A feasibility study of low-dose total body irradiation for relapsed canine lymphoma
Abstract
Seven client owned dogs with confirmed relapsed lymphoma were enrolled in a prospective feasibility study investigating the effects of low-dose total body irradiation (LDTBI) delivered in a single 1 Gy fraction. LDTBI for relapsed lymphoma was safe and well tolerated. The only major side-effect of LDTBI was asymptomatic thrombocytopenia in all dogs. The median platelet nadir was 17 000/μL (range 4000–89 000), which occurred a median of 10 days (range 8–30) post irradiation. Three dogs had short-term partial responses, two stable disease and two progressive disease (PD). Six dogs were euthanatized for PD, and one dog died while in partial remission. No dogs had clinical complications. Survival analysis was not performed, because the study design did not allow for evaluation of survival time. Larger studies incorporating LDTBI in the induction/consolidation phase of treatment need to be performed to determine the therapeutic efficacy of LDTBI.
Introduction
Canine lymphoma is the most common naturally occurring malignancy of the haematopoietic system, accounting for 90% of all canine haematopoietic neoplasias. Lymphoma affects predominantly middle-aged dogs of either gender and any breed and has no identifiable cause.1–3 The most common clinical sign of lymphoma in dogs is generalized peripheral lymphadenopathy. Usually, there are few if any clinical signs of systemic illness, but exercise intolerance, lethargy and inappetence may be possible at presentation. Involvement of abdominal viscera, cranial mediastinum and other extra nodal sites may occur.4,5 The B-cell immunophenotype is most common. Prognosis worsens with the T-cell phenotype or the presence of systemic clinical signs (substage b).1,2 The remission rate for canine lymphoma with chemotherapy has been reported to be between 69% and greater than 90%.6–8 A commonly used five-drug combination chemotherapy protocol resulted in a complete remission (CR) rate of 69% with a median first remission duration of 55 weeks.6 Despite the high rate of clinical remission, the cure rate is less than 15% with current chemotherapy.9
Radiation therapy has not been utilized fully in veterinary medicine for the treatment of canine multicentric lymphoma. Both high-dose total body irradiation with bone marrow transplantation10–12 and half-body irradiation13,14 have shown some efficacy. The goal of both treatment strategies is to eradicate malignant lymphoma cells. These regimens carry a risk of toxicity including death. However, fractionated half-body radiation therapy has diminished toxicity14,15 and can be applied without special supportive care. An alternative treatment strategy is low-dose total body irradiation (LDTBI). Although the mechanism of action of LDTBI in treating lymphoma is not completely understood, experimental data suggest that there are at least three non-mutually exclusive mechanisms. High doses of TBI cause mainly cytotoxicity, but the goal of LDTBI is not just direct cytotoxicity but also to change the immune response of the patients. Some investigators postulate that LDTBI induces an immune effect by enhancing the proportion of helper and cytotoxic T cells, while decreasing the proportion of suppressor T cells.16 The other two postulated mechanisms are the intrinsic hypersensitivity of lymphocytes to low doses of radiation17 and the direct/indirect induction of apoptosis, possibly mediated through BCL-2.18
Johnson et al. in the late 1960s,19,20 used single dose or fractionated whole body irradiation to treat lymphoma in 14 dogs. Although the irradiation protocol was not consistent, a complete or partial response was seen in seven of the dogs. The dose-limiting toxicity was thrombocytopenia, which was asymptomatic in all dogs except one.20 In the 1970s, encouraging results in treatment of human non-Hodgkin's lymphoma were obtained with LDTBI alone. Fifty-seven previously untreated patients received fractionated LDTBI for a total dose of 1.0–1.5 Gy. The overall 5-year survival rate was 66%.21 Several other studies in humans with lymphoma have reported that LDTBI alone resulted in efficacy with acceptable toxicity.17,22,23 In 26 human patients, fractionated LDTBI was used followed by localized high-dose irradiation. Patients received a total of 1.5 Gy in 10 fractions. Twenty-four of 26 patients achieved CR after only the LDTBI with minimal toxicity.18
Several human and canine studies compared single dose TBI versus fractionated TBI. A comparison of canine marrow toxicity from fractionated versus single-dose TBI at a rate of 10 cGy min−1 of 3 showed that there was no difference in the toxicity of the patients.24 Another study showed a strong indication that platelet count decreased more with 60 cGy minute−1 dose rate than 10 cGy minute−1 dose rate as a single dose, although survival was not statistically different.25 In our study, a single treatment at a lower dose rate was chosen to minimize side-effects, cost of therapy, anaesthesia and dogs' time away from home.
