Are Zucker obese rats a useful model for cardiovascular complications in metabolic syndrome? Physical, biochemical and oxidative stress considerations
Abstract
We wondered if Zucker obese (ZO) rats would be a good experimental model to evaluate cardiovascular complications of metabolic syndrome (MS). ZO rats were compared with both their littermate controls, Zucker lean (ZL) rats and to Wistar rats (reference strain). We designed this work:(i) to measure certain physical and biochemical characteristics of MS; (ii) to evaluate coronary and cardiac function in isolated conditions and after ischemia; and (iii) to study plasma and heart tissue oxidative stress markers. In vivo, ZO rats had higher levels of plasma glucose, cholesterol and triglycerides than their ZL littermates, but there was no difference between the groups for systolic arterial blood pressure and heart rate. In vitro, coronary endothelial function was notably impaired in ZO and ZL rats. After global ischemia, the worse ventricular recovery in ZO and ZL rats was associated with arrhythmias during reperfusion. We detected similar levels of plasma ascorbyle free radical, oxygen radical absorbance capacity and vitamin C concentrations in the three groups. Dihydroethidium staining showed higher superoxide production in the coronary vessels of ZO rats than in ZL and Wistar rats. Our results show that ZO might only correspond to early-stage cardiovascular complications associated with MS.
Introduction
Metabolic syndrome (MS) is present in more than 40% of the over sixties in the US population [1] and a recent study [2] has shown that MS is a strong predictor of cardiovascular events in young patients. In 2005, MS was redefined as the association of abdominal obesity and at least two of the following criteria: high fasting blood glucose, high triglycerides, low high density lipoprotein (HDL)-cholesterol or hypertension [3]. Patients with MS have an increased risk for coronary artery disease, which leads to a 2.6-fold increase in cardiovascular mortality [4]. MS also appears to be associated with a worse in-hospital outcome following myocardial infarction, and a higher risk of severe heart failure [5]. Endothelial dysfunction, which is a key early factor in the development of atherosclerosis and a predictor of cardiovascular events, has been found in patients with MS [6]. Oxidative stress, which is one of the causative factors of endothelial dysfunction, is thought to play a major role in the occurrence and complications of MS [7–9]. Though this syndrome has been well described and documented in humans, it is rarely observed in animals and there is a need for a relevant experimental model of MS.
Zucker obese (ZO) rats have a missense point mutation (fa/fa) of the leptin receptor; this mutation impairs the ability of leptin to bind to the receptor in order to induce satiety and finally results in marked obesity. Different metabolic dysfunctions, such as insulin resistance, hyperglycemia or raised lipid levels have been described in these rats [10]. However, only a few studies have been published on the cardiovascular parameters and oxidative status of ZO rats [11,12], and these have often been discordant.
We therefore wondered if ZO rats would be a good experimental model to evaluate cardiovascular complications of MS, in order to determine if they could then be used to assess different drugs for the prevention of MS-related cardiovascular events. We designed this study: (i) to measure in vivo some physical and biochemical features of MS in ZO rats; (ii) to investigate in vitro different cardiovascular parameters, such as coronary reactivity and heart tolerance to ischemia-reperfusion; and (iii) to evaluate oxidative stress levels in plasma and tissue in this model. Therefore, ZO rats were compared with their littermate controls, Zucker Lean (ZL; fa/fa) rats, whereas Wistar rats were considered the reference strain.
Materials and methods
Chemicals
All drugs and chemicals were bought from Sigma-Aldrich Chimie (Saint Quentin Fallavier, France): metaphosphoric acid, 2,2’-azobis(2-amidinopropane) 4-hydrochloride, β-allophycocyanin, (+/−)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), bradykinin (BK), sodium nitroprusside, triphenyl-tetrazolium chloride (TTC).
Animals
The investigators complied with authorization 21CAE057 from the French government, which ensures respect of the Guidelines for the Protection of Experimental Animals issued by the European Convention. Male 12-week-old Wistar (n = 20), Obese: fa/fa (n = 20) and Lean: Fa/fa (n = 20) male Zucker rats were purchased from Charles River Laboratories (Les Oncins, France).
