Use of TLC-FID and GC-MS/FID to examine the effects of migratory state, diet and captivity on preen wax composition in White-throated Sparrows Zonotrichia albicollis
Abstract
Preen wax is important for plumage maintenance and other functions. Its chemical composition is complex, and separating and quantifying its components, commonly by gas chromatography (GC), can be challenging. We present a simple analytical system consisting of thin-layer chromatography/flame ionization detection (TLC-FID) using a solvent system of 100% toluene to analyse the complex compound classes present in preen wax. We used GC and TLC-FID to investigate the effects of migratory status, diet and captivity on the preen wax composition of White-throated Sparrows Zonotrichia albicollis, and to measure the quantity of preen wax on the head, primary and tail feathers. White-throated Sparrows produced preen wax containing only monoesters regardless of migratory state. The monoesters contained several isomers consisting of homologous series of fatty alcohols (C10–C20) and fatty acids (C13–C19) esterified together in different combinations to form monoesters with total carbon numbers ranging from C23 to C38. Weighted average monoester carbon number was greater in captive birds than in wild birds and was greater in captives fed a formulated diet enriched with sesame oil than in birds fed the same diet enriched with fish oil. Captivity and migratory state also affected the complexity of the mixture of monoesters. There was significantly more preen wax on head feathers compared with primary and tail feathers. We suggest that among its many functions, preen wax may play a role in drag reduction by affecting the physical properties of feathers, and/or the fluid flow at their surfaces.
Preen wax is produced in the uropygial (preen) gland of birds. There are many hypotheses about its function, including feather maintenance, waterproofing, ultraviolet (UV) protection, antimicrobial action, olfactory camouflage, chemo-signalling, and sexual attraction (Piersma et al. 1999, Moyer et al. 2003, Haribal et al. 2005, Reneerkens et al. 2005, 2008, Soini et al. 2007, Piault et al. 2008). Its chemical composition is complex (Kolattukudy et al. 1987, Dekker et al. 2000) and can vary among species (Jacob & Ziswiler 1982, Haribal et al. 2005) and seasons (Piersma et al. 1999, Reneerkens et al. 2007b). The influence of captivity and diet on preen wax composition has not been studied before, and both could potentially be important confounding factors in future studies. For example, if preen wax composition is sensitive to dietary change, then perhaps researchers would be able to manipulate it using different diets while birds are in captivity. This would provide further insights into some of the reported changes in preen wax composition noted with changes in seasons and/or migratory state (Kolattukudy et al. 1987, Piersma et al. 1999, Reneerkens et al. 2002, 2007a,b).
In some respects, the complexity of the chemical composition of preen wax has hindered the testing of functional hypotheses. Preen wax is composed predominantly of monoesters consisting of fatty acids esterified to fatty alcohols; the diversity of these components leads to hundreds of monoester products. The fatty acid moiety is nearly always saturated (lacking double bonds), but may be unbranched or branched (e.g. mono-, di- or polymethylated) (Jacob & Ziswiler 1982). The alcohol moiety consists of straight chain or methyl-substituted mono fatty alcohols. Other compounds such as diesters, triesters, alkanes, triglycerides, free fatty acids and free alcohols may also be present in the preen wax (Jacob 1976, Sweeney et al. 2004, Montalti et al. 2005, Salibian & Montalti 2009). Diesters may be present during the breeding season (Kolattukudy et al. 1987, Piersma et al. 1999, Reneerkens et al. 2007b) and they may be of two types: (i) diol-based esters (i.e. 1, 2-diols esterified with two fatty acids) or (ii) diesters based on hydroxy fatty acids (usually at the 2- or 3-position) esterified with an alcohol and a fatty acid (Jacob & Ziswiler 1982, Kolattukudy et al. 1987, Sinninghe Damstéet al. 2000). The diversity and complexity of these compounds in preen wax make chemical analysis by gas chromatography-mass spectrometry (GC-MS) challenging, particularly in resolving and identifying these compounds.
Currently, there is limited information on the molecular composition of intact preen waxes (Dekker et al. 2000, Sinninghe Damstéet al. 2000, Burger et al. 2004, Haribal et al. 2005). Analysis of preen wax using GC-MS usually involves hydrolysis of the preen wax components, leading to loss of information concerning the distribution of molecular species (Kolattukudy et al. 1985, 1987, Gebauer et al. 2004). Knowledge of the molecular distribution of intact wax esters is important in determining their physical properties (Dekker et al. 2000) and could provide further insight into the function(s) of preen wax. We used thin-layer chromatography with automated flame ionization detection (TLC-FID), in combination with GC-FID and GC-MS to provide a detailed analysis of intact preen wax composition. Specifically, TLC-FID enables separation and quantification of the intact preen wax chemical compound classes (monoesters, diesters, triesters, etc.), GC-MS permits identification of the molecular species of the separated compound classes and GC-FID is used for quantification of the molecular species identified in the compound classes.
