Metastatic synovial cell sarcoma in two cats
Abstract
Synovial cell sarcoma (SCS) with metastasis to the regional lymph node was diagnosed in two cats. Synovial cell sarcomas are rare in cats and metastatic SCS has not previously been reported. In both cases, treatment consisted of limb amputation and adjuvant doxorubicin. Local tumour recurrence and pulmonary metastases were diagnosed in one cat 316 days postoperatively. This cat died of chronic renal failure 444 days after limb amputation. The second cat died of an acute pulmonary thromboembolism 41 days postoperatively without evidence of local tumour recurrence or metastatic disease.
Introduction
Synovial cell sarcoma (SCS) arises from synoviocytes of the joint capsule, bursa or tendon sheath. The diagnosis of SCS is controversial in dogs and immunohistochemical staining is recommended to differentiate SCS from other soft tissue sarcomas involving the joint (Whitelock et al., 1997; Craig et al., 2002). In dogs, limb amputation is recommended for the management of the local tumour, while the role of adjuvant chemotherapy remains to be defined, particularly for high-grade SCS (Tilmant et al., 1986; McGlennon et al., 1988; Ogilvie et al., 1989; Vail et al., 1994; MacEwen et al., 2001; Fox et al., 2002). Prognostic criteria in dogs include clinical stage, histological grade, positive cytokeratin immunohistochemical staining and extent of surgical resection (Vail et al., 1994; Fox et al., 2002). Metastasis is more common in high-grade SCS and has been reported in 22–32% of dogs at the time of diagnosis and 41–54% at necropsy (McGlennon et al., 1988; Vail et al., 1994). However, the biological behaviour of feline SCS is unknown and a histological grading scheme or prognostic criteria have not been described. Furthermore, SCS is rare in cats and, to our knowledge, metastatic SCS has not previously been reported (Nielsen, 1952; Hulse, 1966; Engle & Brodey, 1969; Davies & Little, 1972; Thoday & Evans, 1972; Silva-Krott et al., 1993; Borenstein et al., 1999). The present report describes the presentation, treatment and postoperative outcome of two cats with metastatic SCS. Both cats were diagnosed with metastatic SCS within a 12-month period and no other cats were diagnosed with SCS during this same time period.
Case 1
A 15-year-old, female neutered, domestic long-hair cat presented with a history of elevated serum liver activities of unknown cause. A mass, measuring 2.5 × 2.0 cm, encompassing the dorsolateral aspect of the left tibiotarsal joint was detected during physical examination. Lameness was not apparent, palpation of the mass did not elicit a painful response and regional lymph nodes were not palpable. Haematology and serum thyroxine concentrations were within the normal reference ranges. Serum biochemical abnormalities included elevated alanine transferase [282 IU L−1, reference range (RR) 30–100 IU L−1], aspartate transferase (150 IU L−1, RR 15–50 IU L−1) and gamma glutamyltransferase (4 IU L−1, RR 0–2 IU L−1) activities. Laparoscopic biopsies of the liver were submitted for histopathology and consistent with lymphocytic–plasmocytic cholangiohepatitis.
Fine-needle aspiration, incisional biopsy and radiographs of the left hock were performed for diagnostic and staging purposes. Cytology of the fine-needle aspirate was consistent with granulomatous inflammation and radiographs revealed a soft tissue swelling along the dorsolateral aspect of the left tarsus with no underlying osseous involvement (Fig. 1). There was no evidence of metastasis on three-view thoracic radiographs. The mass was diagnosed as a SCS based on histopathological examination of tissue obtained by incisional biopsy.
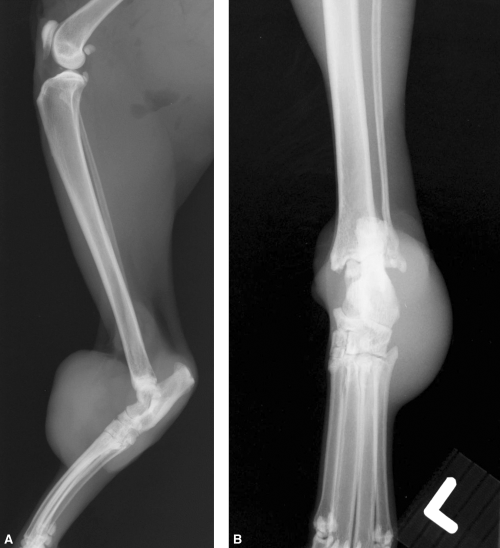
Mediolateral and dorsoplantar radiographs of the left tibiotarsal region showing a large soft tissue mass with no evidence of periosteal or bony involvement in case 1.
