A toxicity study of low-dose rate half-body irradiation and chemotherapy in dogs with lymphoma
Abstract
Thirteen dogs with previously untreated multicentric lymphoma were enrolled in a prospective study investigating the effects of low-dose rate total body irradiation (TBI) and chemotherapy. Dogs received either 6 or 8 Gy TBI in half-body fractions, 2 weeks apart. Toxicity consisted of mild to moderate haematological and gastrointestinal (GI) signs. One dog died from treatment complications. Anorexia was noted independent of dose. Haematological toxicity was more common and more severe after 8 Gy treatment. GI toxicity was more likely postcaudal half-body irradiation with 8 Gy. Other than leukotrichia, late effects from radiation were not observed. Results indicated that haematological and nonhaematological toxicity was dose dependent. However, the protocol was well tolerated and treatment intensification using a 2-week inter-radiation interval was possible in all dogs treated with 6 Gy. Preliminary survival data for these dogs were very encouraging, providing a strong rationale to analyse the efficacy of low-dose rate irradiation (LDRI) in canine lymphoma.
Introduction
Currently, multidrug chemotherapy protocols comprise the standard of care for canine lymphoma. Treatment initially results in high remission rates (up to 85%), but typically they are of short duration (6–12 months).1 Patients almost uniformly die from relapsing disease when neoplastic cells develop multidrug resistance (MDR).2,3 Thus, a need has arisen for complementary treatments to overcome MDR and to target cells within lymphoma sanctuaries inaccessible to chemotherapy. Lymphoma requires systemic treatment and apart from chemotherapy and immunotherapy only wide-field irradiation (WFI), delivered as either total body or sequential half-body treatments, is capable of treating with systemic effect. Its benefit results from the exquisite radiosensitivity of malignant lymphocytes and lack of cross-resistance between chemotherapeutic drugs and radiation.2
In people with non-Hodgkin’s lymphoma, combined chemoradiation protocols have improved remission durations and survival times.3,4 WFI has also been effective in canine lymphoma.5,6 However, dose-limiting bone marrow (BM) and gastrointestinal (GI) toxicities 5 require careful protocol design to limit toxicity while maintaining efficacy. Studies have evaluated the use of radiation alone 6 or in combination with chemotherapy 7,8 and have used either total body irradiation (TBI) with BM transplantation 9,10 or half-body irradiation (HBI) without BM transplantation.7,8 WFI has been evaluated as a rescue protocol 11 and as consolidation of induction chemotherapy,7,9 but the most promising results have been obtained by integrating HBI with induction chemotherapy.8 Current HBI protocols attempt to modulate toxicity by manipulation of total dose administered, use of split-dose delivery or varying the inter-radiation treatment interval and/or eliminating the use of inter-radiation chemotherapy. These protocols have had mixed results and there is no consensus as to the best way to limit toxicity without compromising efficacy.
The goal of this study was to determine the toxicity profile of an intensified chemoradiation protocol, evaluating two dose levels (6 or 8 Gy) of low-dose rate irradiation (LDRI) given as sequential half-body fractions 2 weeks apart. Our hypothesis was that LDRI would reduce toxicity to the lungs, BM and GI tract allowing for a short inter-radiation interval and a therapeutic gain.
Materials and methods
Design
Dogs entered had stage 3, 4 or 5 multicentric lymphoma diagnosed by either cytological or histopathological evaluation of a lymph node. All dogs were clinically staged (3–5) at diagnosis by using the World Health Organization criteria for canine lymphoma.12 Staging included a complete blood count (CBC), chemistry/electrolyte panel, urinalysis, thoracic radiographs, abdominal ultrasound, BM aspirate evaluation and tumour immunophenotype [CD3 (Serotec, Raleigh, NC, USA), Pan T-cell and CD79a (Dako, Carpinteria, CA, USA), Pan B-cell] as previously described.13 Dogs were excluded if previously treated for lymphoma or if they had any concomitant life-threatening disease. Dogs were randomized to receive 6 Gy (group 1, n = 6) or 8 Gy (group 2, n = 7) TBI interposed in a chemotherapy protocol for canine lymphoma. Toxicity criteria for delaying caudal HBI included: mean BM particle cellularity ≤10%, absence of one or more BM cell lineages (granulocytic, erythroid, or megakaryocytic), neutropenia (<2500 μL−1) or > 60% reduction in neutrophil count from baseline value, thrombocytopenia (<100 000 μL−1) or fever (>103.5).