This study was designed to: (i) use TLC-FID to develop a simple analytical system to analyse the complex compound classes present in intact preen wax; (ii) use this analytical system (TLC-FID) along with GC-MS/FID to ascertain how and if the intact preen wax compound classes of White-throated Sparrows Zonotrichia albicollis change with migratory state (autumn, spring and winter), captivity, and diet; and (iii) determine the amounts of preen wax present on head, primary (remiges) and tail (rectrices) feathers.
Methods
Preen wax collection
Preen wax was collected using the methods of Reneerkens et al. (2007b). Cotton or a commercially dewaxed cotton swab (extracted and analysed to confirm its negligible wax content) was gently massaged/squeezed on the preen gland. The sample was wrapped in aluminium foil and placed in a glass sample vial or polyethylene bag and stored at −80 °C. In 2007, samples were collected from wild White-throated Sparrows captured in mist-nets and ground-traps during autumn migration (early October, n = 12) and winter residency (November, n = 8) in Bronx Park, New York, NY, USA, and during spring migration (April/May, n = 12) in 2008 at the Long Point Bird Observatory, Port Rowan, Ontario, Canada.
Additional White-throated Sparrows were captured in early October 2007 on private land near Long Point, Ontario, Canada, and individually caged for approximately 3 weeks at the University of Western Ontario prior to preen wax sampling. They were kept on a photoperiod of 12 h light:12 h dark and fed bird seed (millet, sunflower and cracked corn) well mixed approximately 1 : 1 with one of two powdered formulated diets (n = 8 per diet treatment). The formulated diets both consisted of 13% lipid, 61% starch, 13% protein/amino acids, 5% cellulose and 3% other ingredients by mass (Price & Guglielmo 2009). The ‘sesame oil diet’ contained 10% Loriva extra virgin sesame oil (nSpired Natural Foods, San Leandro, CA, USA) and 3% refined menhaden oil (MP Biomedicals, Solon, OH, USA), while the ‘fish oil diet’ contained 10% menhaden oil and 3% sesame seed oil. These oil mixtures provide a diversity of fatty acids with marked differences in average chain length and in the ratio of n6:n3 fatty acids, a property thought to have an important effect on various metabolic pathways (Watkins 1991). All procedures were approved by the UWO Animal Use Subcommittee (Protocol # 2006-014-020), and permits were obtained from the Canadian Wildlife Service (CA 0170) and United States Geological Survey (23452).
Preen wax extraction
Samples were placed in 2-mL glass vials containing 1 mL of chloroform/ethyl acetate (1 : 1 v/v) as the extraction solvent and sealed with Teflon-lined screw caps. When swabs were used, the cotton head of the swab containing the preen wax was removed and then extracted. Samples were heated at 60 °C for 5 min in an oven, vortexed, and the solvent transferred to new pre-weighed sample vials. The solvent was evaporated under a stream of nitrogen and the vials were re-weighed to obtain the weights of the recovered preen wax. The preen wax was re-suspended in the extraction solvent at a concentration of 0.5 mg/mL for analysis.
Preparative and quantitative TLC
Preparative TLC was used to purify sufficient quantities of preen wax components for detailed analysis by other means. Separation was achieved on activated 20 × 20-cm glass plates coated with silica gel K6F (250 μm layer thickness, pore size 60 Å). Samples (10 μL) were developed with toluene/chloroform (7 : 3 v/v) (Kanya et al. 2007). Hydrocarbons, fatty acids, fatty alcohols and monoesters were obtained from Sigma (St. Louis, MO, USA), while monoesters, diesters and triesters were isolated from beeswax using column chromatography (see below). After development, the plates were dried using a hair dryer, and compounds were visualized using iodine crystal vapour.
