Evaluation of P-glycoprotein expression in feline lymphoma and correlation with clinical outcome
Abstract
P-glycoprotein (Pgp) is a transmembrane protein pump involved in drug resistance in canine and human lymphoma. There are no published clinical studies evaluating Pgp expression in feline lymphoma. The purpose of this study is to evaluate the level of Pgp expression in feline lymphoma and correlate it with clinical outcome. Two human Pgp monoclonal antibodies, C219 and C494, were used to detect Pgp expression in tissue samples from 63 cats with lymphoma. Demographic results appear comparable to recently published feline lymphoma studies. The Kaplan–Meier median remission and survival times were 164 and 571 days, respectively. Fourteen cats had positive expression of Pgp using MAb C219, and 40 were positive with C494. Variables statistically associated with survival included bone marrow involvement, stage, substage, and use of radiation therapy as a part of treatment. Pgp expression as assessed by MAb C219 and C494 is not predictive of remission or survival time in cats with lymphoma.
Introduction
Haematopoietic tumours represent approximately 33% of all feline tumours, with the majority of cases being malignant lymphoma.1 Lymphoma is the most chemotherapy-responsive malignancy treated in cats, and multi-agent chemotherapy is considered the treatment of choice. However, complete response (CR) rates tend to be significantly lower for cats than those reported for humans and dogs using similar protocols, with recently reported complete remission rates ranging from 472 to 75%.3
The majority of patients with lymphoma that initially experience a CR to chemotherapy will ultimately experience a treatment failure. A common cause of loss of responsiveness to previously effective chemotherapeutic agents is drug resistance. In humans, many different mechanisms of drug resistance have been studied and described. In dogs, the most well researched mechanism of chemotherapy resistance is P-glycoprotein (Pgp).4–7
Pgp is a transmembrane protein pump that is normally expressed in many human and canine tissues, including adrenal gland, kidney, liver, intestine, placenta, blood–brain barrier, lung, peripheral blood/bone marrow cells and multiple foetal cell lines.8 Overexpression of Pgp is one of the major factors leading to the development of the multidrug resistance (MDR) phenotype. Cancer cells become simultaneously resistant to a variety of different chemotherapeutic agents commonly employed in lymphoma protocols in MDR.8
Some of the more frequently utilized chemotherapeutic agents that are Pgp substrates include vincristine, vinblastine, doxorubicin, mitoxantrone and corticosteroids.8 Many of these agents are not only substrates pumped out of the cells by Pgp, but they are also capable of inducing the formation of the MDR phenotype and thereby promoting Pgp overexpression.8
In both humans and canines, it has been shown that Pgp expression in lymphoma is inversely associated with remission length and survival time.4 However, despite the abundance of information regarding Pgp and MDR in humans and canines with lymphoma, very little is known regarding their significance in cats. In 2000, an in vitro study was published where a feline lymphoma adriamycin-resistant cell line was developed.9 Subsequently, the authors were able to utilize reverse transcription–polymerase chain reaction (RT-PCR) and gene sequencing to determine that the adriamycin-resistant cell line was expressing the MDR-1 gene, which controls the expression of Pgp. Western blot analysis also showed that Pgp was only expressed in the resistant cell line, not in the parental cell line, which was still susceptible to doxorubicin.
To the authors’ knowledge, there have been no clinical studies regarding Pgp expression and MDR in cats with lymphoma. Therefore, the purpose of this study is to evaluate the level of Pgp expression in feline lymphoma at the time of diagnosis and compare it with clinical outcomes such as first remission length and overall survival time. We hypothesized that cats with increased expression of Pgp in their lymphoma at initial treatment have decreased first remission length and survival times.
Materials and methods
An initial search of the Animal Medical Center oncology department database between December 2000 and July 2004 identified 147 cats with a diagnosis of lymphoma. Cats were included in the study if the diagnosis was histopathologically confirmed, the medical record was complete and available for review to allow adequate follow-up, the paraffin-embedded tissue blocks from the original diagnosis were available and there was adequate tissue available on which to perform immunohistochemistry.
Information obtained from the medical records included age, breed, sex, body weight, results of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) serologic tests, date of diagnosis, anatomic location, clinical stage, clinical substage, initial chemotherapy protocol utilized, inclusion of radiation therapy as part of the initial treatment, length of first remission, number and type of rescue chemotherapy protocols utilized, length of second and all subsequent remissions, overall survival time and cause of death.