Animals
Thirteen dogs were enrolled with the owners’ consent. Group 1 consisted of six dogs (German short-haired pointer, Golden retriever, German shepherd, Irish setter, Labrador retriever and Tibetan terrier); two neutered males and four neutered females; median weight and age were 27.9 kg and 7.9 years, respectively. Group 2 consisted of seven dogs (two Cocker spaniels, German shepherd, Golden retriever, Pit bull, Bull mastiff and Rottweiller cross); two males (one neutered) and five females (four neutered); median weight and age were 29.9 kg and 7.9 years, respectively.
Tumour stages were as follows: group 1: 4a (n = 4), 4b (n = 1) and 5b (n = 1); group 2, 3a (n = 1), 4a (n = 3), 4b (n = 2) and 5b (n = 1). Immunophenotype was: group 1, B-cell lymphoma (n = 6); group 2, B-cell lymphoma (n = 6) and T-cell lymphoma (n = 1).
Treatment
All dogs were treated with a modified Madison-Wisconsin protocol (Table 1).14 After one cycle of induction chemotherapy, dogs received either 6 Gy (n = 6) or 8 Gy (n = 7) TBI calculated at the midplane. Radiation was given in half-body fractions at a 2-week interval with cranial and caudal HBI scheduled for weeks 6 and 8, respectively. Anaesthesia was induced with PropoFlo (Abbott Laboratories, North Chicago, IL, USA) and maintained with isofluorane (generic). LDRI (8–14 cGy min−1) from a Clinac 4/80 (Varian Medical Systems, Palo Alto, CA, USA) was given through bilateral opposed portals at extended source-to-skin distance (180–185 cm). Dogs were placed in lateral recumbency, and cranial and caudal fields were separated by a transverse line drawn between the dorsal spinous process of the 13th thoracic vertebra and the xiphisternum as previously described.15 These landmarks were used to abut the cranial and caudal fields at the 50% isodose line. All parts of the body were included within the treatment field allowing for at least a 2 cm beam fall-off at the edge of the patient. Bolus material was not used and dose corrections for tissue heterogeneity were not made. Source-to-axis distance dose calculations along the central axis were performed using a computerized treatment planning system, Prowess 2000 (Small Systems, Chico, CA, USA) into which hand measurements of patient thickness and extended source-to-skin distances were entered.
| TX week | TX administered | Prescribed dose |
|---|---|---|
| 1 | l-asparaginase | 400 IU kg−1 |
| Vincristine | 0.5 mg m−2 | |
| 2 | Cyclophosphamide | 200 mg m−2 |
| 3 | Vincristine | 0.7 mg m−2 |
| 4 | Doxorubicin | 30 mg m−2 |
| 5 | None | |
| 6 | Cranial HBI | 6 or 8 Gy |
| 7 | Vincristine | 0.5 mg m−2 |
| 8 | Caudal HBI | 6 or 8 Gy |
| 9 | Vincristine | 0.5 mg m−2 |
| 10 | Cyclophosphamide | 200 mg m−2 |
| 11 | Vincristine | 0.7 mg m−2 |
| 12 | Doxorubicin | 30 mg m−2 |
Radiation dosimetry
In vivo skin dose measurements were made on entrance and exit surfaces with a dual diode dosimeter, Model 22D (CNMC Instruments; Nashville, TN, USA). The diode was placed off central axis, 4 cm cranial or caudal from the line delineating body halves, to ensure equivalent patient thickness at both measurement points and eliminate shape as a confounding factor. Skin dose was calculated by adding entrance and exit doses in cranial versus caudal locations and used to estimate the effects of body size and tissue heterogeneity.