Quantitative TLC was done using the Iatroscan MK-6 TLC/FID Analyzer System (Shell-USA, Fredericksburg, VA, USA) to measure accurately and precisely the amounts of different preen wax components in samples. This consists of 10 quartz rods, 15 cm in length and 0.9 mm in diameter, coated with a 75-μm-thick layer of porous silica-gel particles (5 μm diameter) sintered into a supporting glass frit (Ackman et al. 1990, Hudson et al. 2001). Aliquots of preen wax samples (0.5 mg/mL) and standards dissolved in chloroform were used for the analysis. Prior to sample application, chromarods were blank-scanned repeatedly to burn off impurities. A 2-μL Hamilton glass syringe was used to spot 1 μL of sample or preen wax standards. Rods were developed in a TLC chamber using 100% toluene until solvent reached the 100 line mark on the rod holder. Rods were removed and dried at 60 °C in a rod dryer (TK8; Iatron Inc., Tokyo, Japan) for 5 min and then analysed at the following settings: flow rate of 2 L/min for atmospheric air, 160 mL/min for hydrogen and scanning speed of 3.0 cm/s. Preen wax components were identified and quantified by comparing retention times and FID outputs of reference standards loaded at concentrations between 0.1 and 1.0 mg/mL. Fatty acids and alcohols were not clearly resolved using 100% toluene; however, this was corrected by partially burning the rods to remove the previously separated components, and then inverting the rods to develop them in a solvent system of toluene/chloroform (7 : 3 v/v) before a second scan.
Reference standards
Some standards, including monoesters (butyl oleate (C22), myristyl dodecanoate (C26), palmityl dodecanoate (C32), arachidyl dodecanoate (C34), dodecyl dodecanoate (C34) and stearyl arachidate (C38)), octadecane (C18), octadecanoic acid, octadecanol, 3-hydroxypalmitic acid, 2-hydroxypalmitic acid, 1,2-decanediol, 1,2-hexadecanediol and triglycerides (tripalmitin and triarachin) were available commercially from Sigma.
Alkanes, monoesters, diesters and triesters were isolated from beeswax (Tulloch 1971). A 500-mL glass chromatography column was prepared by loading 50 g of silica gel (pore size 60 Å, 230–400 μm mesh) activated at 100 °C for 18 h. Refined beeswax (1.3 g, Sigma) was dissolved in 10 mL of warm hexane (40 °C) and enough silica gel to form a slurry prior to loading on the column. Air pressure was used to assist in pushing solvent through the column. Hydrocarbons were eluted by adding 250 mL of hexane. Monoesters were eluted by flushing the column with 750 mL of hexane/chloroform (9 : 1 v/v). Diesters were eluted with 750 mL hexane/chloroform (4 : 1 v/v) and hexane/chloroform (7 : 3 v/v) and diesters, triesters and free fatty acids were obtained by further elution with 250 mL of hexane/chloroform (7 : 3 v/v). Each fraction was collected in separate clean beakers and evaporated to dryness using a rotary-evaporator (Buchi Rotavapor R-200; Buchi Corporation, New Castle, DE, USA). The dried wax fractions were collected and stored in sample vials. One milligram of each fraction was dissolved in 1 mL of chloroform and 10 μL was spotted on TLC plates along with known commercial standards (monoesters, hydrocarbons and fatty acids). The plates were developed using 100% toluene and toluene/chloroform (7 : 3 v/v) and spots were visualized using iodine. The location of known standards was compared with that of isolated compounds in each fraction to determine purity and identity. Further confirmation of sample identity was done using GC-MS analysis.
GC-MS and GC-FID analysis
The spots obtained from each beeswax fraction separated on preparative TLC plates were scraped and re-suspended in 1 mL chloroform, transferred to clean vials through glass wool and dried under nitrogen. The dried fractions containing fatty acids, diesters and triesters were methylated using BF3 methanol transesterification (Yang & Bernards 2006) followed by trimethylsilylation in 50 μL of pyridine and 50 μL of N,O-bis (trimethylsilyl) trifluoroacetamide (99%) with 1% trimethyl chlorosilane (70 °C for 30 min). Samples were analysed using a CP-Sil 5CB column (25 m × 0.32 mm) as follows: injection at 40 °C, programmed to 200 °C at 4 °C/min, 10 °C/min to 300 °C, where it was held for 15 min (Dekker et al. 2000). Fatty acids were identified by comparison with reference standards (mass spectra and retention time). Diesters and triesters were identified by comparisons of their hydrolysis products, e.g. 3-hydroxy acids and 1,2 diols, with those of reference standards (Sigma).
Extracted preen wax samples (1 μL) were analysed on a GC-MS (GC: Varian 3500, MS: Finnigan MAT 8400) with an HP-5 (5% phenyl methyl siloxane) column (30 m × 0.32 μm ID × 0.25 μm film thickness). The samples were injected at 70 °C (held for 1 min) and the temperature was then ramped to 130 °C at 20 °C/min, then to 320 °C at 4 °C/min where the temperature (320 °C) was held for 10 min.