Five-micrometer-thick tissue sections were prepared from formalin-fixed paraffin blocks and stained with haematoxylin and eosin for histopathological evaluation. All biopsy samples were evaluated by a single pathologist (S. S. C.) and determined to be lymphoma prior to inclusion in the study.
Immunohistochemical procedure
The 5-μm sections were mounted onto positively charged OptiPlus fluid barrier slides (Biogenex, San Ramon, CA, USA) and placed in a 66-°C dryer for 30 min. Adherent sections were manually deparaffinized in histoclear (National Diagnostics, Atlanta, GA, USA) and rehydrated through graded alcohols to water. Antigen retrieval was performed by heating the mounted sections to 120 °C for 3 min in a pressure cooker (Biocare Decloaking Chamber) and then cooled to room temperature for 20 min and rinsed with water. All immunohistochemical processing was performed at room temperature on an automated immunostainer (Optimax; Biogenex), and all automated rinses were performed using phosphate buffered saline (PBS). Endogenous peroxidase activity within the tissue sections was blocked with 3% hydrogen peroxide for 10 min. Sections were incubated independently with mouse anti-human Pgp monoclonal antibody C219 (Signet, Dedham, MA, USA) at 1:20 dilution for 120 min, mouse anti-human Pgp monoclonal antibody C494e at 1:1000 dilution for 30 min, rat anti-human CD310,11 monoclonal antibody (CD3-12; Serotec, Raleigh, NC, USA) at 1:200 dilution for 30 min, mouse anti-human CD7910,11 monoclonal antibody (HM57; Dako, Carpinteria, CA, USA) at 1:600 dilution for 60 min or feline-specific CD1812 monoclonal antibody (FE3.9F2; Dr Peter Moore, Davis, CA, USA) for 30 min. After incubation with the primary antibody, slides were incubated for 20 min with a universal biotinylated secondary antibody (Multilink; Biogenex) followed by 20-min incubation with streptavidin-horseradish peroxidase. The antigen–antibody reaction was visualized using diaminobenzidine as chromogen, and then slides were counterstained with haematoxylin.
Positive and negative controls were used in each immunolabelling run. A feline tissue array consisting of B-cell lymphoma, T-cell lymphoma, kidney, liver, testes, brain, spleen, pancreas, colon, small intestine, thyroid gland, salivary gland, skeletal muscle and cardiac muscle was used as the positive control.13 A positive immunoreactivity pattern similar to what has been previously published for canine tissues was observed.5 The same feline tissue array was used as the negative control, with the primary antibody being replaced with preimmune mouse sera (mouse negative control; Biogenex).
Immunohistochemical evaluation
The slides were assessed using light microscopy by a single pathologist (S. S. C.) blinded to the clinical results. A standardized grading scale was developed for C219 where a score of 0 was assigned if the tumour was negative for expression, (1) if the lymphoma cells displayed weak diffuse cytoplasmic expression; (2) if the lymphoma cells displayed moderate diffuse cytoplasmic expression and (3) if the lymphoma cells displayed strong diffuse cytoplasmic expression as assessed under 40× magnification. A standardized grading scale was developed for C494 where a score of 0 was assigned if the lymphoma cells were negative for expression, (1) if the lymphoma cells contained a perinuclear dot; (2) if the lymphoma cells displayed weak diffuse cytoplasmic expression and (3) if the lymphoma cells displayed strong diffuse cytoplasmic expression. Sections were determined to be positive or negative for CD3, CD79 and CD18 by light microscopic evaluation.