The objectives of this prospective study were to evaluate the feasibility, response and adverse effects of LDTBI in a single 1 Gy fraction administered as rescue therapy for dogs with relapsed lymphoma.
Materials and methods
Eligibility
Any dog presented to the Harrington Oncology Program with relapsed lymphoma that was not responsive to chemotherapy regimens available at the time was eligible. Dogs were staged according to World Health Organization guidelines26 prior to initial chemotherapy, and tumors were immunophenotyped and graded. Restaging procedures performed prior to LDTBI included physical examination, palpation and measurement with or without aspiration of enlarged lymph nodes, complete blood count (CBC), blood chemistry profile, urinalysis, bone marrow cytology and diagnostic imaging as indicated by the clinical presentation. Information of previous chemotherapy was available for review.
LDTBI
A single total body dose of 1.0 Gy at a dose rate of 10 cGy min−1 was given to the mid-plane of the dog using parallel-opposed ports from a Siemens Mevatron 77 (6 MV) linear accelerator. Thermolucent dosimeters (TLD) were not used to evaluate the dose. However, daily constancy meters and machine calibration were performed on the machine as per recommendations of VRTOG.
The dogs were anaesthetized with isoflurane and placed in lateral recumbency on the floor. Tissue equivalent material, including 1.5 cm of Superflab (International Medical-Tek Merrifield, VA, USA) and rice bags, were used over and around the entire body for dose buildup of superficial tissues and to ensure better dose delivery. The superficial lymph nodes are directly below the skin, which makes it necessary to treat full dose to those areas. Bolus is commonly used to ensure that the surface dose is the prescribed dose in human TBI.27 For large dogs, the field was divided in two sections (cranial and caudal halves), and the edges of the light fields were matched to avoid under dosing the area between the cranial and the caudal fields, expecting that a slight overdose between fields would not cause significant toxicity because of the low total dose and relative small volume.
Numbers obtained under standard conditions were used in the determination of the monitor unit settings. Measurements taken at the TBI distance validated the inverse square law factors used to within 2%. The dose rate was calculated and adjusted as closely as possible to 10 cGy min−1 at the axis with lucite-blocking trays previously calibrated by the physicist. Blocking tray attenuation factors were previously determined. A source to axis distance calculation method was used.
Post-radiation care and evaluation of toxicity
After LDTBI, each dog was hospitalized overnight (24–30 h) to monitor for adverse effects, including tumor lysis syndrome (TLS), and to receive maintenance intravenous fluids. The dogs went home the evening after LDTBI with instructions for the owner to monitor carefully for any adverse signs. Dogs were also given 10 mg kg−1 of allopurinol orally one to three times a day for 1 week as a precaution against uric acid nephropathy. One to 3 days after treatment, a physical examination, CBC (n = 7) and serum chemistry profile (n = 6) were repeated to evaluate tumor response and adverse effects of the irradiation. Physical examination and CBC were repeated weekly until the neutrophil and platelet counts were within normal range or the patients died or were euthanatized. One dog lived for 51 days post LDTBI but only underwent CBC/platelets counts until 17 days after treatment. Six dogs had a urinalysis done between 1 and 5 days after LDTBI. At each visit, owners were interviewed regarding the dog's overall well-being, as well as appetite, energy, nausea, diarrhea or any other perceived effect of the treatment.
Evaluation of response
Complete remission (CR) was defined as the disappearance of clinical evidence of disease as evidenced by physical examination and routine imaging. Partial remission (PR) was defined as a reduction in tumor size of greater than 50%, with no new lesions developing. Stable disease (SD) was defined as less than 50% decrease or 25% increase in tumor size, and progressive disease (PD) was defined as an increase in tumor size of greater than 25% or the development of new lesions. Response duration was defined as the time from irradiation until disease progression. Survival time was defined as the time from irradiation until death.