Assessment of radiation toxicity
All dogs were evaluated weekly during induction and toxicity was graded according to VCOG guidelines;16 grades 1–5 being mild, moderate, severe, life threatening and death, respectively. Haematological toxicity (neutropenia or thrombocytopenia) grades 2–4 and GI toxicity (vomiting and diarrhoea) grades 3 and 4 were considered dose limiting when noted within the week following cranial and caudal HBI.
Haematological toxicity was evaluated by CBCs and cytological evaluation of BM aspirates. BM samples were obtained from the humerus and ilium 10–15 min before cranial and caudal HBI, respectively. Samples were obtained from the humerus 2 weeks postcranial HBI and before caudal HBI to evaluate BM recovery at that site. Samples were obtained from the ilium 2 weeks postcaudal HBI to evaluate BM recovery at that site. All BM samples were reviewed post hoc by a single clinical pathologist (M. M. F.). One slide from each sample set was used to estimate overall cellularity and for a 200-cell differential count. Cellularity was estimated based on the proportion of unit particles composed of haematopoietic cells (as opposed to fat) and expressed as a mean percentage. The differential count included categories for early erythroid (rubriblast through basophilic rubricyte), late erythroid (polychromatophilic rubricyte through metarubricyte), early granulocytic (myeloblast through myelocyte), late granulocytic (metamyelocyte through mature granulocyte), monocyte/macrophage, well-differentiated lymphocyte, plasma cell and other (large cells of uncertain lineage, including those with morphology consistent with poorly differentiated lymphocytes). Megakaryocyte numbers were estimated to be within normal limits, increased or decreased, taking into consideration the number of unit particles.
GI toxicity was evaluated by monitoring vomiting and diarrhoea and by faecal occult blood (FOB) testing. Frequency and severity of vomiting diarrhoea and anorexia were monitored immediately after and during the entire interval between irradiation and subsequent chemotherapy for both cranial and caudal halves. FOB testing was carried out in all dogs receiving caudal HBI using standard methods.17 Dogs were fed cottage cheese and rice diet for 6 days, starting 3 days before irradiation to obtain faecal specimens immediately before and 3 days post-irradiation. A positive test would only be considered significant in context of a negative pre-irradiation result and full owner compliance in feeding the prescribed diet.
Toxicity to the liver and kidneys was evaluated by fasting and postprandial bile acid analysis precaudal and 3 days postcaudal HBI and serum chemistry analysis precaudal and 1-week postcaudal HBI.
Statistical analysis
Analysis of all continuous variables including peripheral cell counts and constituent BM cell lineages was carried out using two-way repeated measure ANOVA to analyse the effects of inter-radiation interval, radiation dose and BM sampling location. All data were tested for deviance from normality using Kolmogorov–Smirnov test. All post hoc pairwise comparisons of the mean responses of the different groups were carried out by the Tukey’s test for analysis. A paired t-test was used to analyse differences in skin doses between the cranial and caudal half body. Association between the skin dose ratio of cranial to caudal HBI and body weight was measured by the Pearson correlation coefficient. All computations were performed by use of statistical software version 11.5 (SPSS, Chicago, IL, USA). Statistical significance was set for P < 0.05.
Analysis of outcome
The end-points evaluated were duration of remission and survival times. Variables examined as indicators of prognosis included radiation dose, tumour stage and substage (a or b), immunophenotype, inter-radiation interval (2 or 3 weeks), remission status after irradiation [complete remission (CR) or not] and remission status during the overall treatment (CR or not).
Correlations between prognostic variables were analysed by use of the Pearson χ2. Duration of response to treatment and survival time was computed by use of the product-limit method. Progression-free survival was used to analyse separately response duration in dogs that achieved CR and those that achieved partial remission or had stable disease. Dogs were censored if still alive at the time of analysis or if they died for any reason (including treatment toxicity) other than lymphoma. Actuarial estimates of survival and progression-free survival distributions were tested for statistical differences using log-rank statistics.