Argyrophilic nucleolar organizing region procedure and evaluation
Sections were stained for argyrophilic nucleolar organizing region (AgNOR) histochemical analysis by use of a previously described silver nitrate–formic acid colloid method.14 A 2% solution of gelatin–formic acid was mixed with a 50% silver nitrate solution. The tissue on each slide was covered by 400–500 μL of the resulting solution. Slides were incubated for 25 min in a dark, humidified chamber and then were rinsed three times in distilled water. The AgNOR frequency per tumour cell nucleus was determined by averaging the AgNOR count in 100 cells from representative areas of tumour as viewed by light microscopy under 100× magnification by a single pathologist (S. S. C.) blinded to the clinical results.15,16
Statistical analysis
Kaplan–Meier life-table analysis was utilized to determine median first remission, median second remission and median survival time (MST). Log-rank testing was utilized to determine significance between categorical subpopulations. Remission was defined as the length of time in days from the start of treatment until evidence of the disease recurred. The retrospective nature of the study prevented the determination of CR and partial response (PR). Therefore, treatment response is reported as an overall response rate. The overall response rate was defined as the percentage of treated patients that achieved complete remission or partial remission. Survival time was defined as the length of time in days from the start of treatment until death from lymphoma or the patient was lost to follow-up. Patients who were dead, who were alive in remission or who were lost to follow-up were censored for first remission analysis. Patients who died of disease other than lymphoma, who were lost to follow-up or who were alive at the end of the study period were censored for survival analysis. Patient follow-up information was obtained through regular rechecks or by phone calls to the owners. Factors evaluated to determine whether they were associated with first remission length or survival time included age, breed, sex, body weight at time of diagnosis, results of FeLV and FIV serologic tests, anatomic location, clinical stage, clinical substage, initial chemotherapy protocol, inclusion of radiation therapy as part of initial treatment, length of first remission, number and type of rescue chemotherapy protocols utilized, length of second and all subsequent remissions, AgNOR, immunophenotype and Pgp expression. Statistical significance was set at P < 0.05.
Univariate Cox proportional hazards regression model analysis was performed to evaluate whether variables were associated with first remission length or survival time and as a screen for multivariate analyses. Variables with values of P ≤ 0.2 in the univariate analysis were included in the stepwise multivariate model. Categoric variables were evaluated with χ2 analysis and with the Fisher exact test when there were ≤5 in a cell on χ2 testing. The statview Statistical Package (SAS Institute, Cary, NC, USA) was utilized for all calculations.
Results
Patient characteristics
Out of the 147 cases of feline lymphoma identified in the database search, 71 met the initial inclusion criteria. However, following review of all the histological samples and immunohistochemistry by a single pathologist (S. S. C.), seven of these cases were determined to be inflammatory lesions and one case was a mast cell tumour. This left a final total of 63 cases included in the study. The mean and median ages at the time of diagnosis were 12.5 years and 13.6 years, respectively, with a range of 1–17.4 years. The patient characteristics are summarized in Table 1. Only one of the gastrointestinal cases was small-cell lymphocytic lymphoma, the rest were the large-cell lymphoblastic form.
| Variable | Category | n (%) |
|---|---|---|
| Median age (years) | 13.6 | 63 |
| Breed | Domestic shorthair | 54 (85.7) |
| Domestic longhair | 3 (4.7) | |
| American shorthair | 2 (3.2) | |
| Siamese | 1 (1.6) | |
| Burmese | 1 (1.6) | |
| Maine coon | 1 (1.6) | |
| Ocicat | 1 (1.6) | |
| Sex | Castrated male | 37 (58.7) |
| Spayed female | 26 (41.3) | |
| Stage | I | 18 (28.6) |
| II | 4 (6.3) | |
| III | 25 (39.7) | |
| IV | 11 (17.5) | |
| V | 5 (7.9) | |
| Bone marrow aspirate | Positive | 5 (7.9) |
| Negative | 39 (61.9) | |
| Unknown | 19 (30.2) | |
| Substage | a | 16 (25.4) |
| b | 47 (74.6) | |
| Location | GI | 26 (41.3) |
| Nasal | 13 (20.6) | |
| Hepatic | 7 (11.1) | |
| Mediastinal | 2 (3.2) | |
| Multicentric | 8 (12.7) | |
| Non-lymphoid | 6 (9.5) | |
| Peripheral lymph nodes | 1 (1.6) | |
| FeLV status | Positive | 1 (1.6) |
| Negative | 50 (79.4) | |
| Unknown | 12 (19.0) | |
| FIV status | Positive | 2 (3.2) |
| Negative | 49 (77.8) | |
| Unknown | 12 (19.0) |
Immunohistochemistry and AgNOR results
Fourteen out of the 63 cases were positive with the Pgp C219 monoclonal antibody, with the following score distribution – 5 were grade 1, 6 were grade 2 and 3 were grade 3 (1-4). Forty of the 63 cases were positive with the Pgp C494 monoclonal antibody, with the following score distribution – 8 were grade 1, 20 were grade 2 and 12 were grade 3 (5, 6). Immunophenotyping results yielded 31 positive for CD3 immunoreactivity (Fig. 7), 28 positive for CD79 immunoreactivity (Fig. 8) and 4 tumours negative for both CD3 and CD79 immunoreactivity. The mean AgNOR score was 0.957, with a median score of 0.95 and a range of 0.77–1.29 (Fig. 9).