Data analysis
Established criteria were used for grading of thrombocytopenia (Table 1). Differences in distribution of platelet count at the nadir and time to nadir were analysed by using the Mann–Whitney test. Differences were considered significant at p < 0.05. Statistical analysis was performed with a computer program (SPSS Inc., Chicago, IL, USA). Categorical data were summarized as absolutes, and continuous data were summarized as median and ranges. A straight calculated median was performed for the time to response, duration of response and survival.
| Grade | Criteria (platelet count μL−1) |
|---|---|
| 1 | 100 000–200 000 |
| 2 | 50 000–100 000 |
| 3 | 15 000–50 000 |
| 4 | <15 000 |
Results
Dogs
Seven dogs were enrolled in the study between 1998 and 2001. The median age of the dogs at time of LDTBI was 7 years (range: 4–13). There were three males and four females (two neutered males, one intact male; two spayed females, two intact females). Breeds included three golden retrievers, and one each of poodle, cocker spaniel, bulldog, and mixed-breed. The median weight was 40.7 kg (range: 8.7–50.5). Clinical stage and substage at the time of diagnosis were known for all seven dogs, and immunophenotype of the lymphoma was known in six dogs. Three of the dogs were clinical stage IVa (one T-cell, one B-cell, and one unknown type), two stage IVb (one T-cell and one B-cell) and two stage Vb (two T cell) (Table 2) at diagnosis. All seven were high-grade lymphocytic lymphoma.
| Dog number | Age (years) | Breed | Sex | Weight (kg) | Stage at diagnosis | B/T | Bone marrow at LDTBI | Response | Time to response (days) | Response duration (days) | Survival from LDTBI (days) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | GRET | CM | 50.5 | Vb | T | (–) | SD | 7 | 14 | 53 | Euthanized, lymphoma |
| 2 | 13 | CSPN | F | 21.4 | IVa | T | (–) | PD | – | – | 13 | Euthanized, lymphoma |
| 3 | 6 | Bull dog | F | 21.1 | IVb | T | (+) | SD | 1 | 7 | 10 | Euthanized, lymphoma |
| 4 | 7 | Mix | SF | 46.4 | IVa | B | (+) | PR | 3 | 7 | 13 | Euthanized, lymphoma |
| 5 | 4 | GRET | M | 40.7 | IVb | B | (+) | PR | 1 | 28 | 29 | Died, myocarditis |
| 6 | 7 | Poodle | CM | 8.7 | Vb | T | (–) | PD | – | – | 51 | Euthanized, lymphoma |
| 7 | 9 | GRET | SF | 40.9 | IVa | – | (+) | PR | 6 | 14 | 85 | Euthanized, lymphoma |
| Median | 7 | 40.7 | 3 | 14 | 29 |
- Age, age at LDTBI; B, b-cell immunophenotype; CM, neutered male; CSPN, Cocker spaniel; F, female; GRET, golden retriever; M, male; PD, progressive disease; PR, partial response; SD, stable disease; SF, spayed female; T, t-cell immunophenotype.
- Bone marrow at LDBI: (–), no lymphoma in bone marrow at the time of LDTBI; (+), lymphoma in bone marrow at the time of LDTBI.
Previous treatment
The initial chemotherapy protocol was VELCAP-SC9 in three dogs, VELCAP-S28 in one dog, PVAC29 in one dog and single-agent doxorubicin in two dogs. One dog received maintenance chemotherapy. Dogs were in first remission for a median of 43 days (range 32–161 days). All had received at least one rescue chemotherapy protocol (median 4, range: 1–6) after relapse from initial chemotherapy (Table 3). The dogs had received a median of 10 different drugs (range 8–12) over a median period of 6 months (range 3.8–58) prior to LDTBI. The time interval between last rescue treatment and LDTBI was a median of 16 days (range 8–37 days).
