were killed because of severe gastroenteritis. Additionally, five dogs developed febrile neutropenia and three of these dogs died. Overall, 5% of treated dogs died as a result of toxicity.
| MOPP Day | 0 | 7 | 14 | 21 | 28 (day 0 of next cycle) |
|---|---|---|---|---|---|
| Mechlorethamine 3 mg m−2 i.v. | • | • | • | ||
| Vincristine 0.7 mg m−2 i.v. | • | • | • | ||
| Procarbazine 50 mg m−2 p.o. every 24 ha | •→ → | •→ → | •→ → | ||
| Prednisone 30 mg m−2 p.o. every 24 h | •→ → | •→ → | •→ → | ||
| MPP Day | 0 | 7 | 14 | 21 (day 0 of next cycle) |
|---|---|---|---|---|
| Mechlorethamine 3 mg m−2 i.v. | • | • | • | |
| Procarbazine 50 mg m−2 p.o. every 24 hb | •→ → | •→ → | •→ → | |
| Prednisone 30 mg m−2 p.o. every 24 h | •→ → | •→ → | •→ → |
- a In the MOPP protocol, for dogs >0.8 m2 body surface area (BSA), procarbazine dose was delivered to the nearest 50 mg. For dogs >0.4 m2 but < 0.8 m2 BSA, procarbazine was delivered to the nearest 50 mg but administered every other day. For dogs <0.4 m2 BSA, 10 mg reformulated capsules were prepared and the dose was delivered to the nearest 20 mg.
- b In the MPP protocol, procarbazine was reformulated for each patient to provide a dose of 50 mg m−2 dose−1.
Because the majority of dogs treated with MOPP have failed protocols that include vincristine and because mechlorethamine and procarbazine are not known to modulate mechanisms of resistance to vincristine (VCR),2 we hypothesized that removal of VCR from the protocol would not affect response rates or progression free survival. In addition, because the dose-limiting toxicity of a single dose of VCR in the dog is gastrointestinal effects,3 we hypothesized that removal of VCR would allow increased dose intensity of mechlorethamine and procarbazine without increasing the morbidity associated with the protocol. The purpose of this study was to evaluate a modified MOPP protocol that does not include vincristine (MPP [mechlorethamine, procarbazine and prednisone]) and is administered on a 21-day cycle for the treatment of resistant canine lymphoma.
Methods
Treatment protocol
From January 2001 to December 2005, the oncology service at the University of Georgia, College of Veterinary Medicine treated all dogs that were no longer responsive to L-CHOP drugs (l-asparaginase, cyclophosphamide, doxorubicin, vincristine and prednisone) with mechlorethamine 3 mg m−2 i.v. days 0 and 7, procarbazine 50 mg m−2 day−1 p.o. days 0–13 and prednisone 30 mg m−2 day−1 p.o. days 0–13 or continuously (Table 1). In contrast to MOPP, which is repeated on a 28-day cycle, the MPP cycle was repeated on day 21. Inclusion criteria were histological or cytological diagnosis of lymphoma and resistance to the initially prescribed chemotherapy protocol. Staging tests were performed at the time of diagnosis according to attending clinician and owner discretion, and for most dogs included CBC (complete blood count), serum chemistry profile, urinalysis, thoracic and abdominal radiographs, abdominal ultrasound and bone marrow cytology were performed. Immunohistochemistry for CD79a and CD3 (Dako, Carpinteria, CA, USA) and histological grading were performed for patients diagnosed histologically. Because some dogs were diagnosed by cytology, histological grade could not be assigned, so lymphomas were classified based on cytomorphology as small cell or lymphocytic (well-differentiated cells with a diameter of approximately 1 and 1/2 that of a red blood cell), large cell or lymphoblastic (cells larger than neutrophils with fine chromatin, prominent nucleoli and numerous mitotic figures) or intermediate cell type (cells approximately the same size as neutrophils with fine chromatin with multiple nucleoli or a single prominent nucleolus and few mitotic figures). Dogs were not restaged at the time of MPP chemotherapy.