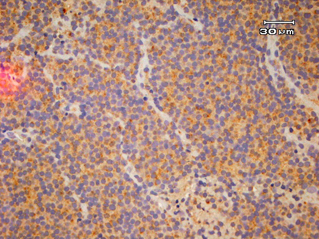
Diffuse, intense positive immunostaining (Grade 3) for C219 monoclonal antibody.

Weak, multifocal positive immunostaining (Grade 1) for C219 monoclonal antibody.
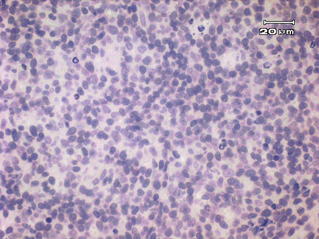
Negative control for C219 monoclonal antibody.
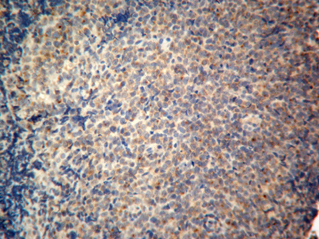
Positive control for C219 monoclonal antibody.
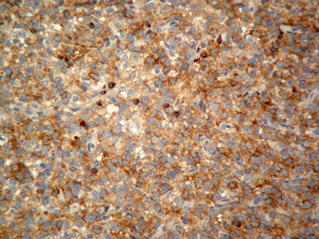
Diffuse, intense positive immunostaining (Grade 3) for C494 monoclonal antibody.
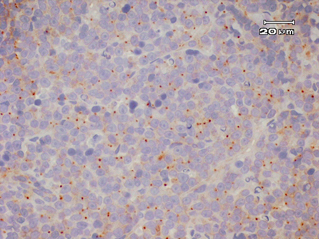
Weak, cytoplasmic dot positive immunostaining (Grade 1) for C494 monoclonal antibody.
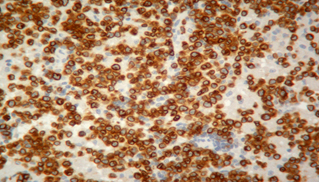
Positive immunostaining for CD3 indicating T-cell lymphoma.
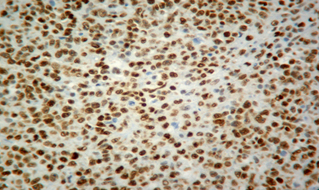
Positive immunostaining for CD79 indicating B-cell lymphoma.
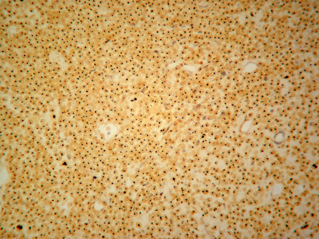
Positive AgNOR stain showing nuclear dots.
Treatment and survival data
All 63 cats underwent therapy with curative intent, and follow-up data regarding response and survival were available for 54 cats. The majority of cats (n = 57) were treated initially with a combination chemotherapy protocol consisting of cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) (either the 25-week University of Wisconsin-Madison protocol17 or The Animal Medical Center protocol18). Other initial protocols utilized included cyclophosphamide, vincristine and prednisolone (COP) (n = 2), l-asparaginase (n = 2), single-agent doxorubicin (n = 1) and alternating vinblastine/lomustine (CCNU) for one cat. Twelve cats received radiation therapy concurrently with chemotherapy. Ten of these patients had nasal lymphoma, and the intent was to treat with 6 weekly fractions of 6 Gy (8/10 completed the protocol, 1/10 received 5 weekly fractions and 1/10 received only 1 fraction). The remaining two patients (1 submandibular lymph node and 1 oral skeletal muscle lesion) each received a single palliative dose of 6 Gy. The initial overall response rate was 70%. Clinical responses and remission status were assessed by clinical signs and physical examination at every treatment visit. Additionally, radiographs and/or ultrasound were rechecked as clinically indicated by the clinician. By Kaplan–Meier analysis, the overall median first remission was 164 days (Fig. 10), the overall second remission was 83 days and the overall MST was 571 days (Fig. 11).