Separation and quantification of the monoesters present in preen wax secretions were carried out on a gas chromatograph with a flame ionization detector (GC-FID) (Agilent 6890N) using an HP-5 (5% phenyl methyl siloxane) column (30 m × 0.32 μm ID × 0.25 μm film thickness). The samples (1 μL) were injected at 70 °C (held for 1 min) and the temperature was then ramped to 130 °C at 20 °C/min, then to 320 °C at 4 °C/min, where the temperature (320 °C) was held for 10 min.
Measurement of preen wax on feathers
Samples of head, primary and tail feathers were taken from 36 spring and 36 autumn migrant Sparrows caught near Long Point in 2006 for an unrelated study, and stored in polyethylene bags at −20 °C until analysis. We measured preen wax on entire head feathers and the distal 30 mm of the primary and tail feathers. To obtain enough material for analysis, for each season feathers of each type from groups of six individuals were combined into three composite samples (n = 3 spring, n = 3 autumn). The composite samples were weighed (12–20 mg), placed in 4-mL glass sample vials with Teflon-lined caps and 4 mL 1 : 1 chloroform/ethyl acetate was added. The samples were heated at 60 °C for 5 min in an oven, vortexed, and the solvent was then transferred to new pre-weighed sample vials. The extraction solvent was evaporated under a stream of nitrogen and the vials were re-weighed to obtain the weights of the recovered preen wax. The preen wax was re-suspended in 1 mL of chloroform and each sample diluted to aliquots of 0.5 mg/mL for TLC, TLC-FID and GC-FID analysis.
Images of head, primary and tail feathers were captured using a stereological microscope (Zeiss Lumar V12; Zeiss, Toronto, ON, Canada) fitted with a Retiga 1300 Series digital camera (Retiga, Vancouver, BC, Canada), at the Biotron, University of Western Ontario, London, Canada. Images were analysed using northern eclipse software (Mempix Imaging, Mississauga, ON, Canada). The nominal surface areas of the captured images were determined by equalizing the image (i.e. making the image black compared with background) in the grey range (135–145) with a threshold setting in the 100 range. The system was calibrated using a millimetre rule and the surface areas of the threshold selected images were auto calculated and reported in square millimetres. Recovered preen wax component (monoesters) were expressed as μg/mm2 feather. Expressing the amount of preen wax per square millimetre gives a more accurate representation of how much preen wax birds apply over the surface of their feathers.
Statistical analyses
The normality of the dataset was assessed using the Wilk–Shapiro statistic/Rankit Plot. Data points departing from the linear trend near the extremes of the plot indicative of outliers (small value for the Wilk–Shapiro statistic) were removed from the model as reflected by the reported degrees of freedom (n = 10 for diet or captives, and n = 31 for migratory state or wild birds). Analysis of variance followed by a post-hoc Tukey’s HSD test (α = 0.05) was used for all analyses. The data were analysed using the statistix software package (Analytical Software, Tallahassee, FL, USA).
Results
Development of TLC rods in 100% toluene resolved the components (alkanes, monoesters, diesters, triesters, triglycerides, and free fatty acids plus alcohols) present in the preen wax standard mix (Fig. 1a). Alcohols and fatty acids could be further resolved from each other by redeveloping the rods in 7 : 3 (v/v) toluene/chloroform (data not shown). When preen wax samples from White-throated Sparrows were analysed, only monoesters were present (Fig. 1b) regardless of migratory state, captivity or diet.
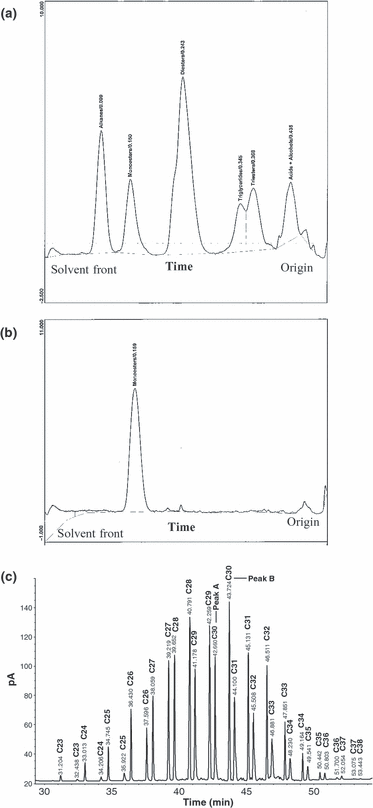
Chromatograms showing TLC-FID analysis of preen wax standard mixture (a), a typical preen wax sample collected from a White-throated Sparrow (b) and GC-FID analysis of preen wax monoesters (c). Peak A and Peak B, denoting two peaks for monoesters of the carbon number, are labelled for C30 as an example.