In vivo measurements
Systolic blood pressure (SBP) and heart rate were measured in vivo by tail cuff plethysmography (LE 5007 pressure meter; Bioseb, Chaville, France) on awake 13-week-old rats. A pneumatic pulse transducer was positioned on the ventral surface of the tail. The SBP and heart rate values recorded were the average of a minimum of six readings. The rats were anaesthetized and killed at 14 and 15 weeks old. At this time body weight and waist circumference were measured.
Blood sampling and biochemical assays
The rats were not fed for the 12 h before being killed. The rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and heparin was intravenously injected (500 UI/kg). Blood samples were collected from the abdominal artery. Glycaemia was directly determined on the samples with a Bayer glucometer 4 (Bayer, Puteaux, France). Other blood samples were centrifuged at 500 gfor 10 min to separate plasma and total cholesterol; HDL, low-density lipoprotein (LDL) and triglycerides were assayed (colorimetric assay, X PAND 2 automaton; Dade Behring, Deerfield, IL, USA).
Preparation and perfusion of isolated hearts
After anesthesia and heparinization of the rats, the hearts were excised and placed in a cold (4 °C) perfusion buffer bath until contractions ceased. Each heart was then immediately cannulated through the aorta and perfused at 37 °C by the Langendorff method at a constant flow corresponding to an initial pressure of 100 cm of water (73 mmHg) in the first protocol and at a constant perfusion pressure equivalent to 80 cm of water (56 mmHg) in the second one. The perfusion buffer consisted of a modified Krebs-Henseleit bicarbonate buffer (KH) (millimolar concentrations: NaCl 118, NaHCO3 25, MgSO4 1.2, KH2PO4 1.2, KCl 4.5, glucose 5.5 and CaCl2 3). The perfusion fluid was filtered through a 0.8 μm Millipore filter to remove any particulate contaminants and gassed with 95% oxygen and 5% carbon dioxide (pH 7.3–7.5 at 37 °C). An elastic water-filled latex balloon (no 4; Hugo Sachs, GmbH, March-Hugstetten, Germany) was inserted into the left ventricle through the mitral valve, connected to a pressure transducer, and inflated to obtain a left ventricular end-diastolic pressure (LVEDP) between 6 and 12 mm Hg (0.8–1.6 kPa). A Gould TA 240 recorder was used to measure intra-ventricular pressures (LVEDP and left ventricular end-systolic pressure (LVSP), left ventricular developed pressure (LVDP = LVSP−LVEDP) and heart rate. The rate-pressure product (RPP) was calculated from the product of LVDP and heart rate. Coronary flow was measured by the timed-collection of the effluent. In order to pool results from both protocols, coronary resistance was calculated as the ratio between the perfusion pressure and coronary flow.
Perfusion protocols
Two different protocols were applied to the isolated hearts.
Coronary reactivity
To evaluate coronary resistance, the hearts were perfused at a constant flow rate; the rate was chosen in order to ensure an initial perfusion pressure of 100 cm of water. After a 10-min stabilization period, BK (5.10−7 m, Sigma), an endothelium-dependent vasodilator, was perfused into the coronary bed for 3 min; then, after a 12-min washout period, sodium nitroprusside (10−6 m, a NO donor, Sigma) was perfused for 3 min in order to evaluate endothelium-independent vasodilatation. At the end of the protocol, the hearts were frozen in liquid nitrogen and stored at −80 °C for further analysis. Those to be used for histology determinations were then embedded in OCT (Dako, Trappes, France) and cut into 10-μm thick sections. The sections were stored at −80 °C.
Ischemia-reperfusion sequence
After a 15-min stabilization period, the hearts were initially observed during 10 min of control perfusion at a constant perfusion pressure of 80 cm of water (pre-ischemic control period). Global no-flow ischemia was then induced by switching off the aortic inflow for 30 min. Throughout this period, a thermoregulated chamber maintained the temperature of the hearts at 37 °C. At the end of ischemia, aortic inflow was resumed for 30 min of reperfusion. An epicardial electrocardiogram was continuously recorded using two fine electrodes.