The cat was premedicated with midazolam and hydromorphone, induced with propofol, and maintained on isoflurane and oxygen. Intraoperative analgesia consisted of an epidural injection of morphine and bupivacaine, and a continuous rate infusion of ketamine (2 µg kg−1 min−1 intravenously) and fentanyl (2 µg kg−1 h−1 intravenously). Cephazolin (25 mg kg−1) was administered intravenously at induction of general anaesthesia. A left pelvic limb amputation was performed with disarticulation of the coxofemoral joint (Fig. 2). Postoperative analgesia included a continuous rate infusion of hydromorphone (0.01 µg kg−1 min−1 intravenously) and ketamine (2 µg kg−1 min−1 intravenously) for 48 h, oral morphine (2.7 mg every 8 h per os) for 3 days, and piroxicam (1 mg every 48 h per os) for 10 days.
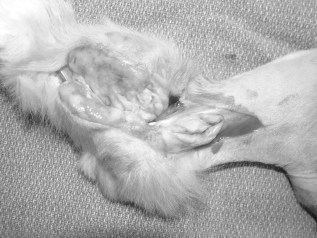
Photograph of the gross cut-section of the tibiotarsal synovial cell sarcoma following pelvic limb disarticulation in case 1.
Histopathological examination of the tarsal mass and ipsilateral popliteal lymph node was performed. Within the local mass, neoplastic round-to-polygonal cells, with moderately pleomorphic round nuclei and abundant eosinophilic cytoplasm, formed broad sheets with interspersed, loose collagenous material. Cleft-like spaces were absent. The nuclei were frequently bizarre with one nucleolus and greater than 30 mitotic figures per 10 high power fields. The percentage of tumour necrosis was estimated at 10% and the tumour was poorly demarcated. There were few giant cells and a moderate number of multinucleated cells (=three nuclei per cell). Approximately 30% of the popliteal lymph node was effaced with the same neoplastic cells observed in the primary mass, whereas the remainder of the node contained abundant lymphoid follicles interspersed between sheets of neoplastic cells. A grade III SCS was diagnosed on the basis of the grading scheme used in dogs with SCS (Vail et al., 1994). These findings were consistent with stage IIIA (T1 N1 M0) SCS, according to a modified staging system for soft tissue sarcomas proposed by MacEwen et al. (2001).
The cat was treated with adjunctive chemotherapy. Doxorubicin (1 mg kg−1 intravenously) was administered every 3 weeks for five treatments. An accidental overdose of doxorubicin was administered on the fifth dose (3.2 mg kg−1 intravenously). The cat was treated with ondansetron (0.3 mg every 12 h per os) for 6 days and enrofloxacin (20 mg every 24 h per os) for 14 days. Serial haematology revealed lymphopaenia (0.3 × 103 µL−1, RR 1.5–6.0 × 103 µL−1) and neutropaenia (1.3 × 103 µL−1, RR 2–12 × 103 µL−1) with a mild left shift (band neutrophils 0.3 × 103 µL−1, RR <0.1 × 103 µL−1) and a low normal platelet count (211 × 103 µL−1, RR 200–500 × 103 µL−1) 7 days postdoxorubicin administration. Granulocyte colony-stimulating factor was administered at this time. Within 4 days, neutropaenia had resolved (10.2 × 103 µL−1), platelet count increased (488 × 103 µL−1) and the lymphopaenia had improved although was still below the reference range (0.8 × 103 µL−1).