GC-FID/MS analysis of Sparrow preen wax revealed several individual monoesters ranging in total carbon number from C23 to C38, with double peaks having the same number of total carbons, but clearly resolved from each other in all the samples (Fig. 1c and Table S1 in the Supporting Information). For simplicity, we denoted the first peak in the doublet as Peak A and the second peak as Peak B (Fig. 1c). The chemical compositions of the two monoester peaks were determined using GC-MS (Fig. 2a,b). Peak A contained one major monoester consisting of a fatty acid esterified to an alcohol moiety. Peak B consisted of a mix of between two to four major monoesters (isomers) with different carbon chain lengths in the acid (C12–19) and alcohol (C10–20) moieties yielding monoesters having the same number of total carbons (Table S1).
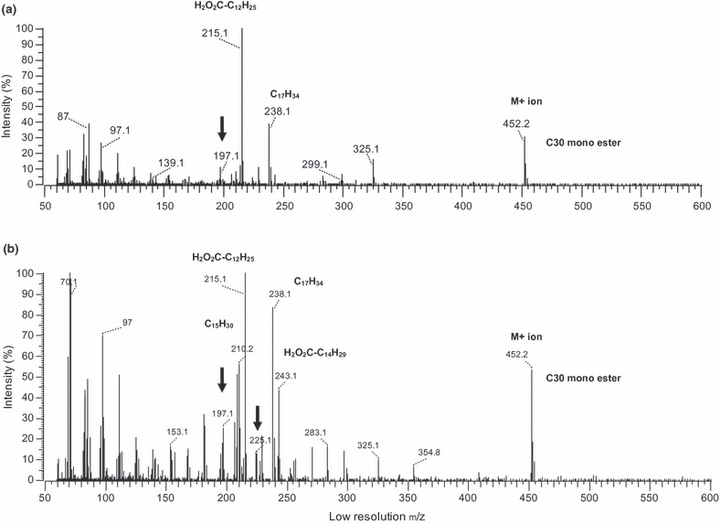
Mass spectra showing an example of preen wax monoesters from a White-throated Sparrow containing 30 carbons (see Figure 1). Peak A consists of one major monoester (a) and Peak B of a mixture of major monoesters (b). Arrows indicate acylium ions of the major acids.
Examples of mass spectra from a monoester having 30 carbons are presented in Figure 2(a,b). Figure 2(a) shows a monoester of Peak A type having one major monoester. The molecular weight of this monoester (452.2 amu) is represented by the molecular ion [M]+ peak. The base peak, mass (m/z) = 215 amu, represents the protonated fatty acid moiety, which corresponds to a C13 fatty acid. The acylium ion (i.e. the organic radical derived from the removal of a hydroxyl group from an organic acid) of this acid peak is also represented at m/z = 197 amu. The molecular ion fragment m/z = 238 amu represents the alcohol ion or the acyl chain of the fatty alcohol moiety that has been dehydroxylated, [M-18]+, corresponding to a C17 alcohol. This mass spectrum showed that Peak A is a C30 monoester consisting of a C13 fatty acid esterified to a C17 alcohol. The mass spectrum for Peak B (Fig. 2b) also showed a C30 monoester having a molecular ion [M]+ of m/z = 452 amu. However, the mass spectrum of Peak B clearly shows the molecular ions of two major protonated acids at m/z = 215 amu (C13) and m/z = 243 amu (C15) and the corresponding acylated alcohol ions at m/z = 238 amu (C17) and m/z = 210 amu (C15). The acylium ions of the acids (C13 and C15) are also present at m/z 197 amu and m/z = 225 amu, respectively. This mass spectrum shows that Peak B is a mixture of at least two major wax ester isomers. One of the isomers consisted of a C13 acid esterified to a C17 alcohol and the other major isomer consisted of a C15 alcohol esterified to a C15 acid. The chain length combination of the acid and alcohol moieties present in Peak A was also observed to occur as one of the major components in the corresponding Peak B (same carbon number; Table S1).