Results
The two groups were comparable with respect to clinical characteristics, tumour stage/substage and immunophenotype.
Treatment feasibility
Four of six (group 1) and three of seven (group 2) dogs received the planned treatment. Five dogs had a 1-week treatment delay postcranial HBI. One dog (group 1) died of progressive disease before caudal HBI. Only three of five delays were associated with defined toxicity end-points. All three occurred in group 2 and resulted from grade 3 neutropenia (n = 1) and inadequate BM particle cellularity (n = 2). One delay (group 1) was because of owner concern with the lack of BM data and one (group 2) because of an inability to return for the scheduled caudal HBI. Two dogs in group 2 required hospitalization for supportive care, one with grade 3 neutropenia postcranial HBI and the other with grade 4 vomiting and diarrhoea postcaudal HBI. The latter was ultimately killed at the owner’s request. Although BM and other toxicity parameters in the remaining dogs were not completely resolved before caudal HBI, none of them developed any complications requiring medical treatment. All 11 dogs that survived longer than 9 weeks were treated with chemotherapy as scheduled in the protocol.
Haematological toxicity
CBC data from 2 weeks postcranial HBI showed significant decreases in the concentrations of neutrophils, lymphocytes, and platelets compared with precranial HBI values (Table 2). No cytopenias were noted in group 1 postcranial HBI but three of seven group 2 dogs developed grade 1 thrombocytopenia, one with concurrent grade 3 neutropenia (Table 3).
| Neutrophils μL−1 | Lymphocytes μL−1 | Platelets 103 μL−1 | |
|---|---|---|---|
| Pre-Cr HBI | |||
| 6 Gy | 8633 (3894–10061) | 1131 (97–1534) | 483 (450–784) |
| 8 Gy | 10004 (6225–16910) | 760 (348–1797) | 392 (279–757) |
| Pre-Cd HBIa | |||
| 6 Gy | 3814 (2550–4836) | 736 (544–825) | 269 (237–396) |
| 8 Gy | 3555 (945–7425) | 450 (270–828) | 223 (120–321) |
| Post-Cd HBIb | |||
| 6 Gy | 3713 (2808–8130) | 507 (432–612) | 162 (132–304) |
| 8 Gy | 1897 (924–4558) | 384 (79–650) | 114 (104–228) |
| Normal range | 3000–15 000 | 1000–4000 | 150–400 |
- Cr HBI, cranial half-body irradiation; Cd HBI, caudal half-body irradiation.
- a (All values) P < 0.001 compared with pre-Cr HBI.
- b (All values) P < 0.001 compared with pre-Cr HBI.
| Toxicity | Grade | Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 6 Gy (n = 6) | 8 Gy (n = 7) | |||||||||
| Postcranial HBI | ||||||||||
| Neutropenia | — | — | — | — | — | — | — | 1 | — | — |
| Thrombocytopenia | — | — | — | — | — | 3 | — | — | — | — |
| 6 Gy (n = 5) | 8 Gy (n = 7) | |||||||||
| Postcaudal HBI | ||||||||||
| Neutropenia | — | — | — | — | — | 2 | 1 | 1 | — | — |
| Thrombocytopenia | 3 | — | — | — | — | 5 | — | — | — | — |
| Vomiting | 1 | — | — | — | — | 2 | — | 1 | 1 | — |
| Diarrhoea | — | — | — | — | — | — | — | — | 1 | — |
| Anorexia | 1 | 1 | 3 | — | — | 1 | 2 | 4 | — | — |
| Lethargy | — | 4 | 1 | — | — | 1 | 4 | 1 | 1 | — |
CBC data from 2 weeks postcaudal HBI showed significant decreases in the concentrations of neutrophils, lymphocytes and platelets compared with baseline precranial HBI values, but no significant decreases in any blood cell counts compared with precaudal HBI treatment values (Table 2). Three of five group 1 dogs developed grade 1 thrombocytopenia postcaudal HBI. In group 2, five of seven dogs developed grade 1 thrombocytopenia, three with concurrent neutropenia (1, grade 3; 1, grade 2 and 1, grade 1). A sixth dog developed grade 1 neutropenia without thrombocytopenia. The seventh dog was killed before obtaining a postcaudal HBI CBC (Table 3).
