Synthesis of neurotransmitter GABA via the neuronal tricarboxylic acid cycle is elevated in rats with liver cirrhosis consistent with a high GABAergic tone in chronic hepatic encephalopathy
Abstract
J. Neurochem. (2011) 117, 824–832.
Hepatic encephalopathy (HE) is a neuropsychiatric complication to liver disease. It is known that ammonia plays a role in the pathogenesis of HE and disturbances in the GABAergic system have been related to HE. Synthesis of GABA occurs by decarboxylation of glutamate formed by deamidation of astrocyte-derived glutamine. It is known that a fraction of glutamate is decarboxylated directly to GABA (referred to as the direct pathway) and that a fraction undergoes transamination with formation of alpha-ketoglutarate. The latter fraction is cycled through the neuronal tricarboxylic acid cycle, an energy-generating pathway, prior to being employed for GABA synthesis (the indirect pathway). We have previously shown that ammonia induces an elevation of the neuronal tricarboxylic acid cycle activity. Thus, the aims of the present study were to determine if increased levels of ammonia increase GABA synthesis via the indirect pathway in a rat model of HE induced by bile-duct ligation and in co-cultures of neurons and astrocytes exposed to ammonia. Employing 13C-labeled precursors and subsequent analysis by mass spectrometry, we demonstrated that more GABA was synthesized via the indirect pathway in bile duct-ligated rats and in co-cultures subjected to elevated ammonia levels. Since the indirect pathway is associated with synthesis of vesicular GABA, this might explain the increased GABAergic tone in HE.
Abbreviations used:
-
- BDL
-
- bile-duct ligation
-
- GAD
-
- glutamate decarboxylase
-
- HE
-
- hepatic encephalopathy
-
- LC-MS
-
- liquid chromatography-mass spectrometry
-
- MCL
-
- molecular carbon labeling
-
- TCA
-
- tricarboxylic acid
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome with symptoms ranging from mild cognitive impairment to coma, which occurs as a consequence of acute or chronic liver disease (Hazell and Butterworth 1999; Muñoz 2008). It is well established that hyperammonemia plays a pivotal role in the pathogenesis but the exact biochemical mechanism is not completely understood (Butterworth 2002; Felipo and Butterworth 2002). Increased ammonia concentrations in plasma, cerebral spinal fluid and brain have been related to HE and there is a significant correlation between the plasma ammonia level and the severity of the disease (Kramer et al. 2000; Ong et al. 2003). It has been suggested that GABAergic neurotransmission is enhanced in HE (Schafer and Jones 1982; Jones and Basile 1998). In line with this, a generalized CNS depression, characterized by impaired motor function and decreased consciousness, is a distinct clinical feature of HE (Basile 2002). In this context, increased GABA release, decreased GABA uptake by astrocytes and increased GABAA receptor stimulation by the neurotransmitter per se or by neurosteroids have been reported (Wysmyk et al. 1992; Oja et al. 1993; Bender and Norenberg 2000; Ahboucha and Butterworth 2007). Ammonia detoxification in the brain occurs primarily via the astrocytic enzyme glutamine synthetase (Schousboe and Waagepetersen 2007; Norenberg and Martinez-Hernandez 1979). Increased glutamine synthesis has been demonstrated in both cultured astrocytes and co-cultures of neurons and astrocytes exposed to ammonia as well as in animal models of HE (Chatauret et al. 2002; Zwingmann and Butterworth 2005; Leke et al. 2011). The enzyme glutamate dehydrogenase, expressed in both neurons and astrocytes, is also likely to participate in the cerebral detoxification of ammonia (Yudkoff et al. 1990; Ott et al. 2005; Schousboe and Waagepetersen 2007; Dadsetan et al. 2011; Leke et al. 2011). In line with this, it has been shown that ammonia via reductive amination by glutamate dehydrogenase of α-ketoglutarate to glutamate can be incorporated into alanine via subsequent transamination catalyzed by alanine transaminase, a mechanism that might be involved in disposal of excess ammonia in GABAergic neurons (Dadsetan et al. 2011; Leke et al. 2011). During physiological conditions, glutamine has a key function in the so-called GABA-glutamine cycle which operates at the GABAergic synapse to replenish neurotransmitter GABA lost by uptake into the surrounding astrocytes (Bak et al. 2006a). In this shuttle, glutamine is synthesized and released by astrocytes and subsequently taken up by neurons where it is deamidated to glutamate by mitochondrial phosphate-activated glutaminase. According to the classical view, glutamate is subsequently decarboxylated to GABA via the cytosolic enzyme glutamate decarboxylase (GAD). However, it has previously been shown that synthesis of vesicular (i.e. neurotransmitter) GABA involves the tricarboxylic acid (TCA) cycle to a significant extent (Waagepetersen et al. 1999; Walls et al. 2011). In previous studies, we have shown that exposure to ammonia leads to an elevated activity in the neuronal TCA cycle of both glutamatergic and GABAergic cultured neurons (Johansen et al. 2007; Leke et al. 2011). Thus, the aims of the present study were to determine if increased levels of ammonia increase GABA synthesis via the indirect pathway in a rat model of HE induced by bile-duct ligation (BDL) and in co-cultures of neurons and astrocytes exposed to ammonia. The BDL rat is a reliable experimental model to study HE since it represents the pathophysiological mechanisms of chronic liver disease and chronic HE (Butterworth et al. 2009). Co-cultures of GABAergic neurons and astrocytes have been previously studied and demonstrated to be a good model for the study of astrocytic-neuronal metabolic interactions (Leke et al. 2008). Hence, metabolism of [U-13C]acetate (3 mM) was investigated in the BDL rats as well as in co-cultures of GABAergic neurons and astrocytes exposed to 5 mM ammonium chloride. [U-13C]Acetate can only be metabolized in astrocytes (Waniewski and Martin 1998) and 13C derived from [U-13C]acetate is incorporated into newly synthesized GABA in neuronal-astrocytic co-cultures via transfer of labeled glutamine from astrocytes to neurons (Leke et al. 2008). Moreover, in order to investigate the fate of exogenously supplied glutamine, co-cultures were incubated with [U-13C]glutamine in the absence and presence of 5 mM ammonium chloride. Extracts of cerebral cortex and cultured cells were analyzed by liquid chromatography-mass spectrometry (LC-MS) to evaluate labeling in GABA as well as glutamate, aspartate, glutamine and alanine.