Several combinations of acid and alcohol moieties were present in the monoesters of preen wax. Both even and odd total carbon number monoesters were present (Table S1). The odd total carbon number monoesters consisted of odd carbon number alcohols esterified to even carbon number fatty acids, and vice versa. The even total carbon number monoesters consisted of even carbon number fatty acids and alcohols esterified to each other, and odd carbon number fatty acids and alcohols esterified to each other. Averaged across all samples, the distribution of monoesters was bell-shaped with a mode at C30 (15.89% of the total preen wax).
For each individual bird a weighted carbon number was calculated as the summation of each carbon number multiplied by its relative proportion of the monoester mix. There was a marked difference in carbon number distribution between captive and wild birds (F4,36 = 31.34, P < 0.001; Fig. 3). Post-hoc analysis indicated that captive birds had longer chain monoesters in their preen wax compared with wild birds, and that captives fed the sesame oil diet had longer chain monoesters than those fed the fish oil diet. The average percentage Peak B monoester content was calculated by summing the total relative percentage peak area of Peak B monoesters for each individual. Only one peak type was chosen (peak B) because both types sum to 100%, and a difference in one peak indicates an opposite difference in the other peak. The relative amount of Peak B monoesters varied among migratory states and captivity (F4,36 = 3.62,P = 0.014; Fig. 4). Post-hoc analysis indicated that captive birds tended to have more Peak B monoesters compared with wild birds (and significantly so compared with autumn migrants and wintering birds).
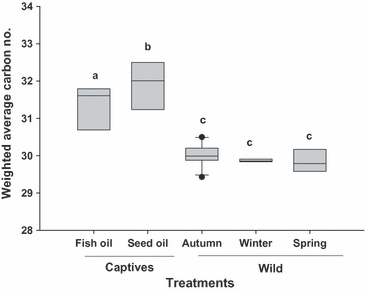
Weighted average carbon number of preen wax monoesters in captive and wild White-throated Sparrows at different seasons and eating different diets. Solid line represents the median. Bars sharing the same letters are not significantly different from each other (P > 0.05), n = 10 for captives, and n = 31 for wild.
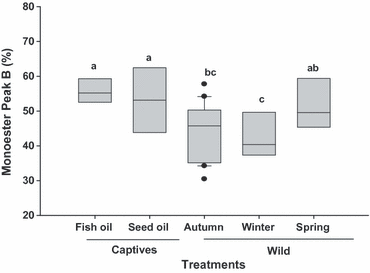
Relative percentage peak area of Peak B of preen wax monoesters in captive and wild White-throated Sparrows at different seasons and eating different diets. Solid line represents the median. Bars sharing the same letters are not significantly different from each other (P > 0.05), n = 10 for captives, and n = 31 for wild.
Preen wax collected directly from the preen gland was similar in chemical composition to that extracted from the feathers (data not shown). Significantly greater amounts of preen wax were present on head feathers in both autumn and spring compared with the primary and tail feathers (F5,12 = 8.38, P = 0.001; Fig. 5). It is important to point out here that to obtain enough material for analysis, feathers from six individuals were pooled into one sample for each feather type (head, tail and primary), and three of these pooled samples were analysed per season (n = 18 total birds per season, but only three pooled samples) for each feather type. Although this method allowed us to obtain sufficient sample material for chemical analysis, it precluded more in-depth statistical analysis (e.g. repeated measures analysis) due to the small independent sample size.
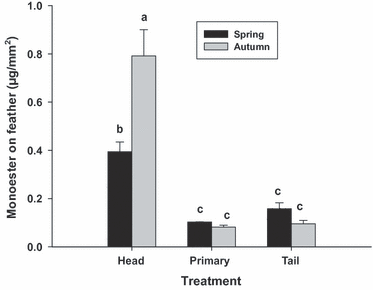
The amounts of monoester preen wax present on the feathers of wild White-throated Sparrows in spring and autumn. Values represent means ± se. Bars sharing the same letters are not significantly different from each other (P > 0.05); n = 3 composite samples per season, each combining six individuals.
Discussion
The complex compound classes of intact preen wax could be easily separated and quantified via thin-layer chromatography with flame ionization detection using a simple solvent system of 100% toluene, followed by redevelopment in 7 : 3 (v/v) toluene/chloroform to resolve alcohols and fatty acids if desired. This method is not only applicable for the easy (compared with GC-MS/FID) analysis of preen wax, but also for the analysis of other waxes found in animals and plants.