Measurement of myocardial infarct size
At the end of the ischemia-reperfusion protocol, the hearts were removed from the perfusion apparatus and immediately frozen for 1–4 h. Then, the frozen hearts were cut into five slices of approximately equal thickness (1 mm), which were incubated in 10% TTC in phosphate buffer at 37 °C for 10–20 min. The viable tissue was TTC-positive and stained red, whereas the necrotic tissue was TTC-negative. After staining, the heart slices were fixed in 10% formaldehyde overnight and the area of infarcted tissue was measured by computer morphometry (Histolab, Microvision, Evry, France). The infarct size was expressed as the percentage of the left ventricular surface area.
Determination of lactate dehydrogenase activity
Coronary effluents were sampled during reperfusion and kept at 4 °C until dosage (within a day) with a Roche Diagnostics GmbH (Mannheim, Germany) kit using an ultraviolet spectroscopic method with pyruvate and reduced nicotinamide adenine dinucleotide (NADH) as substrates. Lactate dehydrogenase (LDH) activity was determined by the rate of NADH oxidation by following its change in extinction at 340 nm and at 37 °C. The accumulated amount of LDH released was obtained by integrating the area under the time course curve during the 30-min reperfusion period. Enzyme activity was normalized against coronary flow, heart weight and expressed as units/min/g.
Oxidative stress parameters
Plasma vitamin C measurements
Two volumes of 5% metaphosphoric acid solution were added to plasma samples before freezing and storage. Ascorbate concentration in the plasma was then measured by high performance liquid chromatography using fluorometric detection at 360 nm excitation and 440 nm emission, as previously described [13].
Determination of plasma oxygen radical absorbance capacity
Oxygen radical absorbance capacity (ORAC) of the plasma was determined according to a modified method of Cao et al. [14], which has been described in detail elsewhere [15]. Briefly, the reaction mixture contained a final concentration of 37.5 nmβ-allophycocyanin in 75 mm phosphate buffer, pH 7.0, at 37 °C in the presence or the absence of trolox or diluted plasma (dilution 1 : 500). The reaction was initiated by the introduction of 3 mm of 2,2’-azobis(2-amidinopropane) 4-hydrochloride, and followed spectrophotometrically by the decrease in fluorescence at 598 nm excitation and 615 nm emission. Trolox was used as a reference antioxidant for calculating the ORAC values, with 1 ORAC unit defined as the net protection area provided by 1 μm final concentration of Trolox.
Plasma ascorbyle free radical levels
Plasma ascorbyle free radical (ASC) levels were detected directly on plasma samples by electron paramagnetic resonance (EPR) spectroscopy. EPR spectra were recorded with an ESP 300E-X band spectrometer (Bruker, Wissenbourg, France) using a TM110 cavity and an aqueous flat cell. The following parameters were selected: microwave power, 20 mW; microwave frequency, 9.74 GHz; modulation amplitude, 1.6 G; modulation frequency, 100 kHz; gain, 3.2.106; scan rate, 0.95 G/s; time constant, 163.84 ms; and conversion time, 82 ms. Relative radical concentrations (in arbitrary units, AU) were determined by the measurement of line intensities on spectra recorded with identical spectrometer settings.
Myocardial superoxide-dependent fluorescence
Myocardial superoxide-dependent fluorescence was assessed in heart slices using dihydroethidium (DHE). It is cell permeable and reacts with O2− to form ethidine, which in turn intercalates with DNA and provides nuclear fluorescence. To control for differences in cellular densities, DAPI (4’,6-diamidino-2-phenyindole, Sigma) was used to determine cellular density. Fresh-frozen heart tissues were fixed in acetone for 10 min. Slices were incubated in a dark humidified chamber at room temperature with DHE (5 μm) for 5 min. To verify the specific detection of O2− with DHE, some slices were incubated with superoxide dismutase (SOD, 300 U/mL, Dako) before DHE incubation (data not shown). The slices were immediately analyzed with a computer-based digitizing image system (Microvision) using a fluorescent microscope (Eclipse 600; Nikon, Champigny-Sur-Marne, France) connected to a video camera (Tri CCD; Sony, Paris, France). Fluorescence was detected at 510–560 nm excitation and 590 nm emission filters. Automatic computer-based analysis was performed with the same threshold for all sections (×500 magnification). Results are expressed as the percentage of fluorescence/nuclei.