The cat was examined 316 days postoperatively. A firm, non-painful mass, measuring 1.5 × 3.0 cm, was present at the amputation site. Local recurrence of the SCS at the amputation stump was confirmed by fine-needle aspiration of the mass. Two lung lesions, consistent with pulmonary metastases, were detected on three-view thoracic radiographs (Fig. 3) at this time. Chronic renal failure, possibly secondary to the overdose of doxorubicin (O'Keefe et al., 1993) or age-related disease, was suggested by the presence of a non-regenerative anaemia (packed cell volume 26%, RR 30–46% and reticulocyte count 11 680 µL−1, RR 0–50 000 µL−1) and azotaemia (blood urea nitrogen 89 mM, RR 16–35 mM and creatinine 3.9 mM, RR 1.3–2.6 mM). The anaemia and azotaemia persisted, despite management with potassium gluconate (2 mEq every 12 h per os) and subcutaneous fluid administration, and the cat was euthanized 444 days postoperatively as a result of progressive and unresponsive chronic renal failure.
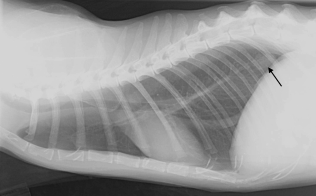
A pulmonary nodule (arrow), consistent with metastatic disease, is present on a lateral thoracic radiograph 316 days postoperatively in case 1.
Case 2
A 12-year-old, male neutered, domestic short-haired cat presented with chronic asthma and a 5-week history of right pelvic limb swelling and lameness. On physical examination, the cat was overweight (7.6 kg body weight) with a grade 4 to 5 right pelvic limb lameness and diffuse swelling distal to the right stifle. Haematology and serum biochemistry results were within normal limits. A peribronchial pulmonary pattern with atelectasis of the right middle lung lobe, both consistent with chronic bronchitis secondary to asthma, was evident on three-view thoracic radiographs. Radiographs of the right pelvic limb revealed intra- and extra-articular mineralization of the stifle. Ultrasonographic examination of the right stifle confirmed the presence of areas of poorly circumscribed soft tissue mineralization and also identified an enlarged popliteal lymph node. Ultrasound-guided fine-needle aspirates of the caudal stifle region and popliteal lymph node were performed. An undifferentiated sarcoma with ipsilateral popliteal lymph node metastasis was suspected on the basis of cytological examination of these aspirates. The cat was treated with albutenol for 3 days in an attempt to improve respiratory function prior to anaesthesia and surgery.
The cat was premedicated with atropine and hydromorphone, induced with midazolam and ketamine, and maintained on isoflurane and oxygen. Bronchoscopy and bronchoalveolar lavage were performed prior to surgery. Bronchoscopy revealed a flaccid airway with increased secretions and polypoid bronchial masses consistent with bronchomalacia and polypoid hyperplasia. Infectious organisms were not identified from aerobic and mycoplasmal cultures of respiratory secretions collected by bronchoalveolar lavage. Intraoperative analgesia consisted of an epidural injection of morphine and bupivacaine and a continuous rate infusion of ketamine (2 µg kg−1 min−1 intravenously) and fentanyl (2 µg kg−1 h−1 intravenously). Cephazolin (25 mg kg−1) was administered intravenously at induction of general anaesthesia. A right pelvic limb amputation was performed with disarticulation of the coxofemoral joint. The semimembranosus and semitendinosus muscles were surrounded by oedematous tissue, which extended to the level of the ischiatic tuberosity. Postoperative analgesia consisted of a continuous rate infusion of hydromorphone (0.0125 µg kg−1 min−1 intravenously) and ketamine (2 µg kg−1 min−1 intravenously) for 24 h and a transdermal fentanyl patch (25 µg) for 3 days.
A diagnosis of an incompletely resected, grade III SCS with ipsilateral lymph node metastasis was made on the basis of histopathological examination of the stifle mass and popliteal lymph node. Both the mass and popliteal lymph node contained highly pleomorphic round-to-polygonal cellswith marked anisocytosis, moderate anisokaryosis, multiple prominent nucleoli, greater than 30 mitotic figures per 10 high power fields, few giant cells and numerous multinucleated giant cells (4, 5). The degree of tumour necrosis was estimated at 30% and cleft-like spaces were present. The tumour was poorly demarcated and the cranial and caudoproximal margins of the amputated limb contained scattered nests of neoplastic cells approximately 5 mm distant to the mass, consistent with incomplete resection. On the basis of clinical and histological findings, a stage IIIA (T1 N1 M0) SCS was diagnosed.