Comparison of cranial BM samples before versus after cranial HBI revealed significant findings that were independent of the radiation dose (Table 4). The postcranial HBI sample had a decreased ratio of granulocytic to erythroid cells (0.37) compared with the precranial HBI value (1.46). Megakaryocytes were interpreted to be decreased in 4 of 13 dogs (two from each group) postcranial HBI. The estimated cellularity of the caudal BM sample collected 2 weeks postcranial HBI was significantly higher than the cranial BM sample collected at the same time (60 versus 38%, respectively). No significant differences in the proportions of different cell types were detected between these two samples.
| Precranial HBI | Postcranial HBI | |
|---|---|---|
| Particle cellularity (%) | 40 | 38 |
| Early granulocytic (%) | 7 | 7 |
| Late granulocytic (%) | 50 | 18a |
| Early erythroid (%) | 10 | 16a |
| Late erythroid (%) | 29 | 52a |
- a Significant difference from pre-HBI value.
Comparison of caudal BM samples before versus after caudal HBI revealed significant findings that were independent of the inter-radiation interval (2 or 3 weeks) and radiation dose (Table 5). The postcaudal HBI sample had a decreased ratio of granulocytic to erythroid cells (0.3) compared with the precaudal HBI value (1.0). Megakaryocytes were interpreted to be decreased in 9 of 12 dogs postcaudal HBI (three in group 1 and six in group 2).
Nonhaematological toxicity
Gastrointestinal
No dog vomited immediately (0–2 h) postcranial HBI or at home in the interval between irradiation and subsequent chemotherapy. One of five (group 1) and four of seven (group two) dogs experienced grade 1 vomiting immediately postcaudal HBI. Of these five dogs, zero of one (group 1) and two of four (group 2) continued to vomit at home. One dog experienced grade 3 and the other grade 4 vomiting. Diarrhoea (grade 4) manifested in 1 of 13 dogs and was in the same dog with grade 4 vomiting postcaudal HBI.
Anorexia was noted uniformly postcaudal HBI. In group 1, anorexia was categorized as grade 3 (n = 3), grade 2 (n = 1) and grade 1 (n = 1), while in group 2, it was grade 3 (n = 4), grade 2 (n = 2) and grade 1 (n = 1).
Twelve of 13 dogs had negative FOB tests precaudal HBI. Two of 13 dogs had positive FOB tests postcaudal HBI, 1 of which was positive before irradiation and the other had been fed a meat-based diet before testing.
Kidney and liver
No dog showed any significant serum biochemistry abnormalities including preprandial and postprandial bile acids.
Skin
Eight dogs had coat changes. The specific contribution of irradiation and chemotherapy could not be determined. Changes likely to be associated with radiation exposure included diffuse cranial half-body leukotrichia (Fig. 1) in 2 of 11 dogs, 1 in each group. Two of 11 dogs had diffuse greying of the coat affecting both body halves. Two of 11 dogs experienced patchy alopecia, which resolved despite continued chemotherapy and 2 of 11 experienced patchy alopecia, which did not resolve during ongoing chemotherapy (104 weeks).

Radiation field-associated leukotrichia.
Dosimetry
The measured skin dose of the cranial half body was significantly higher than the caudal half body (P < 0.001). The average (±SEM) skin dose ratio (cranial:caudal) was 1.15 (±0.03). The higher skin dose for cranial versus caudal halves reflects the increased transmission of radiation through lung tissue. No significant association was found between increased dose to the cranial half body and body weight.