Statistical analysis
All data are presented as means ± SEM, and tests were performed with α = 0.050. Data were evaluated by one-way anova followed by intergroup pairwise comparisons using Tukey’s test.
Results
In vivo evaluation of physical, cardiovascular and biochemical parameters related to MS
(1) Both body weight and waist circumference of ZO rats were higher than those of ZL rats, which in turn were lower than those of Wistar rats. Nevertheless, no differences were found in heart weights, which were 1.03 ± 0.03 g in the Wistar group, 1.07 ± 0.03 g in the ZL group, and 1.02 ± 0.02 in the ZO group (Table I).
| Wistar (W) (n = 20) | Zucker lean (ZL) (n = 20) | Zucker obese (ZO) (n = 20) | |
|---|---|---|---|
| Body weight (g) | 396 ± 5a | 333 ± 6b | 420 ± 6c |
| Heart weight (g) | 1.03 ± 0.03 | 1.07 ± 0.03 | 1.02 ± 0.02 |
| Waist circumference (cm) | 18.6 ± 0.2a | 17.3 ± 0.2b | 21.2 ± 0.4c |
| Systolic blood pressure (mmHg) | 131.3 ± 2.8 | 126.6 ± 2.2 | 129.1 ± 1.3 |
| Heart rate (beats/min) | 384.2 ± 13.7 | 383.8 ± 9.3 | 401.5 ± 65.9 |
| Glucose (g/L) | 1.09 ± 0.05a | 1.16 ± 0.03a | 1.45 ± 0.05c |
| Total cholesterol (mm) | 1.38 ± 0.08a | 1.75 ± 0.05a | 3.86 ± 0.10c |
| HDL-cholesterol (mm) | 0.29 ± 0.02a | 0.31 ± 0.01a | 0.83 ± 0.03c |
| LDL-cholesterol (mm) | 0.86 ± 0.06a | 1.13 ± 0.05b | 2.48 ± 0.15c |
| Triglycerides (mm) | 0.33 ± 0.07a | 0.24 ± 0.05a | 1.77 ± 0.31c |
- Values are means ± SEM. Means in a row without a common letter differ; P < 0.05.
(2) Wistar rats and ZL rats were comparable with regard to glycemia, total and HDL-cholesterol and triglyceride levels. However, plasma LDL-cholesterol was 30% higher in ZL rats than in Wistar, but the HDL or LDL/total cholesterol ratio was comparable in the three groups. In contrast, ZO rats were found to have higher biochemical markers of MS than ZL rats: 25% higher glycemia, more than 2.2 times the level of cholesterol, and more than seven times the level of triglycerides (Table I).
(3) The SBP and heart rates of the rats, measured in vivo, were around 129 mmHg and 390 beats/min; for these variables, there was no difference between the three groups (Table I).
In vitro cardiovascular parameters
Evaluation of the initial cardiac parameters in isolated hearts
Coronary resistances, intra-ventricular pressure, heart rate and RPP were recorded in basal Langendorff perfusion conditions for 10 min, and the results obtained at the 8th min of perfusion in the two protocols were pooled and are presented in Table II. Initial levels of coronary resistances were similar in all groups. While isolated cardiac parameters were similar for Wistar and ZL rats, ZO hearts were found to have a lower heart rate and a higher LVDP than both ZL and Wistar hearts (P < 0.05).
| Wistar (W) (n = 16) | Zucker lean (ZL) (n = 16) | Zucker obese (ZO) (n = 16) | |
|---|---|---|---|
| Coronary resistances (mmHg min/mL) | 5.07 ± 0.22 | 4.57 ± 0.28 | 4.75 ± 0.21 |
| LVDP (mmHg) | 95.7 ± 3.7a | 92.9 ± 4.2a | 112.5 ± 6.7b |
| Heart rate (beats/min) | 293 ± 4a | 292 ± 10a | 252 ± 9b |
| RPP (mmHg beats/min) | 26 814 ± 1399 | 27 376 ± 1881 | 28 060 ± 1630 |
- LVDP, left ventricular developed pressure; RPP, rate-pressure product.