| Initial chemotherapy | Rescue Chemotherapy | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dog number | VELCAP-SC | VELCAP-S | Single agent doxorubicin | PVAC | Number of rescue treatments | MOPP/DOPP | Cytosine arabinoside | CCNU | L-asparaginase | COP | Mitoxantrone | Doxorubicin | Etoposide | Vinblastine | Ifosfamide | Total duration on Cx (months) | Total number of Cx drugs prior to LDTBI |
| 1 | 1 | 4 | 1 | 1 | 1 | 1 | 5.27 | 8 | |||||||||
| 2 | 1 | 4 | 1 | 1 | 1 | 1 | 5.13 | 8 | |||||||||
| 3 | 1 | 1 | 1 | 8.57 | 8 | ||||||||||||
| 4 | 1 | 1 | 3 | 1 | 1 | 3.83 | 11 | ||||||||||
| 5 | 2 | 4 | 1 | 1 | 1 | 10.00 | 12 | ||||||||||
| 6 | 1 | 6 | 1 | 1 | 2 | 1 | 1 | 6.20 | 10 | ||||||||
| 7 | 1 | 5 | 2 | 1 | 1 | 1 | 57.97 | 10 | |||||||||
Restaging
At the time of LDTBI, diagnostic tests included CBC (n = 7), platelet count (n = 6), platelet comment (n = 1), chemistry profile (n = 7), urinalysis (n = 4), thoracic (n = 4) and abdominal radiographs (n = 3), abdominal ultrasonography (n = 1) and bone marrow cytology (n = 7). Four dogs were thrombocytopenic (44 000/μL, 118 000/μL, 158 000/μL, and a comment of significantly decreased platelet number). Both dogs with lymphoma in the bone marrow at the time of initial diagnosis had no evidence of infiltration in repeat aspirates before LDTBI. However, four dogs that originally had normal bone marrow had evidence of lymphoma in their marrow at the time of LDTBI, including the four dogs with thrombocytopenia. The other three dogs had normal platelet counts and cytologically normal bone marrow. All dogs had measurable disease. At the time of LDTBI, the dogs were restaged as one 3a, two 4a, and four 5a.
Toxicity
Weekly CBCs revealed that the major haematologic toxicity in all dogs was thrombocytopenia. Platelet counts decreased in all dogs, including those that were thrombocytopenic before treatment. The median platelet nadir was 17 000/μL (range 4000–89 000), which occurred a median of 10 days (range 8–30) post irradiation. No dogs had any signs of bleeding. Although six dogs died or were euthanatized because of disease progression before platelet counts returned to normal, the platelet count of one dog returned to normal 34 days after LDTBI (Fig. 1). There was a trend for the platelet count at the nadir to be lower in dogs with lymphoma in the bone marrow (median 13 500/μL, range: 6000–26 000) than in the dogs with cytologically normal marrow (median 36 000/μL, range: 26 000–89 000), however, the difference was not statistically significant (p = 0.05). The time to the nadir was not significantly different between dogs with cytologically normal marrow and those with lymphoma infiltration in the marrow.
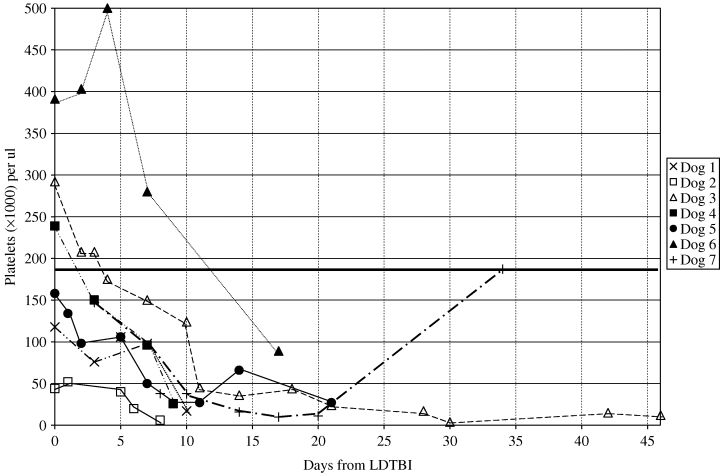
Platelet responses of six dogs after 1 Gy low-dose total body irradiation (LDTBI). The platelet counts in all dogs decreased after LDTBI. Median platelet count on day of radiation was 198 500 μL−1[normal > 180 000 μL−1 (solid horizontal line)]. After 7 days, the median platelet count was 98 000 μL−1, and after 14 days, it was 36 000 μL−1. Platelets had returned to normal in only one dog on day 34.