Outcome
The median follow-up time to last contact or death was 850 days. All dogs were available for remission and survival analysis. A negative correlation was found between radiation dose and substage (P = 0.033), and positive correlations were found between stage and substage (P = 0.009) and between achieving CR and remission status before irradiation (P = 0.001).
The median remission duration was 1126 days (SE, 287 days) and the median survival time was 1131 days (SE, 391 days). Univariate analysis of progression-free survival revealed that remission status after completion of irradiation protocol was significantly associated with duration of response and prognosis (P = 0.03) (Fig. 2). In dogs that were in CR before irradiation, the 1-year and 3-year progression-free survival rates were 89 (SE, 10.4%) and 64% (SE, 16.9%). Although median survival times were longer in dogs that were in CR before irradiation (median not reached) than in dogs that were not in CR (median 93 days), the difference in survival distribution was not statistically significant (P = 0.08) (Fig. 3). None of the other variables evaluated was found to affect remission or survival. However, these findings should be interpreted cautiously because of the low statistical power because of the small sample size.
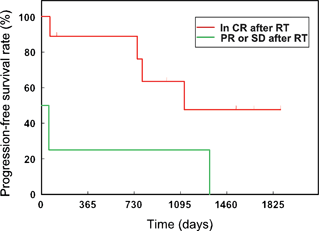
Progression-free survival rate: comparison of dogs in CR versus PR or SD postcaudal HBI. CR, complete remission; PR, partial remission; SD, stable disease (P = 0.03).
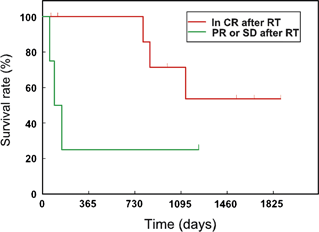
Survival rate: comparison of dogs in CR versus PR or SD postcaudal HBI. PR, partial remission; SD, stable disease (P = 0.08).
Discussion
This LDRI protocol attempted to enhance the therapeutic gain of a combined chemoradiation protocol by shortening the inter-radiation interval. Classically, decreased tissue toxicity associated with LDRI results from the repair of sublethal damage that occurs during prolonged radiation exposure.18 We hypothesized that LDRI would decrease normal tissue toxicity and increase the therapeutic index by minimizing tumour cell repopulation during a shortened inter-radiation interval. A dose rate of 8–14 cGy min−1 was selected based on previously published data.17,18 This resulted in treatment times ranging between 60 and 80 min. A lower dose rate was considered but would have resulted in significantly prolonged treatment time and increased anaesthesia costs thought to be impractical.
Decreases in the concentrations of circulating neutrophils, platelets and lymphocytes were evident postcranial HBI and persisted postcaudal HBI; however, dose-limiting cytopenias were rare and only occurred in group 2. Cytopenias were more common postcaudal HBI in both groups but were more frequent and severe in group 2. Our assumption is that cytopenias were a direct result of radiation-induced damage to BM haematopoietic elements and impaired granulopoiesis and thrombopoiesis. The increased frequency postcaudal HBI most likely reflects the fact that caudal HBI always followed cranial HBI resulting in diminished BM stores with which to replenish cell populations. With respect to severity, haematological findings were consistent with those of other investigators 7,8,19 where fractionation and/or prolonged intertreatment intervals were used.