- Values are means ± SEM. Means in a row without a common letter differ; P < 0.05.
Evaluation of coronary reactivity during isolated heart perfusion
To assess endothelial coronary function in the different groups of rats, we examined endothelium-dependent vasorelaxation in response to 5.10−7 m BK. An 11.2 ± 6.0% decrease in coronary resistances was observed in Wistar rats during BK infusion, suggesting preserved endothelial function (1). BK-induced endothelium-dependent vasorelaxation was significantly lower in Zucker rats. This was evidenced by only a slight decrease or even a paradoxical increase in the coronary pressure in these two groups. Moreover, BK also induced a decrease in the LVDP (−21.95 ± 5.23 mmHg ZO; −15.20 ± 4.01 mmHg ZL; −19.87 ± 6.05 mmHg Wistar groups) and an increase in the heart rate (13.61 ± 7.98 bpm ZO; 4.29 ± 2.16 bpm ZL; 6.05 ± 1.43 bpm W), but there was no difference between the three groups for either variable.
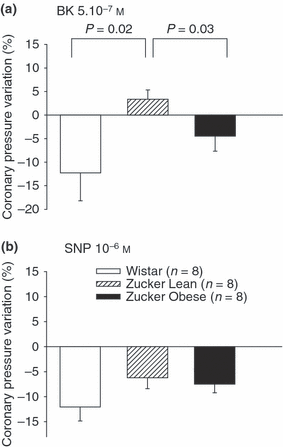
Percentage of coronary pressure variations measured in isolated Wistar, Zucker lean and obese rat hearts after perfusion with (a) bradykinin (BK, 5.10−7 m) and (b) sodium nitroprussiate (SNP, 10−6 m). Values are means ± SEM. The percentage of coronary pressure variation recorded between the beginning and the end of perfusion of the drug is shown.
When 10−6 m sodium nitroprussiate (SNP) was perfused, the coronary pressure dropped by about 7–11% in the three groups. In Wistar rat hearts, 10−6 m SNP-induced vasodilatation was similar in extent to that induced by 5.10−7 m BK, showing that these doses were equally able to dilate the coronary arteries in an endothelium-dependent or independent manner (1).
Evaluation of the hearts’ tolerance to ischemia-reperfusion
The myocardial recovery of functional parameters was evaluated during 30 min of reperfusion following 30 min of total global ischemia.
Coronary flow was only partially restored after 30 min of ischemia, with a level of recovery reaching about 60% (W 61.34 ± 6.23; ZL 57.50 ± 8.87; ZO 59.39 ± 5.87) of the initial value in the three groups (2).
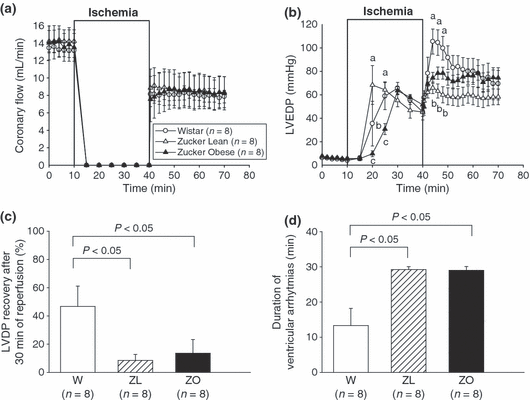
Evolution of different functional parameters of isolated Wistar, Zucker lean and obese rat hearts during 30 min of total global ischemia and 30 min of reperfusion. (a) coronary flow; (b) left ventricular end diastolic pressure (LVEDP); (c) percentage of left ventricular developed pressure (LVDP) recovery between the end of reperfusion and the pre-ischemic period; (d) duration of ventricular arrhythmias occurring during the 30 min reperfusion period. Values are means ± SEM. Means at times with different letters differ; P < 0.05.