Evaluation of response to treatment and toxicity
Tumour response was evaluated by physical examination and calliper measurement of tumour diameter in the largest dimension and then perpendicular to that diameter at days 7 and 21 of each cycle. Response was categorized as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) using the modified World Health Organization criteria described in the study describing MOPP rescue therapy for dogs1 where CR indicates 100% reduction in size of all measurable disease for at least 21 days, PR indicates >50%, but <100% reduction of all measurable disease for at least 21 days, SD indicates <50% reduction or no change in size of all measurable disease and lack of appearance of new neoplastic lesions for at least 21 days and PD indicates an increase of >25% of all measurable disease or the appearance of new neoplastic lesions. Progression-free survival (PFS) was defined as the time from initiation of MPP until PD, and remission duration was defined according to the previous study of MOPP1 as the time from initiation of MPP until PD in dogs achieving CR or PR. PFS of the initial combination protocol was defined as the time from initiation of the first chemotherapy protocol (at diagnosis) until PD.
CBCs were performed on days 7 and 21. The Veterinary Co-operative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG CTCAE, version 1)4 were applied retrospectively to categorize documented toxicities. Toxicity was graded retrospectively because the criteria were published after the dogs were treated.
Statistical analysis
Statistical analysis was performed by a biostatistician using commercially available software (SAS Statistical Software, version 9.1.3; SAS Institute, Cary, NC, USA). The Kaplan–Meier product-limit method was used to estimate PFS and remission duration. Dogs dying of intercurrent disease or lost to follow-up while still in remission were included in the analysis and censored at that point.
Differences in response associated with grade, stage, substage, B-cell versus T-cell immunophenotype and response to initial protocol were tested by analysis of variance. Kaplan–Meier survival curves for PFS and remission duration were compared for association with histological/cytological morphology, stage, substage, B-cell versus T-cell immunophenotype and response to initial protocol using log-rank tests. Cox proportional hazards regression was used to test for relationships of weight, age, number of drugs before MPP and PFS with the initial combination protocol to the PFS and remission duration with MPP. Logistic regression was used to test for an association between parameters and likelihood of gastrointestinal toxicity or thrombocytopenia in dogs treated with MPP. All hypothesis tests were two-sided with α = 0.05.
Results
Forty-one dogs were treated with MPP. Thirty-four dogs had multicentric lymphoma and 7 had extranodal primary sites. Characteristics of MPP-treated dogs, including stage and anatomic site for dogs with stage V lymphoma are summarized in Table 2. The intended initial treatment protocol for 38 of the dogs was a standard 6-month canine lymphoma protocol consisting of L-CHOP.5 Six dogs received additional or alternative chemotherapeutic agents because of alterations in the intended initial protocol: lomustine (n = 1; included because of central nervous system [CNS] involvement), vinblastine (n = 2; substituted in one dog’s protocol after vincristine-induced ileus and in a second dog’s protocol because of concurrent metastatic mast cell tumour) and mitoxantrone (n = 3; substituted for doxorubicin in two dog’s protocols because of Boxer cardiomyopathy and in a third dog’s protocol when he reached the scheduled cumulative doxorubicin dose). Three dogs had different intended initial protocols. Two of these dogs started treatment elsewhere, one with COAP (cyclophosphamide, vincristine, cytosine arabinoside and prednisone) and one with cytosine arabinoside, vincristine and prednisone. The remaining dog was initially treated with prednisone and single doses of l-asparaginase, vincristine and lomustine for epitheliotropic lymphoma.
