Survival analysis of one versus two treatments of local delivery cisplatin in a biodegradable polymer for canine osteosarcoma
Abstract
The purpose of this study was to evaluate one versus two doses of local delivery cisplatin in a biodegradable polymer (OPLA-Pt) for the treatment of osteosarcoma (OSA) after amputation in dogs. Medical records were reviewed retrospectively, and 105 dogs were included in the study; 39% of dogs received one treatment (surgical implantation) of OPLA-Pt and 61% of dogs received two treatments of OPLA-Pt after amputation. Administration of two doses of OPLA-Pt did not have a significant effect on disease-free interval or survival time compared to one dose. The anatomic site of the tumour was identified as a prognostic factor, and dogs with proximal humeral OSA had the shortest disease-free interval and survival times. There was no advantage to giving a second dose of local delivery cisplatin following amputation for the treatment of OSA in dogs.
Introduction
Osteosarcoma (OSA) is the most common primary bone tumour in dogs and accounts for up to 80% of all malignant tumours of bone and up to 98% of primary appendicular bone tumours.1 It has a high rate of metastasis, with over 90% of cases having micrometastases at the time of initial diagnosis.2 Treatment of OSA generally involves the removal of the primary tumour, either by amputation or limb-sparing procedures, followed with adjuvant chemotherapy.3 Studies have shown that dogs treated with amputation alone had a median survival time of 18–20 weeks, whereas dogs treated with amputation and adjuvant chemotherapy had a median survival time of 37–43 weeks.2,4,5 One widely used and studied chemotherapeutic agent is cisplatin with proven efficacy for delaying the time to metastasis.6,7 Median survival times have ranged from 35.5 to 45 weeks using variable cumulative cisplatin doses and treatment protocols.1 Systemic toxicity, mainly renal and bone marrow, limits the maximal single dose and cumulative doses that can be administered to dogs.
Intracavitary chemotherapy (placed in a wound or body cavity before closure) can result in adjacent cells being exposed to drug concentrations far exceeding those obtainable with systemic treatment without the systemic toxicities. Cisplatin has been used for intracavitary therapy in a limited number of dogs.7,8 The use of a biodegradable polymer sponge (Open cell PolyLactic Acid or OPLA, OSMED, THM Biomedical Inc., Duluth, MN, USA) containing cisplatin (OPLA-Pt) within the wound cavity may reduce local recurrence in dogs undergoing marginal resection of OSA during limb-sparing procedures.6 One dose of intracavitary OPLA-Pt has also been shown to significantly improve survival times in dogs undergoing amputation for OSA.7 Additionally, local delivery of cisplatin has been evaluated in dogs to treat soft tissue sarcoma and OSA and in mice to treat mammary carcinoma.6–12
Results of pharmacokinetic studies demonstrated that local release of cisplatin by OPLA-Pt delivered low sustained serum concentrations of cisplatin, with an area under the curve (AUC) for whole body drug exposure 27 times greater than that achieved with an equivalent dose of cisplatin given intravenously.7,11 This method of sustained release of low concentrations of cisplatin may be more beneficial than standard pulse dose intravenous infusions by exposing tumour cells to the cisplatin for a longer time period at lower peak concentrations, which decreases systemic toxicities. Systemic toxicity of intracavitary OPLA-Pt is rare even at doses exceeding the maximally tolerated intravenous dose of 70 mg m−2. When OPLA-Pt was implanted in normal dogs, no systemic toxicity or impedance of cortical allograft healing was identified at doses of up to 80.6 mg m−2.11
Based on the success of systemic micrometastasis treatment using a single implantation of OPLA-Pt, the authors investigated the effect of two implantations on outcome. The goals of this study were to determine the median disease-free interval and survival in dogs treated with one versus two doses of OPLA-Pt after amputation for the treatment of OSA and to identify prognostic factors for disease-free interval and survival times.
Materials and methods
This project was approved by and adhered to the guidelines of the Animal Care and Use Committee of Colorado State University.