During global no-flow ischemia when coronary flow was set at zero, myocardial contractions ceased rapidly, whereas the intraventricular pressure progressively increased (ischemic contracture) reaching a maximum of 65.71 ± 4.91 mmHg in the Wistar group, 66.71 ± 19.18 mmHg in the ZL group, and 64.57 ± 3.70 mmHg in the ZO group after respectively 30, 20 and 30 min of ischemia. The maximum value was reached earlier in ZL rats (2). LVEDP increased rapidly at the onset of the reperfusion and remained high throughout this period (post-ischemic contracture). This post-ischemic contracture was initially higher in Wistar (97.5 ± 12.4 mmHg; at the fourth min of reperfusion) than in Zucker Lean (66.0 ± 4.9 mmHg) or obese (74.5 ± 4.7 mmHg) but, at the end of the reperfusion period, the LVEDP was similar for the three groups (2).
Recovery of LVDP at the end of reperfusion reached 46.68 ± 14.38% in Wistar rat hearts (2); this recovery was significantly lower in both Zucker lean (8.45 ± 4.27%, P < 0.05, ZL vs. Wistar) and obese (13.56 ± 9.72%, P < 0.05, ZO vs. Wistar).
Electrocardiogram monitoring allowed us to evaluate ventricular arrhythmias, such as tachycardia and fibrillation which occurred during reperfusion. These ventricular disturbances were frequently observed after 30 min of a global normothermic ischemia and had an average duration of 13.3 ± 4.9 min in Wistar hearts. The duration of these arrhythmias was significantly higher in both Zucker lean (29.3 ± 0.8 min, P < 0.05) and obese (29.0 ± 1.0 min, P < 0.05) hearts (2).
Coronary effluent was analyzed for LDH, an intracellular enzyme released during post-ischemic reperfusion, to assess the degree of cardiac injury. The release of LDH was not significantly different for the three groups: Wistar, 93.1 ± 4.5 units/min/g; ZL, 76.0 ± 9.1 units/min/g; ZO, 92.6 ± 12.2 units/min/g.
Thirty min of ischemia followed by 30 min of reperfusion was associated with an infarct size of 32.6 ± 3.2% and 33.1 ± 4.5% in Wistar and ZL groups respectively (n = 10 in each group); it increased to 42.5 ± 3.8% in ZO, but this difference was not significant.
Oxidative stress parameters
In the plasma, there was no difference between the three groups for plasma vitamin C concentration, antioxidant capacity (ORAC), or the level of ascorbyle free radical (Table III).
| Wistar (W) (n = 20) | Zucker lean (ZL) (n = 20) | Zucker obese (ZO) (n = 20) | |
|---|---|---|---|
| Vitamin C (μm) | 42.4 ± 3.6 | 39.8 ± 5.3 | 37 ± 4.9 |
| Plasma ORAC value (AU) | 0.29 ± 0.07 | 0.16 ± 0.05 | 0.25 ± 0.05 |
| ASC level (AU) | 5.6 ± 2.5 | 5.7 ± 1.2 | 4.6 ± 1.9 |
- AU, arbitrary units; ORAC, oxygen radical absorbance capacity; ASC, ascorbyle free radical.
We evaluated myocardial superoxide production with DAPI-DHE double staining (3) in frozen heart slices from Wistar, ZL and ZO rats. We observed that DHE mainly stains coronary arteries rather than cardiomyocytes, and observed a significant increase (P < 0.05) in ethidium fluorescence (related to cellular density) in the coronary vessels of ZO as compared with Wistar and ZL rats (respectively 30.40 ± 1.94%/105 cell vs. 18.82 ± 1.66%/105 cell vs. 18.06 ± 1.73%/105 cell).