| Characteristic | Number of dogs (%) |
|---|---|
| Age (years) | |
| Median (range) | 8 (4–13) |
| Gender | |
| Female | 10 (24) |
| Male | 31 (76) |
| Weight (kg) | |
| Median (range) | 28.9 (5.5–73.5) |
| Breeds | |
| Mixed | 10 (24) |
| Golden retriever | 7 (17) |
| Boxer | 5 (12) |
| Labrador retriever | 3 (7) |
| Other purebred | 16 (39) |
| Received prednisone prior to chemotherapy | |
| Yes | 9 (22) |
| No | 32 (78) |
| Initial WHO stage | |
| III | 5 (12) |
| IV | 9 (22) |
| V | 24 (59) |
| Blood/bone marrow | 14 |
| Cutaneous | 4 |
| Gastrointestinal | 3 |
| Pulmonary | 2 |
| CNS | 2 |
| Osseous | 1 |
| Gingival | 1 |
| Incompletely staged | 3 (7) |
| WHO substage at diagnosis | |
| a | 23 (56) |
| b | 18 (44) |
| Lymphoma histological grade/cytological appearance | |
| Large cell/lymphoblastic | 29 (70) |
| Small cell/lymphocytic | 5 (12) |
| Intermediate cell | 5 (12) |
| Epitheliotropic T cell | 2 (5) |
| Immunophenotype | |
| B cell | 13 (32) |
| T cell | 11 (27) |
| B and T cell | 1 (2) |
| Unknown | 16 (39) |
| PFS – initial combination chemotherapy protocol (days) | |
| Median (95% CI) | 92 (53–121) |
| Number of chemotherapy drugs prior to MPP | |
| 1 | 0 |
| 3 | 1 |
| 4 | 0 |
| 5 | 33 |
| 6 | 6 |
| 7 | 1 |
| 8 | 0 |
| Median (range) | 5 (3–6) |
- WHO, World Health Organization.
Twenty-four dogs (63%) had a CR, 11 (29%) had a PR and 5 (13%) had SD with the initial combination chemotherapy protocol. One dog treated elsewhere had a partial or complete (could not be distinguished based on information in medical record) response to vincristine, cytosine arabinoside and prednisone. The median PFS with the initial combination protocol was 92 days (95% confidence interval [CI] 53–121). Only four dogs completed the initial planned protocol in CR.
Dogs were treated with MPP a median of 123 days (95% CI 92–156) after initiation of their first chemotherapy protocol. Twelve dogs received MPP as the first rescue protocol. Twenty-nine dogs were treated with other rescue chemotherapy prior to receiving MPP. For these 29 dogs, the first rescue therapies included l-asparaginase (n = 25), doxorubicin (n = 1), lomustine (n = 1), alternating doxorubicin and lomustine (n = 1) and vinblastine (n = 1) and resulted in CR in 9 dogs (31%), PR in 11 dogs (38%), SD in 3 dogs (10%) and PD in 6 dogs (21%). Of these 29 dogs, 10 dogs received a second rescue therapy prior to MPP: doxorubicin (n = 4), lomustine (n = 3), vincristine (n = 2) and l-asparaginase (n = 1). Responses to the second rescue before MPP included CR in one dog, SD in three dogs and PD in six dogs. Of these 10 dogs, 2 dogs received a third rescue therapy before MPP. One was treated with doxorubicin and the other with vincristine, and both dogs experienced PD.
Response to MPP
Response rates and durations are described in Table 3. Response could not be assessed in three dogs because at the time of starting MPP, they were in CR following the previous rescue agent. These dogs remained in CR for 50, 81 and 347 days after the initiation of the MPP protocol. The median PFS for all dogs was 24 days (95% CI 16–54; Fig. 1), and the median overall remission duration was 56 days (95% CI 30–238; Fig. 2). Three dogs were censored from the PFS and remission duration analyses. One had megaoesophagus and died of pneumonia while in CR at 198 days after initiation of MPP, one died of Boxer cardiomyopathy while in CR at 50 days and one dog with low-grade lymphoma stopped chemotherapy after 11 months of MPP. This dog was still in CR and alive when lost to follow-up at 539 days. One additional dog was censored from the PFS analysis at 21 days because the clinician changed the treatment protocol despite SD.
| Response | Number of dogs (%) | PFS (95% CI) |
|---|---|---|
| Overall (CR+PR) | 14 (34) | 56 (30–238) |
| CR | 7 (17) | 238 (22–upper level undefined) |
| PR | 7 (17) | 56 (53–135) |
| SD | 13 (32) | 24 (16–64) |
| PD | 11 (27) | |
| NA | 3 (7) |
- NA, not available.