Preen wax composition sometimes changes between seasons or migratory states (Reneerkens et al. 2002, 2007a,b). We did not measure preen wax in the breeding season, when diesters are often observed. However, the preen wax of migrating and winter resident White-throated Sparrows contained only monoesters. Analysis of the molecular species of the monoesters revealed many individual monoesters, each having several isomers with varying number of carbons in both the alcohol and acid moiety making up each isomer. Identification of the molecular weights and distribution of the alcohol and acid moieties of the monoester isomers was based on the interpretation of mass spectra of commercial and isolated standards and chromatographic retention data of the resolved peaks. Using mass spectrometry we were able to identify the wax ester isomers present in each chromatographic peak according to methods described in Moldovan et al. (2002).
There have been several studies of the preen wax composition of birds (Kolattukudy et al. 1985, Dekker et al. 2000, Gebauer et al. 2004, Haribal et al. 2005, Montalti et al. 2005). In most studies, preen wax composition was determined by the analysis of fatty acids and alcohols released after hydrolysis of the monoester preen waxes, resulting in the loss of information concerning the molecular distribution of the alcohols and fatty acids making up the preen wax. It was only recently that analysis of the intact preen wax composition has been reported (Dekker et al. 2000, Haribal et al. 2005). These reports provided meaningful information on the molecular distribution of the preen wax components. However, there are still problems encountered with resolution of individual monoesters, with GC traces often showing groups of peaks clustered together (Dekker et al. 2000). Sometimes these clusters confound the interpretation of associated mass spectral data because some molecular ions of interest can show low intensities or may not be present as they are masked by the fragments of co-eluting peaks. We were able to achieve excellent resolution of intact monoesters present in the preen wax and consequently could derive added information on the identity of many of the isomers present in it. The monoesters consisted of homologous series of alcohols (C10–C20) and acids (C13–19) esterified together ranging in chain length of C23–C38. This is in agreement with the composition of White-throated Sparrow preen wax reported by Haribal et al. (2005). These authors found that monoester composition ranged between C24 and C36 with the ester consisting of homologous series of acids ranging from C13 to C17 and alcohols ranging from C13 to C20.
Our study demonstrated the presence of two monoester peaks (in GC-FID/MS) for nearly every carbon number of monoester. The close elution between isomers with the same total carbon number (denoted Peak A and Peak B) and the fact that monoesters having the same combination of acids and alcohols occurred in both peaks suggest that these structural isomers have different amounts of branching on the alcohol or acid moiety or both (Nelson et al. 2001, Moldovan et al. 2002). The acid moiety of monoester preen wax can be un-branched or branched, and often consists of monomethyl, dimethyl and polymethyl fatty acids (Dekker et al. 2000, Sinninghe Damstéet al. 2000, Sweeney et al. 2004). We were unable to confirm whether the location of the branching was on the alcohol or acid moiety of the isomers present in our samples because we did not analyse the hydrolysis products of the monoesters. However, the hydrolysis products of preen wax of White-throated Sparrows were previously analysed and found to consist of between 60 and 80% of 3-methyl-branched fatty acids (Haribal et al. 2005). Therefore, it is likely that the branching is on the acid moiety of the isomer.
Effects of migratory state and diet
Dietary lipids can be incorporated directly into storage, structural and signalling molecules, and thus affect the composition of various avian tissues (Watkins 1991, Xu et al. 1994, Klasing 1998, Thil et al. 2003, Pierce & McWilliams 2005). There has been speculation that at least some components of preen wax are affected by diet (Haribal et al. 2005). However, the fatty acid moieties of preen wax monoesters, particularly methyl-branched fatty acids, are known to be synthesized in the uropygial gland from acetate and propionate precursors (Jacob & Ziswiler 1982, Stevens 1996). Thus, it is unclear how dietary lipids could have altered the composition of preen wax monoesters in our captive Sparrows. Notably, fish oil fatty acids have longer average chain lengths than those in sesame seed oil, yet Sparrows fed the fish oil diet had shorter monoesters than those fed the sesame oil diet. A simple model of direct incorporation of dietary fatty acids into the monoester biosynthetic pathway therefore seems unlikely.
Our results suggest that diet could account for some of the differences in preen wax composition observed among species (Haribal et al. 2005). This finding also demonstrates that diet could play an important role in seasonal changes to preen wax composition, as birds often switch diets between wintering, migratory and breeding phases (Bairlein & Gwinner 1994). Diet should therefore be carefully considered when inferring functional properties of preen wax based on seasonal changes or species differences. Nonetheless, diet cannot account for all seasonal changes in preen wax composition, as Reneerkens et al. (2007b) have demonstrated that Red Knots Calidris canutus exhibit seasonal changes in preen wax composition even with a constant controlled diet. It also seems unlikely that diet can explain switches between monoesters and diesters.