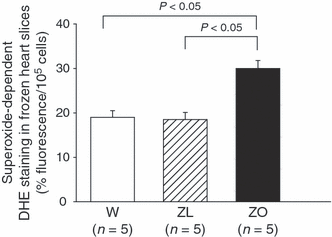
Quantification of superoxide-dependent DHE fluorescence in frozen slices of Wistar, Zucker lean and obese rat hearts, subjected to 30 min of global total ischemia and 30 min of reperfusion. Values are means ± SEM.
Discussion
Our primary goal was to determine whether Zucker rats would be a good experimental model for MS. First, 13-week-old ZO rats presented global obesity, with abdominal pre-dominance (greater waist circumference). This abdominal obesity is known to be linked to insulin resistance, one of the underlying pathophysiological elements of MS [7,16]. Moreover, as has already been reported [17], ZO rats have significantly higher levels of fasting glucose, triglycerides (six–seven times those of ZL and Wistar rats) and LDL-cholesterol. Even though ZO rats presented high HDL-cholesterol, usually considered to be a marker of a good prognosis in cardiovascular disease, this was probably linked to an increase in total cholesterol. The only difference between ZL rats and Wistar rats was the higher level of LDL-cholesterol in the former. Insulin dosage and insulin resistance would also have been relevant early markers of MS [16]. In vivo, systolic arterial blood pressure and heart rate were similar in all of the 13-week-old non-anesthetized rats. However, according to the literature, hypertension seems to appear later in ZO rats, around the 4th month of life [18,19]. It seems therefore that our 3-month-old ZO rats could represent a model for the early stages of MS.
The second aim of this study was to appreciate in vitro, with Langendorff isolated perfused heart preparations, the cardiovascular function of ZO rats. Regarding initial cardiovascular parameters, ZO rat hearts presented higher LVDP and lower heart rates than in the other rats. This intrinsic lower heart rate has already been described by Wang et al. in 6-, 12- and 24-week-old ZO rats. Wang also found a higher LVDP, but the difference was not significant [20]. Studies on in vivo ventricular function assessed by echocardiography gave mixed results; in some, improved function in ZO rat hearts [21] was found, while other authors reported a reduction in contractile function [22]. In our study, ZO rats tended to be tachycardiac in vivo; this tendency was reversed in vitro, where neurohumoral influences disappeared, suggesting for instance an increase in circulating catecholamines, which has already been observed in ZO rats [23], and in obese humans [24].
In isolated heart experiments, bradykinin perfusion showed that coronary endothelial function was impaired in both ZO and ZL rats, as we observed the absence or even a paradoxical contractive response to bradykinin perfusion, while endothelium-independent vasorelaxation assessed by SNP was preserved in the three groups. Several studies have already demonstrated that ZO presented impaired endothelium-dependent vasorelaxation induced by different drugs on different vessels: adrenomedullin on aortic rings [25], and arachidonic acid on small mesenteric arteries [26]. Circulating hyperleptinemia, which is due to the presence of the fa allele in the Zucker strain [27] may be involved in coronary endothelial dysfunction in ZO rats. Impaired BK-induced endothelium-dependent vasorelaxation has already been observed in different ‘pathologic’ animal strains such as hypertensive deoxycorticosterone acetate-salt rats [28]. Nevertheless, it was surprising to find this impaired response in ZL rats, which have few clinical-biological components of MS. Several hypotheses can be raised: either this endothelial alteration is related more to the difference of strain than to the MS, or raised LDL-cholesterol was sufficient to impair coronary BK response, or ZL rats represent an incomplete form of MS, which may be responsible for this impairment.
Another finding of our work was the poor recovery of LVDP in ZO and ZL rats, associated with the persistence of ventricular arrhythmias during reperfusion following 30 min of total global ischemia. In fact, coronary endothelial dysfunction appears to play an important part in the ventricular arrhythmias occurring during reperfusion. Regarding other data published on the tolerance of Zucker rat hearts to ischemia-reperfusion, we can note that results diverge. Some studies showed good recovery after 30 min of low-flow ischemia [20], whereas others demonstrated impairment [17]. Surprisingly, in ZO and ZL rats, neither LDH release nor the extent of the infarcted zone increased significantly during reperfusion, even though, in ZO hearts, the extent of the infarct tended to be greater than that in the other two groups. Hoshida et al. [29] showed that there was no difference between ZO and ZL rats for infarct size after 30-min of left coronary artery occlusion followed by 24-h reperfusion. Several physiopathological mechanisms could explain this slight increase in cell death: first, the absence of increased oxidative stress in plasma, but which was present in vascular tissue; secondly, the endothelial dysfunction seen in both Zucker groups.