A minimal effect of LDTBI on neutrophil counts was observed. All dogs had normal neutrophil counts before LDTBI, with a median count of 9504/μL (range: 6976–21 086). Seven days post LDTBI, the median neutrophil count was 4788/μL. The median low point in the neutrophil count was 4328/μL (range: 2244–10 022), occurring most commonly on day 7 (range: 3–14). Overall, the changes in neutrophil numbers were not clinically significant.
One dog had mild self-limiting gastrointestinal signs (vomited once) 2 days after LDTBI that lasted for only 1 day, and a second dog had mild diarrhea 3 days post irradiation, which was resolved 7 days after LDTBI. A third dog had a cough starting 11 days post LDTBI and had progressive lymphoma at that time. No dogs required hospitalization for toxicity following LDTBI.
No dog manifested clinical evidence of TLS. Serum phosphorus concentration was increased in one dog, 1 day after LDTBI; however, the concentration returned to normal by day 3. The median phosphorus concentration on the day of LDTBI was 4.6 mg dL−1 (range 3.7–5.6) (normal range: 3.0–6.0). One day post LDTBI, the median phosphorus level was 5.0 mg dL−1 (range 4.3–7.1). No other notable changes were noted on chemistry profiles (n = 7) and urinalyses (n = 6).
Tumor response
Three of the seven dogs had objective partial responses to LDTBI for 7, 14 and 28 days, respectively. The median time to a partial response was 3 days (range: 1–5). Two dogs had SD for 7 and 14 days, respectively, and the other two dogs had PD (Table 2).
The median survival time after LDTBI was 29 days (range: 10–84). Six dogs were euthanatized because of PD, and one died of myocarditis while in PR 28 days after LDTBI (Table 2). Four of the dogs had necropsies done, and in those, no evidences of radiation toxicity were seen.
Discussion
This study investigated the feasibility of single dose LDTBI for the treatment of relapsed canine lymphoma. Although the remission rate for canine lymphoma with chemotherapy has been reported to be between 69% and greater than 90%6–8, the prognosis for long-term survival has been poor. Therefore, the development of a new effective treatment is needed.
A human study looked at 1 Gy total body irradiation for refractory haematologic malignancy. Sixteen percent of the patients had a sustained CR of the cancers.30 Our study was based on the results of that human study. Fractionated whole body irradiation has been used to treat canine lymphoma.20 In the canine study, a single dose of 180 rads (three dogs) or fractionated doses of 15 or 30 rads daily for several weeks (11 dogs) was given from a 2 MV photon accelerator. A complete or partial response was seen in seven of 14 dogs, and the main toxicity was thrombocytopenia.20
LDTBI showed an antitumor effect for relapsed canine lymphoma in three of seven dogs, although the durations of response were brief. These findings should be viewed in light of the very late stage in the clinical course at which these dogs were treated. All dogs had relapsed at least once, and their lymphomas were resistant to available established chemotherapy, so any objective response in this setting should be considered encouraging from the standpoint of a pilot study.
Thrombocytopenia was also observed in this study. Platelets in dogs have a life span of 7–9 days with a production time of 4 days. Following a myelosuppressive insult, the platelet nadir usually occurs in 10–12 days, which is consistent with that seen in this study. The exact mechanism by which this thrombocytopenia occurs is unknown. The cause could be direct radiation toxicity to megakaryocytes or their immediate precursors. Another potential or exacerbating cause could be the depletion of stem cells caused by extensive previous chemotherapy. It has been reported that haematologic toxicity is more severe in human patients who have received cytotoxic drugs before total body irradiation because of their diminished bone marrow reserve.31 Bone marrow infiltrated with lymphoma, as it was in four of the dogs treated here, could also have reduced regenerative potential. Although we were unable to evaluate the time to platelet recovery in six of the seven dogs, there was a trend for the dogs with infiltrated bone marrow to have a lower platelet count at the nadir than in those with cytologically normal marrow.