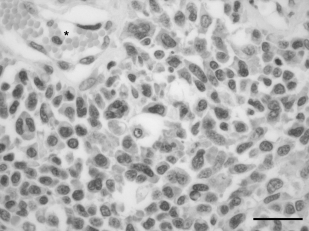
Haematoxylin and eosin stain of the synovial cell sarcoma in case 2. The tumour is composed of sheets of histiocytic cells with abundant cytoplasm and pleomorphic nuclei with moderate anisokaryosis. Multinucleated cells are numerous. *, vessel; bar = 25 µm.
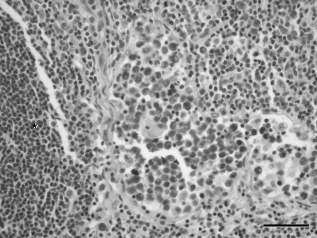
Haematoxylin and eosin stain of the ipsilateral popliteal lymph node of case 2 with a synovial cell sarcoma of the right stifle. There is invasion and expansion of the sinus regions by histiocytic neoplastic cells. *, lymphoid follicle; bar = 50 µm.
The cat was scheduled to receive doxorubicin every 3 weeks for five treatments. However, 7 days after the second dose of doxorubicin (1 mg kg−1 intravenously) and 41 days postoperatively, the cat died following an acute episode of respiratory arrest. Necropsy was performed and revealed firm and adherent thromboemboli in the pulmonary arteries of the cranioventral lung lobes, marked and diffuse bronchial thickening, and no gross evidence of cardiac abnormalities. The cause of pulmonary thrombosis remains undetermined as there was no gross or histological evidence of cardiac disease, the pulmonary thrombosis was not neoplastic in origin, and the cat was in otherwise good physical condition.
Discussion
Synovial cell sarcoma is rarely diagnosed in cats with four reports of malignant SCS (Nielsen, 1952; Engle & Brodey, 1969; Silva-Krott et al., 1993; Bornstein et al., 1999) and three reports of benign synovioma (Hulse, 1966; Davies & Little, 1972; Thoday & Evans, 1972) in the veterinary literature (Table 1). Signalment details were reported in six of these cases with age ranging from 6 to 9 years, four males and two females, and one British and four Domestic short-haired cats. Of these cases, three originated from a joint (carpus, elbow and tarsus) and four from tendinous structures adjacent to the carpus (n = 1), antebrachium (n = 2) and hind paw (n = 1). Lameness and osseous invasion were only apparent in cats with tumours arising from periarticular tissue. Periosteal reaction and bony lysis are common radiographic findings in dogs with SCS (McGlennon et al., 1988; Vail et al., 1994). The lack of both clinical lameness and radiographic bone involvement may cause uncertainty in the diagnosis of SCS in the present cases. However, it is possible that these tumours originated from adjacent tendinous structures rather than periarticular tissue, or SCS in cats may not have the same tendency to involve bone as in dogs. In humans, SCS is considered a misnomer as these tumours do not arise from synovial tissue and frequently occur in extra-articular locations (Greenspan & Remagen, 1998; Pool & Thompson, 2002).
| Case | Nielsen (1952) | Hulse (1966) | Engle & Brodey (1969) | Davies & Little (1972) | Thoday & Evans (1972) | Silva-Krott et al. (1993) | Borenstein et al. (1999) |
|---|---|---|---|---|---|---|---|
| Age (years) | 8 | 6 | NR | 8 | NR | 9 | 6 |
| Sex | Male | Male | NR | Female | Female | Male | Male |
| Breed | NR | DSH | NR | ||||
| British short hair | DSH | DSH | DSH | ||||
| Site | Antebrachium* | Antebrachium* | Tarsus | Hind paw* | Carpus* | Elbow | Carpus |
| Lameness | No | No | Yes | No | No | Yes | Yes |
| Bone Invasion | No | No | Yes | NR | No | Yes | Yes |
| Metastasis | No | No | No | No | No | No | No |
| Surgery | No | Local | No | Local | Local | Amputation | Amputation |
| Recurrence | NA | Yes | NA | Yes | No | No | No |
| Survival | NA | 18 months | NA | 4 months | 7 months† | 8 months† | 7 months† |
| Histology | SCS‡ | Synovioma‡ | SCS | Synovioma‡ | Synovioma‡ | SCS‡ | SCS‡ |
- DSH, domestic short hair; NR, not recorded; SCS, synovial cell sarcoma.