As the imbalance between oxidative species and antioxidant components is often associated with MS, for the third aim of this study, we investigated basal oxidative stress in the plasma and tissue of 14-week-old Zucker and Wistar rats. Considering the numerous cardiovascular risk factors present in ZO rats, we could expect to find increased oxidative stress in them. However, in our experiments, we detected similar plasma ASC• levels via EPR, similar ORAC values and similar plasma vitamin C concentrations in the three groups. Indeed, whereas ORAC allows global evaluation of plasma oxidative defences, it does not allow determination of the part played by every ‘protagonist’ involved in these antioxidant defences. Some studies demonstrated a paradoxical increase in antioxidant enzyme activity (catalase, SOD) in 12 or 13-week-old ZO rats [11,12]. In heart tissue, however, our results using DHE showed that the vascular superoxide producing capacity was markedly greater in ZO than in Wistar controls or ZL, and that this staining was highly reduced by SOD (data not shown). We observed that the increase in superoxide levels was not confined to endothelial cells but also occurred in the media and adventitia; this vascular distribution has previously been reported [30].We had previously observed that aging was associated with increased production of oxidizing species evaluated by ethidine fluorescence in vascular tissues [31]. In our experimental conditions, the SOD-inhibitable increase in superoxide production in vascular tissue was not associated with the development of hypertension. Our results are in accordance with previous studies which found higher myocardial tissue levels of lipid peroxidation in 12-week-old ZO rats [12]. However, only a few data are available in the literature concerning the measurement of oxidative stress in ZO rats by EPR. Sonta et al. [32] observed that the spin clearance rates of 3-carbamoyl-2,2, 5, 5-tetramethylpyrrolidin-1-oxyl assessed by in vivo EPR were higher in 11-week-old ZO rats than in ZL rats, which corroborates our findings of a higher degree of oxidative stress in the hearts of ZO rats as compared to ZL. Therefore, while the evaluation of global markers of plasma oxidative stress does not show that 13-week old ZO rats are more exposed to radical species, it is noticeable that their coronary vessels produce higher amounts of superoxide anion. This early and much localized event could later induce in older ZO rats a higher susceptibility to cardiovascular complications of MS.
In conclusion, 13 to 15-week-old ZO rats appear to be an interesting experimental MS model, as they associate several clinical, biological and metabolic alterations: abdominal and global obesity, hyperglycemia, and raised plasma cholesterol and triglyceride levels. We also demonstrated that tissue oxidative stress was higher in the coronary arteries of ZO rats than in the other two groups. Studies on isolated hearts revealed higher LVDP and a lower heart rate in ZO rats, suggesting increased catecholamine activity in these obese animals, which is similar to what has already been observed in obese humans. However, despite this higher vascular superoxide production, 12-week-old ZO rats are too young to present systolic hypertension. It seems therefore that our 3-month-old ZO rats could represent a model for the early stages of MS. On the other hand, both ZO and ZL rats present coronary endothelial dysfunction, with a paradoxical vasoconstrictive response to BK coronary perfusion, associated with impaired recovery after ischemia-reperfusion due to prolonged ventricular arrhythmias. In our model, the endothelium could be responsible for the poor tolerance of Zucker rats to the myocardial I/R sequence. Finally, our results show that 3-month-old ZO rats, despite several biochemical, physical and oxidative stress markers for MS are not distinctly more susceptible to ischemia reperfusion injury than their lean littermates, and might therefore correspond to the early stages of cardiovascular complications associated with MS.
Acknowledgements
This work was supported by Region Bourgogne, and Association de Cardiologie de Bourgogne. We kindly thank Franck Ménetrier for the histological preparation, and Philip Bastable for the English revision.