The average carbon number of monoesters did not vary among different parts of the non-breeding season. The wild Sparrows did show a seasonal pattern in the distribution of monoester isomers; specifically, Peak B monoesters increased in relative abundance during spring compared with winter. This change is reminiscent of the seasonal switch to ‘Monoesters B’ in Red Knots that precedes the switch to diesters during breeding (Reneerkens et al. 2007a,b), and we can only speculate that the functional consequences of these changes are similar in Sparrows.
The functions of preen wax and its potential importance to flight
Many functions of preen wax have been proposed (Jacob & Ziswiler 1982). Among these are maintenance of feather quality (Jacob & Ziswiler 1982, Moyer et al. 2003), mate attraction (Piault et al. 2008), scent suppression during incubation (Reneerkens et al. 2005), waterproofing (Elowson 1984) and anti-dermatophytic properties (Jacob et al. 1997). Previous studies have shown that monoesters were the predominant or only compound present in the preen wax of several bird species (Dekker et al. 2000, Sweeney et al. 2004, Haribal et al. 2005). However, just prior to breeding, a shift from monoester to diester preen wax can occur (Kolattukudy et al. 1987, Piersma et al. 1999, Reneerkens et al. 2007b), with incubating birds almost always producing diesters (Reneerkens et al. 2002, 2007a). These findings indicate that certain preen wax compositions are probably optimal for breeding, and thus they provide support for the mate attraction and/or scent suppression hypotheses. But they also suggest that a different composition, such as monoesters, is optimal outside the breeding season either because they are less costly to make or somehow fulfil another function better. Moreover, Reneerkens et al. (2002) reported that the composition of monoesters shifted from smaller to larger carbon numbers during migration, raising the possibility that particular monoester compositions might be advantageous during this life history stage, or as we found, simply reflect changes in diet.
With this in mind, we propose a new hypothesis for a function of preen wax, which is that preen wax can reduce aerodynamic and/or hydrodynamic drag. The phenomenon of drag reduction in a fluid field using wax polymers has been known for the last 50 years (Gyr & Bewersdorff 1995). However, most of the research has been focused on the reduction of drag in hydrodynamic environments due to the physico-chemical interaction between the medium and the wax polymer/surfactant. Several species of fishes and swimming mammals use these waxes on their surface to control the interaction between their bodies and the fluid flow to reduce drag, conserve energy or move faster (Fish & Lauder 2006). In aerodynamics, there are no apparent interactions between the air and surface polymers; nonetheless, the presence of wax on the feathers could aid in smoothing the surface by affecting feather interactions and forcing the fluid flow to align itself over the body. This layer could thus serve as a mechanism to suppress turbulence during locomotion in a fluid field.
Birds appear to have inexplicably low drag coefficients, and live birds have much reduced flow separations compared with dead frozen or mounted birds (Pennycuick 2008). During flight, the head is the first part of the body to penetrate the fluid field and consequently is the first part of the body that experiences fluid resistance (drag) where vortices are created. The greater amount of preen wax on the head than on the primary or tail feathers that we measured could be explained by more efficient application of preen wax to the head by the bird. Yet, it could also be related to a role in reducing drag. Additional support for the drag reduction hypothesis could be found among the families of birds in which most species lack a uropygial gland and are either not long-distance migrants or are entirely flightless (e.g. ostriches (Struthionidae), rheas (Rheidae), cassowaries (Casuariidae), mesites (Mesitornithidae), bustards (Otididae), frogmouths (Podargidae), woodpeckers (Picidae); Johnston 1988, Moyer et al. 2003). Although speculative, it would be valuable to measure the effects of preen wax on fluid flow and drag in flying birds. The analytical methods we describe will be useful in such studies.
Acknowledgments
The authors wish to thank Dr Roi Gurka of the UWO Department of Mechanical and Materials Engineering and Ben Gurion University, Israel, for his enthusiasm, insights into aerodynamics and fluid mechanics, and editorial contribution to the manuscript. Richard Harris of the UWO Biotron provided assistance with microscopy and digital image analysis. Doug Hairsine provided help with GC/MS analysis. Funding was provided to C.G.G. by NSERC (Discovery Grant), the Canada Foundation for Innovation (Leader’s Opportunity Grant), and the Ontario Ministry of Research and Innovation (CFI matching funds and an Early Researcher Award). Funding was provided to M.A.B. by NSERC (Discovery Grant) and the University of Western Ontario Academic Development Fund.