Similar results have been seen in studies in humans using fractionated whole body irradiation where patients with advanced stage non-Hodgkin's lymphoma (NHL) (stage III-IV)32 had more severe thrombocytopenia than patients with stage I–II NHL.18 Although these studies are not direct correlations with the present study, they also show that more severe thrombocytopenia is associated with greater disease burden.
Advanced clinical stage, bone marrow infiltration and extensive previous chemotherapy possibly potentiated the thrombocytopenia seen in the dogs in this study. Despite this, none of the dogs showed any clinical complications of thrombocytopenia, and none were admitted to the hospital.
Another potential toxicity of LDTBI in humans with a high tumor burden is TLS, characterized by hyperphosphatemia, hyperkalemia and metabolic acidosis, with or without azotemia. These chemical changes are due to the acute release of intracellular phosphate, potassium, uric acid and nucleic acid metabolites released from dying tumor cells. Clinical signs include vomiting, depression, diarrhea which may be haemorrhagic, and cardiovascular collapse and shock. Aggressive fluid therapy and correction of the electrolyte imbalances are needed if this occurs. The clinical signs of TLS in humans are partly due to secondary uric acid production, and dogs may be more resistant to TLS, because they can more easily metabolize uric acid. However, because lymphoma cells can be rapidly destroyed through apoptosis, and these dogs all had heavy tumor burdens, it was considered prudent to provide TLS prophylaxis consisting of intravenous fluids and allopurinol after LDTBI. None of the dogs had clinical or laboratory signs of TLS.
LDTBI can have limitations for the veterinary radiation oncologist for several reasons. For example, it is important that a low dose rate or fractionated schedule is used to prevent adverse effects. Not all radiation equipment is able to deliver the appropriate (low) dose rate to the dog, hence fractionated treatment could be used instead. The linear accelerator used in this study could deliver a reduced dose rate of 50 cGy min−1. Using filters and an extended distance set up, the dose rate to the patient was decreased to 10 cGy min−1. Second, LDTBI can be delivered to awake human patients while standing, however, in this study, dogs were anaesthetized and placed on the floor to achieve the extended distance necessary. Third, due to the large size of the dogs, two separate fields were often necessary to include the whole dog. These challenges were surmountable and feasible in this study. None of the dogs in this study developed any known toxicity except thrombocytopenia that was perhaps exaggerated by the extent of tumor and by prior chemotherapy.
This study showed that LDTBI is feasible in dogs with lymphoma. The responses did not appreciably influence the dogs' survival times, therefore LDTBI does not appear to be promising as a single modality in the salvage setting. However, this study had a very small number of patients, and results may have been more encouraging with a larger group in an earlier stage of disease. LDTBI may be more effective and less toxic earlier in the disease process, during remission, and in combination with chemotherapy. An alternative approach that could be studied to determine any differences in toxicity and survival would be fractionated TBI with a higher dose rate. By fractionating, each fraction would be a lower dose allowing more of an immune stimulatory response to occur and less of a cytotoxic response. Due to the small fraction size, influence from patient contours, inhomogeneities, and internal organ motion, the accuracy in dose delivery to the individual dog may have been non-ideal. A flaw in this study was that patient dosimetry was not performed. The definitive mechanism to determine the actual dose delivered to an individual dog is through in vivo dosimetry. At several human hospitals, in vivo dosimetry is used routinely during TBI to measure entrance/exit doses at various treatment sites. This technology exists for dogs, but it is technically difficult and expensive. The use of TLDs or diodes should be considered in future TBI studies. Also, whole body CT scans for the purpose of treatment planning can be performed with the dogs in treatment position to determine the isodose curves. This will help determine the dose being received by the dogs with such low doses of radiation.
While changes in platelet counts in this study were substantial, clinical toxicity was acceptable, and the thrombocytopenia may have been exaggerated by extensive stage of disease and prior chemotherapy in these dogs. Therefore, LDTBI may be of some benefit to canine lymphoma patients during first remission in a multidrug chemotherapy protocol. Studies of LDTBI in this setting are warranted to determine if LDTBI in conjunction with chemotherapy would increase remission duration or survival rates for dogs with lymphoma.