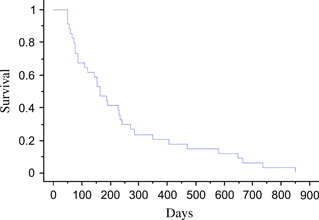
Kaplan–Meier first remission duration estimates. Median first remission = 162 days.
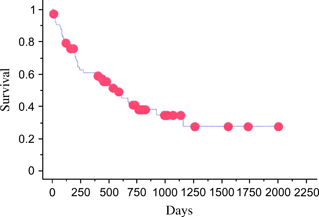
Kaplan–Meier overall survival time estimates. MST = 571 days. Red dots ( ) represent censored observations.
) represent censored observations.
The only categorical variable found to be prognostically significant for first remission length by log-rank analysis was primary location of disease (P = 0.001), with patients having nasal lymphoma having the longest first remission. The sole variable found to be prognostically significant for first remission length by univariate Cox proportional hazards analysis was starting weight (P = 0.025; hazard ratio = 1.1; 95% confidence interval [CI], 1.018–1.312), with higher starting weight correlating with longer first remission. There were no statistically significant prognostic variables for first remission length with multivariate Cox proportional hazards analysis.
The categorical variables delineated by log-rank analysis to be prognostically significant for survival were stage (P = 0.0003, with stage of disease being inversely related to survival), substage (P = 0.03, with substage b patients having shorter survival), bone marrow involvement (P = 0.0013, with patients having a positive bone marrow aspirate having shorter survival), radiation therapy included as part of the initial treatment protocol (P = 0.03, with patients that did receive radiation therapy having longer survival) and second remission treatment protocol (P = 0.02), where patients treated with mustargen, vincristine, procarbazine and prednisolone (MOPP) as their second remission treatment lived significantly longer than patients treated with CCNU at that stage. Univariate Cox proportional hazards analysis for survival identified the following prognostically significant variables: stage (P = 0.001; hazard ratio = 9.0; 95% CI, 2.695–30.3), substage (P = 0.036; hazard ratio = 2.5; 95% CI, 1.061–6.211), bone marrow involvement (P = 0.004; hazard ratio = 3.7; 95% CI, 1.293–10.989), radiation therapy included as part of the initial treatment protocol (P = 0.044; hazard ratio = 2.9; 95% CI, 1.028–8.282), second remission treatment protocol (P = 0.037; hazard ratio = 12.6; 95% CI, 1.341–118.405) and first remission length (P = 0.002; hazard ratio = 1.003; 95% CI, 1.001–1.006). Multivariate Cox proportional hazards analysis revealed second remission treatment protocol to be prognostically significant for survival (P = 0.004; hazard ratio = 31.6; 95% CI, 2.741–364.780). Stage did not reach significance for second remission survival duration (P = 0.069).
The levels of Pgp expression at the time of diagnosis as assessed by the human monoclonal antibodies C219 or C494, immunophenotype and AgNOR counts were not statistically significant prognostic indicators for first remission length or overall survival time.