OPLA-Pt consisted of a porous body composed of biodegradable polymer. The internal architecture was an association of partially enclosed, randomly sized, shaped and positioned intercommunicating interstices. All voids of the delivery unit were incompletely partitioned, communicating through multiple openings with all neighbouring voids with multiple avenues of communication to any location on the surface of the unit. Interstices of the device were described by their feret diameters, which ranged from 12 to 1200 µm. The void partitions were platforms of polymer riddled with additional porosities between 2 and 20 µm in diameter. Both polymer groups were synthesized from the same monomer, differing only in weight average, molecular weight (Mw) and polydispersities. Prior to radiation sterilization (2.5 megarads gamma radiation), 50% of the polymer was high molecular weight (Mw = 350 000) and 50% was low molecular weight (Mw = 34 000). Following radiation, molecular weights of the high and low fraction were altered to 235 067 and 52 938, respectively. Cisplatin was joined to the low molecular weight fraction, yielding 8% cisplatin by weight of polymer. The polymer was available in approximately 1 cm × 1 cm × 0.25 cm pieces, each containing approximately 5 mg platinum.7
Medical records of all dogs that had histologically confirmed OSA treated by amputation and local delivery OPLA-pt and all dogs that were operated for OSA at Colorado State University between 1995 and 2002 were reviewed. Dogs were excluded if there was evidence of metastatic disease at the time of diagnosis, if the animal received intravenous chemotherapy or if the dog had clinicopathologic changes that would preclude them from receiving cisplatin.
All dogs had a histologically confirmed OSA. Informed consent was obtained from all owners. Preoperative screening included a physical examination, complete blood count, serum biochemical analysis and thoracic radiographs. All dogs underwent an amputation and OPLA-Pt was implanted within the surgical site at the time of surgery. Dependent on fore or hind leg amputation and level of resection (coxofemoral disarticulation versus partial pelvectomy/amputation), OPLA-Pt sponges were implanted between muscle bellies at the first level of wound closure. The dose of OPLA-Pt implanted was based on the maximum tolerated systemic dose of cisplatin delivered from the polymer. The OPLA-Pt was implanted into the amputation site, and the amputation site was closed without surgical drainage. Dogs received one or two doses of OPLA-Pt based on chronological presentation, dogs presenting between 1995 and 1999 received one dose of OPLA-pt and dogs presenting between 1999 and 2002 received a second dose of OPLA-pt. Disease-free interval was defined as the time interval between the date of amputation and date of detection of metastasis, and survival was defined as the time interval between the date of amputation and the date of death.
The surgical sites were evaluated 2, 4 and 6 weeks postoperatively. Four weeks after surgery, dogs that received two doses of OPLA-Pt underwent a short general anaesthesia and had an additional dose of OPLA-Pt implanted into the amputation surgical site. Complete blood count and serum biochemical profile were obtained prior to re-implantation and if toxicity was suspected. An attempt was made to place the sponges deep to the most superficial fascial layer encountered on surgical exploration immediately within or adjacent to the initial surgical wound. Complications were assessed and recorded. Physical examination and thoracic radiographs were recommended at 3-month intervals to evaluate for metastasis, but was not performed in all cases. In cases where bone metastasis was suspected, nuclear scintigraphy was recommended to localize the site of metastatic disease. Disease failure was considered at the time of radiographically evident pulmonary or bone metastasis or by metastasis at other sites documented through imaging, cytology or biopsy. Following death or euthanasia, an attempt was made to obtain a complete necropsy with gross and histologic confirmation of disease.
Statistical analysis
A software package was used for statistical analysis (Egret for Windows, Cytel Software Corporation © 2001, Cambridge, MA, USA). Multifactorial analysis using a Cox proportional hazards regression model to evaluate the effects of age, breed, weight, sex, anatomic site of tumour, local or systemic toxicity, and one or two doses of OPLA-Pt on disease-free interval and survival following limb amputation was performed. Anatomic sites evaluated were divided into the following locations: humerus, radius, femur, tibia and others. Results are presented as hazard rate ratios (HRR) and 95% confidence intervals (95% CI). The Kaplan–Meier (product-limit) method was used to determine disease-free and overall survival probability curves. Dogs that were lost to follow-up or died of causes not related to OSA or treatment were censored at the date of last contact or date of death, respectively. Significance was set at a P-value less than or equal to 0.05.
Results
One hundred and five adult dogs with a mean weight of 34 ± 11 kg were used in this study. Median age was 9 years, ranging from 2 to 16 years. There were 15 Labrador retrievers, 17 Golden retrievers, 11 Rottweilers, eight sight breeds (Greyhounds, Borzoi), seven Doberman Pinschers, six dogs of Giant Breeds (Dane, Saint Bernards, or Irish Wolfhound), two Nordic breeds, 25 mixed breeds and 14 other breeds (one of each). There were 56 spayed females, two intact females, 39 castrated males and eight intact males, with a male-to-female ratio of 0.81 : 1.0. The primary tumour was recorded in 104 dogs, and the following sites were reported: femur (24 dogs), tibia (27), humerus (33), radius (14) and other (six) [pelvis (two), scapula (two), digit and extraskeletal (one each)].