Materials and methods
Materials
Female NMRI mice of 15-days gestation and 12- to 13-week-old female Wistar rats (mean body weight 200 g; range 192–214 g) were from Taconic (mice from Borup, rats from Ry, Denmark). Plastic tissue culture dishes were purchased from NUNC A/S (Roskilde, Denmark), fetal bovine serum from GIBCO, Invitrogen (Taastrup, Denmark). Culture medium and poly-d-lysine (MW > 300 000) were from Sigma Chemical Co. (St. Louis, MO, USA). Penicillin was from Leo (Ballerup, Denmark). Isotopically labeled compounds (13C) were all labeled > 95% and were from either Cambridge Isotopes Laboratories, Inc. (Cambridge, MA, USA) or Isotec (a subsidiary of Sigma Chemical Co.). All other chemicals used were of the purest grade available from regular commercial sources.
Experimental cirrhosis in rats
Experimental cirrhosis in rats was induced by ligation of the common bile duct 6 weeks before the experiment and evaluated as previously described by Bak et al. (2009). Liver tissue was sampled for histological examination using Haematoxylin & Eosin and Masson’s Trichrome staining. Three primary changes for cirrhosis were assessed: disturbed architecture, bile duct proliferation with displacement of hepatocytes, and formation of septa between portal areas containing bile duct cells and connective tissue. The control group consisted of sham-operated animals.
Cerebral metabolism of [U-13C]acetate in BDL rats
The rats were fasted for 3 h prior to the experiment. At the time of the experiment the rats were weighed and subsequently given i.p. injections of [U-13C]acetate four times during a 24-min time period totaling a dose of 3.2 μmol [U-13C]acetate per g of body weight. Two min after the last injection, the animals were subjected to inhalation anesthesia and the abdomen was opened. Blood (1 mL) was taken from the aorta for measurement of arterial blood ammonia. The rats were decapitated and the brains removed and frozen in liquid nitrogen within 40–50 s after the blood sampling. Within 1 week, the brains were dissected and the forebrain was used for analysis. The tissue samples were extracted in 70% v/v ethanol (ice–cold) and the extracts centrifuged (20 000 g, 20 min). The supernatants were analyzed for labeling in amino acids as described below.
Co-cultures of cortical neurons and astrocytes
Co-cultures of cortical (GABAergic) neurons and astrocytes were prepared employing cerebral cortices from 15 gestation day mouse fetuses, as previously described by Leke et al. (2008). The cultures were maintained in 6-well culture dishes (35 mm). This co-culture system has been described as a useful tool to study metabolic interactions between astrocytes and GABAergic neurons, since it exhibits functional exchange of metabolites such as glutamine (Leke et al. 2008). Moreover, this cell culture model has recently been used to study the effect of high ammonia levels (Dadsetan et al. 2011; Leke et al. 2011).
Incubation experiments
After 7–8 days in vitro, co-cultures were pre-incubated in the presence or absence (controls) of 5 mM NH4Cl for 1 h at 37°C. At the end of the incubation period the medium was aspirated and the cultures were rinsed twice in phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 7.3 mM Na2HPO4, 0.9 mM CaCl2, 0.5 mM MgCl2, pH 7.4, 37°C). Subsequently, they were incubated for 2.5 h in serum free culture medium (2 mL per well) containing one of the following four different combinations of substrates in the presence or absence of 5 mM NH4Cl: (i) 3 mM [U-13C]acetate, 2.5 mM glucose and 1 mM lactate; (ii) 0.1 mM [U-13C]glutamine, 2.5 mM glucose and 1 mM lactate; (iii) 0.3 mM [U-13C]glutamine, 2.5 mM glucose and 1 mM lactate; or (iv) 0.5 mM [U-13C]glutamine, 2.5 mM glucose and 1 mM lactate. After termination of the incubation, the medium was aspirated and the cultures washed twice with ice-cold phosphate buffered saline. Subsequently, the cells were extracted in 70% v/v ice-cold ethanol and the extracts centrifuged (20 000 g, 20 min, 4°C). Cell extracts were lyophilized and reconstituted in water for biochemical analyses.