Discussion
We hypothesized that cats with increased expression of Pgp in their lymphoma at initial treatment have decreased first remission length and survival times. This hypothesis was not supported by the results of the present study. Pgp expression, as assessed by the human Pgp monoclonal antibodies C219 and C494, was not prognostic for first remission length or overall survival time. The human monoclonal antibodies did bind to the paraffin-embedded, feline tissue samples, as evidenced by malignant lymphoid cells staining positive in 14 out of the 63 cases with the C219 monoclonal antibody and in 40 out of the 63 cases with the C494 monoclonal antibody. This was expected based on the fact that the feline MDR-1 gene, which controls the expression of Pgp,19 displays >90% amino acid homology to that of human MDR-1, when analysed by RT-PCR and gene sequencing.9
The disparity between the numbers of cases that were positive with each of the Pgp monoclonal antibodies is most likely explained by a high level of non-specific background staining seen with the C494 monoclonal antibody.5 Also, a score of 1 for the C494 monoclonal antibody was defined as a malignant lymphoid cell containing only a perinuclear dot. This same pattern of staining was occasionally seen in normal lymphocytes from the lymph node positive-control sample. Therefore, the 8 cases that were scored 1 may represent false positives. Other possible explanations are false-negative results with the C219 monoclonal antibody secondary to prolonged formalin fixation or nonprotein-producing Pgp mutations.20
The demographic results of the cats included in the present study are similar to those previously reported,21 although the median age of 13.5 years is somewhat older than that reported in other recent studies.2,16,22–25 Similarities, other than age, in the present study’s population to those previously reported include the majority of cats being domestic shorthair (85.7%),2,16,22–25 a low percentage of cats testing positive for the feline leukaemia virus on serologic testing (4%),2,24 alimentary lymphoma representing the most common anatomic location affected (41.3%),2,16,22,23 and the majority of cats being systemically ill (substage b) at the time of diagnosis (74.6%).16,24
The immunophenotype results from this study population of cats with lymphoma showed that 49.2% (31/63) of cases were T cell in origin, 44.4% (28/63) were B cell in origin and 6.3% (4/63) were non-T cell, non-B cell in origin. This distribution differs somewhat from previous reports. In one report of 109 Australian cats with lymphoma, 70% (76/109) were B cell, 26% (28/109) were T cell and 4% (5/109) were null cell.10 Another report of 145 cats with lymphoma showed that 25.4% (33/130) were positive for CD3 immunoreactivity, indicating T-cell origin.16 Finally, a report of 23 cats with alimentary lymphoma revealed 65% to be of B-cell origin and 26% of T-cell origin.11 The lack of prognostic significance for immunophenotype, seen in this study, is in agreement with other published reports.10,11,16 This is in direct contradiction to canine studies, which have consistently shown immunophenotype to be strongly prognostic for disease-free interval and survival length.26–29
Nucleolar organizer regions are sites for transcription of ribosomal RNA found within the nuclei of cells that can be selectively stained using a silver reaction.30 The average number of AgNOR/nucleus is a measure of the proliferative rate of that tumour30 and has been shown to be prognostic in both human non-Hodgkin’s lymphoma31 and canine lymphoma.32 In this study, the mean AgNOR count was 0.95/cell and the AgNOR values were not found to be predictive of either first remission length or overall survival time. This is consistent with previous reports that also failed to show prognostic significance for AgNOR in feline lymphoma.15,16
The evaluation of treatment and survival data in the current study is difficult because of the lack of standardization of staging, treatment protocols and follow-up schedules. Kaplan–Meier life-table analysis revealed that the median first remission length was 164 days. This compares favourably with previous reports.2,16,25,33 The only factor that proved significant for predicting first remission length on Kaplan–Meier analysis was primary location of the lymphoma. Cats with nasal lymphoma experienced the longest disease-free interval, followed in decreasing order of length by alimentary, multicentric, hepatic, atypical (or extranodal) and mediastinal cases. Previous studies have also supported the longest response to therapy for nasal lymphoma.3,16,34 An interesting finding on univariate analysis of potential prognostic factors for first remission length was the fact that body weight at the time of instituting treatment was predictive, with cats having a higher starting weight experiencing a longer first remission. This may represent a shortcoming of the body surface area dosing method, which does not allow for significant dose variations within the limited range of body weights for domestic cats.35 It may also be a reflection of cats with higher starting weights having a better performance status and, therefore, being able to better tolerate chemotherapy. Both of the combination chemotherapy protocols utilized in this study call for a doxorubicin dose of 1 mg kg−1 for cats, which is significantly lower than the standard canine doxorubicin dose of 30 mg m−2. It is suspected that doxorubicin at a dose of 25 mg m−2 may represent a more reasonable starting dose for cats. The basic principles of chemotherapy dictate that all drugs should be utilized at their maximally tolerated dose and at the ideal dosing intervals in order to achieve maximal dose intensity.36 It would seem advisable to always begin at the upper end of the dosing range and only reduce the dose later for patients that experience unacceptable levels of toxicity.