The effect of dose rate versus fractionation has been evaluated in dogs.5,17 LDRI resulted in the best therapeutic ratio of inactivation of haematopoietic precursor cells to acute toxicity. While inactivation and recovery of precursor cells was not dose rate dependent, the nadirs of leucocyte, lymphocyte and platelet counts were lower in dogs treated at higher dose rates.5 Additionally, it was shown that a single dose of TBI administered at 60 cGy min−1 versus 10 cGy min−1 was more BM toxic as indicated by more profound nadirs in peripheral blood counts.19 This result agreed with a number of previous studies showing greater organ toxicity with higher dose rates.18,19 In dogs, toxicity to the lymphoid system increases significantly when dose rate is increased by 1 log.20
BM cells represent the most radiosensitive mammalian cells.18In vitro experiments have demonstrated that BM has virtually no capacity for repair.18 However, it has been demonstrated that only 10% of the body’s BM needs to be spared to allow the otherwise lethally irradiated portion to be repopulated.21 Repopulation may occur as a result of surviving stem cells within the irradiated BM compartment or through migration of stem cells from the unexposed BM compartment.22,23 Humerus BM particle cellularity ≥10% was set as our threshold for the administration of caudal HBI, although in most instances cellularity exceeded this value. Only two dogs had treatment delay because of inadequate BM cellularity. BM toxicity of fractionated versus single-dose TBI has been shown to be identical when single doses are administered at an LDR (10 cGy min−1).24 As dose rate is lowered and treatment time is lengthened, greater cellular recovery (because of repair or repopulation) of radiation damage occurs during exposure.18 This results in decreased toxicity to BM, lungs and GI organs.21,23 We predict the resultant decrease in inter-radiation interval will outweigh any sparing effect of LDRI on lymphoid tissue resulting in an overall therapeutic gain.
The lack of difference in estimated cellularity of humerus BM samples before and after cranial HBI was an unexpected finding, especially in light of the CBC findings. The most likely explanation for this is that there was decreased cellularity, mostly because of decreased granulopoeisis, in the cranial marrow compartment postcranial HBI, but we failed to detect it because of the limitations of aspiration cytology (core biopsy and histopathology is more reliable,25 but was not carried out).
The estimated cellularity of the caudal BM samples before caudal HBI was significantly higher than the cellularity of the cranial samples before cranial HBI. Although this may indicate compensatory hyperplasia in the caudal compartment after cranial HBI, the study design did not allow us to test this hypothesis. It is also possible that we underestimated the cellularity of the cranial BM samples before HBI, which may explain why no apparent difference in the cellularity of cranial BM samples before and after cranial HBI was noted.
Although the changes were not statistically significant, neutrophil, lymphocyte and platelet counts all decreased in both treatment groups following caudal HBI, consistent with the significant decrease in estimated cellularity of caudal BM samples obtained precaudal and postcaudal HBI. Compensatory hyperplasia in the cranial compartment after caudal HBI may have prevented a more dramatic decrease in peripheral cell counts, but direct evidence of this is lacking.
The lower proportions of granulocytic cells in BM samples post-HBI compared with pre-HBI, and the lack of a similar pattern in the erythroid lineage, suggests that the granulocytic lineage was more susceptible than the erythroid lineage to radiation-induced damage. Researchers have shown that in people, radiation does not significantly alter the function or longevity of mature erythrocytes and nucleated erythroid precursors appear more radiation resistant than granulocytic precursors.26 Our data suggest that the canine erythroid lineage is also more resistant than the granulocytic lineage to the effects of ionizing radiation.
In this study, thrombocytopenia and decreased megakaryocyte numbers were more common postcaudal HBI but was mild in both groups. In people, thrombocyte lifespan is only 8–9 days in peripheral blood, but the levels are not immediately affected by ionizing radiation because of a protracted megakaryocyte response. Only the earliest progenitors undergo immediate lethal changes. Most of the remaining megakaryocytes, although damaged, will continue some degree of maturation and some will be capable of limited platelet production. This provides a slowly diminishing source of platelets.26
Acute toxicity of TBI other than diminished haematopoiesis is primarily GI. The progression of the GI syndrome post-irradiation is characterized by anorexia, abdominal cramping, vomiting, diarrhoea, lethargy, dehydration and infection.26 In this study, GI signs were much more likely after caudal HBI, which likely reflects that no significant portion of the GI tract was included within the cranial half-body field. We speculate that vomiting immediately after caudal HBI was likely a serotonin-mediated event because of direct action of ionizing radiation on enterochromaffin cells of the GI lining.27 The acuteness of onset is consistent with the prodromal stage of GI syndrome. The two dogs that continued to vomit at home may still have been exhibiting signs of the prodromal stage, bordering on the latent stage of GI syndrome at which point cells lining the GI tract are dying.28 Apart from anorexia, very little GI toxicity (vomiting or diarrhoea) was noted in this study, but was more common in group 2. Anorexia, starting within 1–2 days postcaudal HBI was likely associated with the prodromal stage. We speculate that prolonged anorexia, 8–10 days post-irradiation was likely associated with general malaise secondary to myelosuppression. No dog showed evidence of GI ulceration as evaluated by FOB testing. In dogs, LDRI has been significantly associated with increased treatment tolerance with respect to GI toxicity.5,28 Kidney and liver toxicity were not clinically relevant issues in this study.