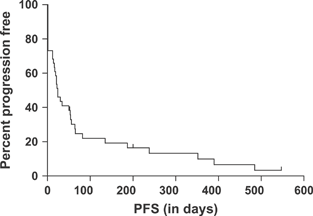
PFS for 41 dogs with resistant lymphoma treated with MPP. Tick marks indicate dogs that were progression free at last follow-up.
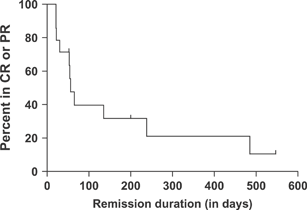
Remission duration of 14 dogs with resistant lymphoma achieving complete or partial remission for at least 21 days with MPP. Tick marks indicate dogs in remission at last follow-up.
There was a significant association of response to MPP with histological grade or cytological interpretation of cell morphology (P= 0.04). Dogs with low-grade, small cell, lymphocytic lymphoma were most likely to respond (75%, all CR). Two of these dogs had B-cell immunophenotype lymphoma, two had T-cell immunophenotype and one was not typed. All five dogs had multicentric lymphoma: one stage IIa, three stage IIIa and one stage Vb (involvement of lymph nodes, spleen, liver and bone marrow). Dogs with intermediate-grade, intermediate cell type lymphoma were least likely to respond (20%). Thirty-seven percent of the dogs with high-grade, large cell, lymphoblastic lymphoma responded to MPP. No other significant associations of patient factors with response, or PFS were identified. Of the dogs with known B-cell phenotype, four had CR, one PR, five SD and three PD. Of the dogs with known T-cell phenotype, three had CR, four PR, two SD and two PD. One dog positive for both B-cell and T-cell markers had PD. Of the stage V dogs experiencing CR, the affected site resulting in stage V classification was blood/bone marrow (n = 2), bone marrow and skin (n = 1), CNS (n = 1, based on clinical signs) and lung (n = 1). Stage V dogs with PR had involvement of blood/bone marrow (n = 2) or bone (n = 1). The remaining stage V dogs did not respond.
Eight dogs had longer PFS after MPP than the initial chemotherapy protocol (seven L-CHOP and one COAP). Three of these dogs had B-cell immunophenotype lymphoma, two had T-cell immunophenotype and the phenotype was unknown for three dogs. Six of the dogs had histologically high-grade or cytologically large cell/lymphoblastic lymphoma and two had histologically low-grade or cytologically small cell/lymphocytic lymphoma.
Toxicity
Ten (24%) MPP-treated dogs experienced grade 1 gastrointestinal toxicity, including mild vomiting (n = 3), diarrhoea (n = 5) or loss of appetite (n = 4). One dog experienced grade 3 acute vomiting starting 6 h after the first treatment with mechlorethamine and required hospitalization for anti-emetic therapy and intravenous fluids. The mechlorethamine dose was reduced by 20%, and prophylactic metoclopramide was instituted for all subsequent treatments (n = 5). With these alterations in protocol, the dog experienced grade 1 vomiting or ptyalism 6–10 h after each treatment. Eight dogs (17%) experienced grade 1 thrombocytopenia at a median of 50 days of MPP therapy (95% CI 21–407). Two were already thrombocytopenic at the time of MPP treatment and one had PD at the time of thrombocytopenia (day 49). Two dogs in CR developed thrombocytopenia after being treated with MPP for 258 and 407 days, suggesting cumulative bone marrow injury. Neutropenia was not observed in any of the dogs. No patient factors were associated with toxicity in dogs treated with MPP.