The overall survival time in the present study of 571 days is dramatically longer than previously published reports for feline lymphoma.2,3,16,24,25 There are several possible explanations for this finding. The first is that there was a high overall response rate of 70% to the various treatment protocols. This is similar to a reported overall response rate of 84% (47% CR, 37% PR) in another recent study.2 The retrospective nature of this study precluded the segregation of response to treatment into complete remission and partial remission categories. Therefore, the results are reported as overall response rate. One paper shows a clear survival benefit to cats achieving complete remission versus a partial remission, with MSTs of 654 days and 122 days, respectively.2 Therefore, it is possible that the majority of responding cats in this study experienced a complete remission, and subsequently, longer survival. A second contributing factor to the long MST in the current study could be the relatively high percentage of nasal lymphoma cases (13/63 = 20.6%). When analysed separately, the MST for these 13 cases of nasal lymphoma had yet to be reached with a median follow-up time of >2 years.
Numerous factors evaluated for significance regarding survival proved to be significant. Univariate Kaplan–Meier analysis revealed stage, substage, bone marrow involvement, the inclusion of radiation therapy in the original treatment and the protocol utilized to treat following recurrence for the second time to all be prognostic for survival. Stage and substage have previously been reported, although the more cumbersome nature of the feline-staging system makes it more difficult to apply and less frequently utilized by clinicians.16,25 The fact that not all patients received a bone marrow aspirate might result in some stage migration affecting the prognostic significance of stage. The use of radiation therapy in the treatment plan as a prognostic factor is most likely a function of the high number of nasal lymphoma cases. There were a total of 12 cases that received radiation therapy, 10 of which had nasal lymphoma, 1 had multicentric disease and the other had a solitary peripheral lymph node involved.
Cats that received MOPP survived significantly longer than the cats that received CCNU as their second remission protocol. At the Animal Medical Center, the patients that completed their multi-agent chemotherapy protocol still in remission would subsequently be retreated with a modified version of the same protocol after the first remission ended. Once the original protocol clearly failed, then they would most commonly be switched to MOPP as the first rescue protocol. Therefore, the fact that MOPP performed better as a rescue protocol after the second remission ended indicates that those patients likely experienced a second remission with the repeated original protocol. Logically, these patients would be expected to experience longer survival than patients that had to be switched to CCNU earlier in the course of treatment because of the failure of the multi-agent protocol and MOPP protocol.
Univariate Cox proportional hazards analysis revealed all of the same aforementioned survival prognostic factors as well as first remission length. Intuitively, considering the fact that the first remission with lymphoma tends to be the most durable, a patient that experiences a longer first remission is likely to survive significantly longer than a patient with a short first remission.37 The sole factor found to be an independent prognostic factor for survival on multivariate analysis was the treatment protocol utilized after the second remission ended. Stage of disease was not significant for survival after the second remission; however, the P = 0.06 value obtained suggests further evaluation following inclusion of a larger number of cases is warranted.
The authors acknowledge the fact that there were numerous shortcomings with the present study. The retrospective nature of the study precluded a lack of uniformity in staging procedures, treatment protocols and recheck schedules. There was a relatively high number of lost to follow-up cases (9/63 = 14.3%). Also, the original intent of this study was to compare Pgp expression at three different time frames within the disease process – at diagnosis, at first recurrence and at necropsy. However, there were too few cases in which histopathology samples were available at the time frames other than first diagnosis to allow any statistical analysis. Finally, the human Pgp monoclonal antibodies have not been previously validated on feline tissues. In order to counteract this potential problem, positive and negative controls were included with all samples to ensure that the results were valid. Also, the subcellular staining patterns seen in this study’s population of feline lymphoma cases were consistent with Pgp expression in other species.5 This suggests that the use of the human Pgp monoclonal antibodies, such as C219 and C494, is a valid methodology in cats.
In conclusion, unlike in humans and dogs, the level of Pgp expression at the time of diagnosis is not prognostic for first remission length or overall survival time in cats with lymphoma. Based on other findings in the present study, the authors suggest that the clinical staging system for feline lymphoma adapted by Mooney et al. be applied more consistently, that bone marrow aspirates be considered as part of complete staging whenever possible, that the CHOP-based chemotherapy protocol that induced the first remission be restarted at the first recurrence if the patient has not been actively receiving chemotherapy and that dose intensity be maximized whenever possible. Future studies should continue to explore the possibilities of other intrinsic chemotherapy resistance mechanisms in feline lymphoma.38