Field-related alopecia has been reported in dogs undergoing WFI.8 In this study, radiation-related leukotrichia was seen in two dogs. Increased skin surface dose measured in the cranial half body most likely reflects a greater transmission of radiation through less dense air-filled lung. Clinical evidence of lung toxicity (pneumonitis and/or fibrosis) was not observed in this study but may have occurred at a subclinical level. Although this study mostly evaluated acute toxicity, long-term follow-up in all dogs was available as all dogs returned for follow-up chemotherapy until completion of the protocol at 2 years or death. During the entire evaluation period, no dog showed any evidence of clinically significant kidney, liver, lung or BM late effects.
Current TBI protocols involve sequential HBI with either 8 Gy (two consecutive 4 Gy fractions 24 h apart) 7,8,29 or 6 Gy (single fraction) 29 with a 3- to 4-week inter-radiation interval. These protocols use high dose rate irradiation and presumably rely on prolonged inter-radiation intervals to avoid significant toxicity. While successful in limiting toxicity, these protocols have not resulted in a significant improvement in survival.7,8 This is most likely because of the rapid doubling time (28–32 h) of lymphoma cells 30,31 and subsequent repopulation of the irradiated half body by cells mobilized from nonirradiated tissues. Our study attempted to minimize tumour repopulation by shortening the inter-radiation interval to 2 weeks and by administering chemotherapy in between radiation doses. The vincristine dose (weeks 7 and 9) was decreased as a precautionary measure to minimize the risk of severe BM toxicity following irradiation. Our results suggest that this protocol allows for the maximal benefit of combined chemoradiotherapy, although it should be noted that high dose rate protocols with 2-week inter-radiation intervals have not been directly evaluated and may also be feasible.
As with most cancer therapies, it has been shown that the initial performance status of patients treated with HBI is one of the strongest determinants of outcome.32 Patients with more complete initial responses ultimately have longer overall median survival times.33 This was consistent with our results as two of three dogs that did not achieve CR postcaudal HBI died within 153 days, whereas the third dog had an indolent form of the disease associated with improved survival. In this study, radiation was performed at weeks 6 and 8, respectively, as most patients on multidrug protocols will have achieved CR by this time. No benefit could be seen to waiting any longer before initiating the respective HBI treatments. We also speculated that a relatively fresh BM would be more likely to recover from radiation exposure than marrow that had received multiple insults from previous chemotherapy exposures.
Our results show that this LDRI protocol was generally well tolerated in dogs treated with 6 Gy. Treatment intensification using a short 2-week inter-radiation interval was possible in all dogs treated with 6 Gy. Our preliminary data suggest that significant improvements in first remission duration and survival time can be obtained with combination treatment protocols. One important limitation of this study is the small number of patients evaluated especially in light of several treatment delays that occurred. Currently, a larger clinical trial is underway to evaluate the efficacy of this protocol. Another aspect to evaluate is whether or not inter-radiation vincristine is beneficial or if it contributes to radiation treatment delays.
Acknowledgments
The authors thank Paul Primas, Brooke Jones and Jaque Young for their assistance in sample collection and radiation treatments. The study received grant support from the Center for Companion Animal Health (CCAH).