- * Tendon origin.
- † Alive.
- ‡ Multinucleated giant cells.
The diagnosis of SCS is controversial. In humans, SCS is classified as monophasic or biphasic depending on the proportion of malignant mesenchymal and epithelial cells in the tumour (Greenspan & Remagen, 1998; Pool & Thompson, 2002). Epithelial cells are rarely identified in canine SCS, however, and hence the use of human criteria to classify SCS in cats and dogs has been questioned (Pool & Thompson, 2002). Immunohistochemistry has been recommended for the diagnosis of SCS in dogs as histopathology can be unreliable in differentiating SCS from other soft tissue sarcomas (Whitelock et al., 1997; Craig et al., 2002). In the present series, SCS was diagnosed on the basis of histological appearance and periarticular location of the tumours. In contrast to dogs where the majority of SCS are fibroblastic (Pool & Thompson, 2002), both cats in the present report were diagnosed with histiocytic SCS. Synovial cell sarcoma arises from synovial cells of joints or tendon sheaths. Two types of synoviocytes have been identified. Type A synoviocytes are migratory, resemble macrophages, and have a phagocytic role within the joint. Type B synoviocytes are ultrastructurally similar to fibroblasts and are responsible for the production of synovial fluid and extracellular matrix of the synovium (Pool & Thompson, 2002). Canine SCS most likely originates from type B synoviocytes due to their predominantly fibroblastic nature. In the two cats described herein, neoplastic proliferation of type A synoviocytes is suspected, as both tumours were primarily histiocytic. This difference may represent an interesting variation in SCS between cats and dogs.
Surgery was performed in five of the previously reported cats with SCS. Limb amputation was performed in two cats and both cats were alive at 7 and 8 months after surgery (Silva-Krott et al., 1993; Borenstein et al., 1999). In contrast, local resection was performed in the other three cats with local recurrence in two cats 4 and 7 months postoperatively (Hulse, 1966; Davies & Little, 1972) and the other cat alive without recurrence 7 months following surgery (Thoday & Evans, 1972). Limb amputation is associated with a lower local recurrence rate and longer survival time than marginal resection in dogs with SCS (Vail et al., 1994; Fox et al., 2002).
Doxorubicin was administered to both cats due to the presence of regional metastasis. Doxorubicin may be efficacious in dogs with SCS (Tilmant et al., 1986; Ogilvie et al., 1989), but other larger studies have shown no survival benefit with adjuvant doxorubicin (McGlennon et al., 1988; Vail et al., 1994). Similarly, in humans with SCS, single-agent therapy with either doxorubicin or ifosfamide is not associated with a significant survival benefit (Brennan et al., 2001). Doxorubicin-induced chronic renal failure was suspected in one cat following an accidental overdose on the fifth and final treatment. Azotaemia and decreasing urine-specific gravity were observed in two of six cats receiving an accumulative dose of 300 mg m−2 doxorubicin (O'Keefe et al., 1993).
The biological behaviour of SCS in both cats in the present series is similar to canine SCS. A histological grading system has not been reported for cats with SCS and it is unknown whether the canine SCS grading scheme has any prognostic significance in cats. One cat was diagnosed with local stump recurrence following histologically complete resection of a grade III SCS, whereas the SCS in the other cat was poorly defined with diffuse neoplastic extension from the stifle to the ischium. Furthermore, both cats were diagnosed with grade III SCS and both had histological confirmation of regional lymph node metastasis at the time of amputation. Unlike most other types of soft tissue sarcoma, canine and human SCS have a predilection to metastasize to lymph nodes and, although local tumour recurrence of soft tissue sarcomas following incomplete surgical resection is relatively common, recurrence at the amputation site following apparently complete resection is characteristic of SCS (Vail et al., 1994; Blazer et al., 2003).
Conclusion
The present report details two cats with regional metastasis to the popliteal lymph node from pelvic limb SCS. Both cats died due to unrelated causes 41 and 444 days postoperatively, although one cat died with evidence of local recurrence and pulmonary metastasis. Limb amputation is recommended for the management of SCS of the extremities, although the role of chemotherapy is unclear.