All dogs had OPLA-Pt implanted at the time of amputation. Sixty-four (61%) of these dogs had a second OPLA-Pt reimplanted by opening part of the previous surgical scar 4 weeks after the first surgery under general anaesthesia.
Median and mean dose of cisplatin was 86 mg m−2 and 95.7 ± 18 mg m−2, respectively, with a range of 60–120 mg m−2 for each treatment. Swelling and/or drainage occurred at the surgical site in 14 dogs that received a single treatment of OPLA-Pt. Twenty-four dogs that received a second dose of OPLA-Pt had signs of local toxicity, which included drainage (20 dogs), swelling (three) and dehiscence (two). Systemic complications were observed in four dogs that underwent two OPLA-Pt treatments and complications included azotemia, vomiting, fever, anorexia and lethargy.
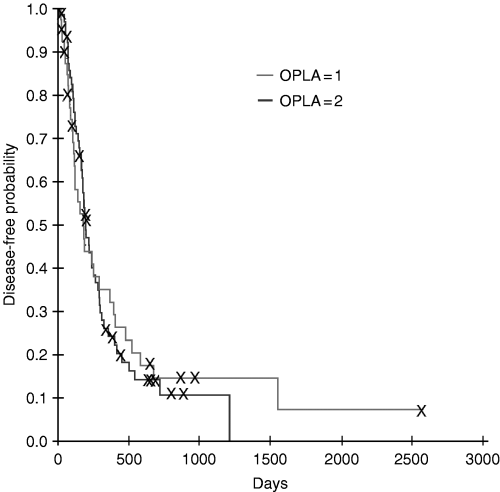
The median disease-free interval in dogs that received one dose of OPLA-Pt was 130 days, with a range of 19–1551 days. Dogs that received two doses of OPLA-Pt had a median disease-free interval of 183 days, with a range of 16–1215 days. There was no difference in the disease-free interval between the two groups (HRR = 1.02, 95% CI 0.65–1.60, P = 0.92) (Fig. 1). The median survival time in dogs that received one dose of OPLA-Pt was 233 days (range 5–2570 days), whereas dogs that received two doses of OPLA-Pt had a median survival time of 235 days (range 49–1215 days). There was no difference in survival times between dogs that received one versus two doses of OPLA-Pt (HRR = 1.23, 95% CI 0.78–1.93, P = 0.37) (Fig. 2). All dogs but one died in the study period. The other dog was lost to follow-up 242 days after the amputation, at which time metastasis was detected.
Comparison of Kaplan–Meier disease-free interval estimates for dogs with osteosarcoma treated with amputation and one dose of OPLA-Pt (n = 41) and for dogs treated with two doses of OPLA-Pt (n = 64). There was no difference in the disease-free interval between the two groups.
Comparison of Kaplan–Meier survival estimates for dogs with osteosarcoma treated with amputation and one dose of OPLA-Pt (n = 41) and for dogs treated with two doses of OPLA-Pt (n = 64). There was no difference in the survival times between the two groups.
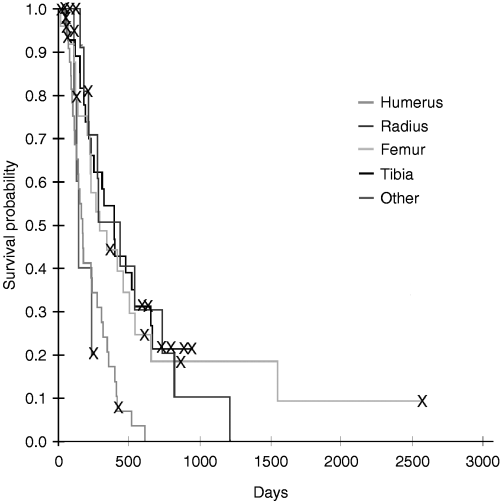
Of all prognostic factors evaluated, only anatomic site of tumour was identified as a prognostic factor for both disease-free interval (P = 0.025) and survival (P = 0.001), with the humerus having the worst prognosis (3, 4). There was no interaction between anatomic site of tumour and the number of OPLA-Pt treatments, and therefore, the number of OPLA-Pt treatments did not affect disease-free interval and survival, even when correcting for anatomic site of tumour.