Discussion
MPP demonstrated activity in dogs with resistant lymphoma. In this study, it is tempting to compare the response and response duration of the population of dogs treated with MPP with the historical population of MOPP-treated dogs described by Rassnick et al.; however, this would not be valid. The use of a historical control group in a clinical trial is appealing because it reduces the numbers of cases that must be enrolled; however, there are several weaknesses to this approach.6,7 Treatment groups may not be truly comparable, and, over time, there may be changes in non-treatment factors that might affect outcome (e.g. stage at diagnosis and referral, clinicians managing the cases, criteria for selection of patients for treatment and supportive care abilities). To determine whether MPP and MOPP were equally effective, a randomized prospective clinical trial with stratification for relevant prognostic factors would be needed.
The response rate for MPP was lower than expected. However, MPP-treated dogs had a very short PFS with the initial chemotherapy protocol, which might suggest an inherently resistant population. A 94.2% remission rate and remission duration of 282 days were reported for dogs treated with the 25 week L-CHOP protocol used initially for the majority of MPP-treated dogs.5 It is not surprising that the MPP-treated dogs would have had less durable remissions with the initial chemotherapy protocol because MPP was used in this study as a rescue protocol. Consequently, there is selection bias in the evaluated patients that results in the inclusion of only dogs that have failed chemotherapy. Other reports of rescue protocols show similarly short remission durations with initial combination chemotherapy.8–11 In addition, the fact that 9 of the 41 MPP-treated dogs did not receive the intended initial L-CHOP protocol may have contributed to the observed inferior response rate and durations with the initial protocol.
The MPP protocol was very well tolerated. Only one dog experienced clinically significant toxicity, acute emesis with each mechlorethamine treatment. This adverse effect was ameliorated but did not completely resolve with dose reduction and prophylactic metoclopramide. With the increased availability of superior anti-emetics for prevention of acute chemotherapy-induced nausea and vomiting, including the NK-1 inhibitor maropitant citrate and the 5-HT3 antagonists dolasetron and ondansetron, it likely that this adverse effect could be avoided. Because MPP was so well tolerated, it is likely that further dose intensification or addition of another active agent would be possible. This is supported by preliminary information describing a modified MOPP protocol that substitutes vinblastine for vincristine (D. Bailey, personal communication, Cornell University College of Veterinary Medicine, Ithaca, NY).
It is interesting to note that eight dogs experienced longer PFS with MPP than with the initial protocol. Factors that predict PFS with MPP were not identified in this study. The only factor associated with the likelihood of response to MPP was the histological grade or cytological appearance of the tumour cells. Dogs with histologically low-grade or small cell/lymphocytic appearance on cytology were most likely to respond to MPP, followed by those with high-grade or large cell/lymphoblastic appearance, and dogs with intermediate grade or intermediate cell type were the least likely to respond to MPP. This study was limited by the fact that some dogs were diagnosed cytologically and some histologically, so we were not able to classify lymphomas further according to standard grading systems. Lymphomas are a heterogeneous group of diseases and differences in response rate and duration between high-grade and low-grade canine lymphomas have been recognized.12–14 High-grade lymphomas have been associated with greater likelihood of CR to chemotherapy, but dogs with low-grade lymphoma may live longer because of its more indolent nature. In this study, the difference in response to MPP based on cell type/grade suggests that different chemotherapy protocols may be appropriate for different types of lymphoma. As clinically relevant systems that use morphologic criteria and biomarkers to classify lymphomas become established in veterinary oncology, oncologists may better tailor treatment protocols based on known optimal strategies for specific subtypes of lymphoma. Additionally, novel therapies may be better evaluated in the type of lymphoma suspected to be most likely to respond. This study had limited power to detect associations of patient factors with response or response duration because the numbers of dogs in each group were low. It is possible that additional associations would be detected in a larger study.
Acknowledgment
This study was funded by the University of Georgia CaRES (Cancer Research, Education and Service) for Pets Fund.