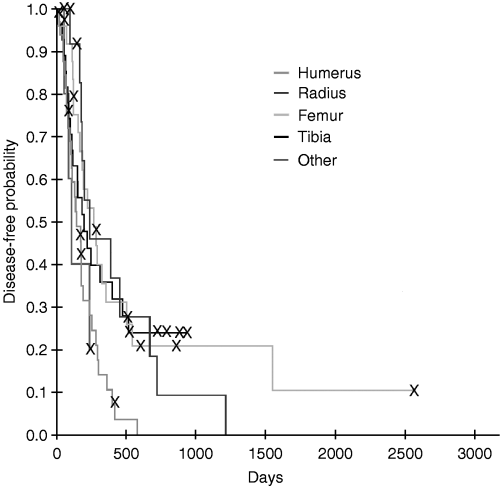
Kaplan–Meier disease-free interval estimates for dogs with osteosarcoma (OSA) treated with amputation and one or two doses of OPLA-Pt comparing anatomic site for tumour; dogs with humeral OSA had shorter disease-free intervals.
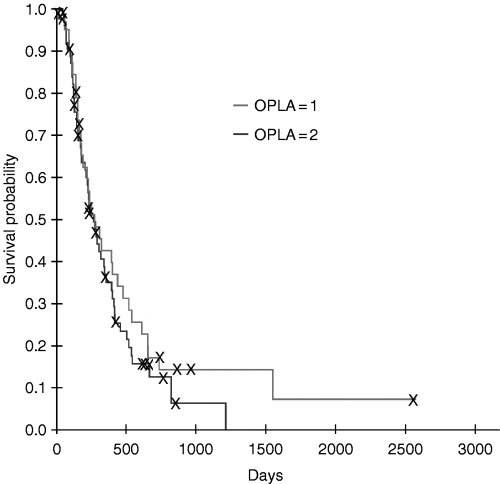
Kaplan–Meier survival estimates for dogs with osteosarcoma treated with amputation and one or two doses of intracavitary OPLA-Pt comparing anatomic site for tumour.
Discussion
The results of this study showed that there was no difference in time to metastasis or survival for dogs that had one versus two doses of OPLA-Pt. The median survival time for dogs of this study compares with the medial survival time for dogs of a previous study where one dose of OPLA-Pt was used. In the previous study, 39 dogs with appendicular OSA were treated with amputation and one dose of OPLA-Pt was implanted in the muscles of the amputation site at the time of surgery.7 The median survival time was 240 days. This was at least equivalent to the improvement in survival probability when two intravenous doses of cisplatin are used after amputation. We expected that dogs treated with two doses of OPLA-Pt would have a longer disease-free interval and survival time based on a larger total AUC for whole body drug exposure. When the effect of drug AUC on survival was evaluated in the group of dogs from the previous study, dogs with an AUC of greater than 7 µg mL−1 min−1 had a significantly longer survival time than those dogs with an AUC below 7.7 It is possible that the rate of delivery (µg mL−1 min−1) is an important factor for killing efficacy for sustained release chemotherapy systems, even more so than the total AUC. The second implantation of OPLA-Pt would not necessarily improve the rate of delivery and consequently may not improve disease-free interval and survival. In the current study, we were not able to determine the AUC and rate of delivery and could not test this hypothesis. Other possible explanations for the results of this study include:
- 1
In healthy dogs with this delivery system of cisplatin, although the AUC for whole body drug exposure exceeded, the AUC determined after an equivalent dose administered intravenously, peak serum concentrations were much lower for the group of dogs with OSA treated with OPLA-Pt.11 It is possible that select tumour types are more susceptible to peak serum concentrations of a given chemotherapeutic agent.10
- 2
The time interval between the first and second treatment with OPLA-Pt, approximately 30 days, may have allowed cells to develop a tolerance to cisplatin, or may have selected for resistant tumour cells.
In this study, anatomic site of tumour had an effect on disease-free intervals and survival times; dogs with OSA of the humerus had shorter disease-free intervals and survival times compared to dogs with tumours at other locations. This is consistent with another study where OSA affecting the humerus was also associated with a poorer outcome.13 One possible explanation for dogs with humeral tumours having a shorter disease-free interval and survival time is that this anatomic site may be associated with a larger tumour volume. In previous studies, large tumour size was a negative prognostic factor for survival in dogs and human beings.14–16
The major limitation of this study was its retrospective nature. Although the diagnostic and therapeutic protocol was fairly consistent, complete data were not available for each dog. As well, dogs were not randomized into treatment groups, and the two cohorts of dogs were treated at different periods of time. Regardless, dogs in this study appeared to have a survival advantage over dogs that had been treated by amputation alone.17 As well, a previous study has shown adjuvant sustained released low dose cisplatin does have a therapeutic benefit for dogs with OSA.7