Biochemical analysis
The Phenomenex EZ:faast amino acid analysis kit for LC-MS was used for analysis of labeling in glutamate, aspartate, GABA and glutamine. Mass spectrometric analysis was performed on an LC-MS system consisting of a Shimadzu LCMS-2010 mass spectrometer coupled to a Shimadzu 10A VP HPLC system (Shimadzu corp., Kyoto, Japan). Plasma ammonia levels were determined as previously described (Van Anken and Schiphorst 1974).
Data analysis
Data analysis was performed employing Microsoft Excel 2007 (Microsoft Denmark, Hellerup, Denmark) and GraphPad Prism v4.01 software (San Diego, CA, USA). All labeling data were corrected for natural abundance of 13C by subtracting the mass distribution of a standard containing the relevant metabolites. Isotopic enrichment was calculated according to Biemann (1962). The average percent of 13C-labeled carbon atoms, i.e. the percent molecular carbon labeling (MCL) was calculated as initially introduced by Bak et al. (2006b) in glutamate, GABA, aspartate, alanine and glutamine. All data are presented as averages ± the standard error of the mean (SEM). Differences between groups were analyzed statistically by one- or two-way anova followed by Bonferroni post hoc test except for the plasma ammonium levels where a Student’s t-test was employed. A p-value of less than 0.05 was considered statistically significant.
Results
Experimental liver cirrhosis
At 6 weeks post-surgery, bile duct-ligated (BDL) rats exhibited yellowish color of the tail and fur and one-third had ascites. All BDL rats had severe microscopic changes in the liver showing cirrhosis as evaluated by a trained pathologist, and all sham-operated rats had normal histology of the liver. Liver weight was significantly higher in the BDL rats (on average 16.8 g, range 10.8–21.3 g) than in the sham-operated rats (on average 8.0 g, range 7.2–10.2 g; p < 0.001). Both sham and BDL rats gained weight during the post-surgical period. Plasma levels of ammonia were significantly higher in BDL rats compared to sham rats (95 ± 16 and 37 ± 7 μmol/L in BDL and sham-operated rats, respectively; p < 0.05).
Labeling patterns in GABA, glutamate, glutamine and aspartate from [U-13C]acetate and [U-13C]glutamine
A simplified schematic cartoon of 13C-labeling in glutamine, glutamate and GABA resulting from metabolism of [U-13C]acetate and [U-13C]glutamine, respectively, is provided in Fig. 1(a and b). [U-13C]Acetate is selectively taken up by astrocytes, transformed to [1,2-13C]acetyl-CoA and subsequently metabolized in the astrocytic TCA cycle giving rise to α-[4,5-13C]ketoglutarate which is subsequently converted to [4,5-13C]glutamate. [4,5-13C]glutamate then serves as substrate for formation of [4,5-13C]glutamine (i.e. double-labeled glutamine as indicated in Fig. 1a). [4,5-13C]Glutamine is subsequently released into the extracellular compartment and taken up by neurons where it is deamidated to [4,5-13C]glutamate. This can be converted to GABA via either one of two different pathways: (i) it can be decarboxylated directly to [1,2-13C]GABA (i.e. double-labeled GABA as indicated in Fig. 1a; referred to as direct synthesis) or (ii) enter the TCA cycle via transformation to α-[4,5-13C]ketoglutarate and after one full turn of the cycle be converted to α-[3-13C]- or α-[1,2-13C]ketoglutarate, which are then transformed to [3-13C]- or [1,2-13C]glutamate giving rise to [3-13C]- or [4-13C]GABA (i.e. mono-labeled GABA as indicated in Fig. 1a); referred to as indirect synthesis; see Waagepetersen et al. 2001). However, mono-labeling of GABA could also be the result of astrocytic metabolism of [4,5-13C]glutamate via the TCA cycle prior to conversion to glutamine resulting in mono-labeled ([3-13C])glutamine which may be transferred to the neurons for GABA synthesis via the direct pathway. The fact that mono-labeled glutamine is only formed in minute amounts (Fig. 2d) shows that this was not the case. Thus, double-labeled GABA reflects direct synthesis and mono-labeled synthesis via the TCA cycle in the neurons (Fig. 1a). It is important to realize that GABA can only be labeled if labeled glutamine is released from the astrocytic compartment and taken up into neurons where synthesis of GABA takes place. Double-labeled aspartate (i.e. [1,2-13C]- or [3,4-13C]aspartate) might be formed from further metabolism of α-[4,5-13C]ketoglutarate in the neuronal or astrocytic TCA cycle and subsequent transamination of the resulting 13C-labeled oxaloacetate to aspartate. [1-13C], [2-13C], [3-13C] or [4-13C]aspartate will be formed during the second turn of the TCA cycle if labeled oxaloacetate condenses with an unlabeled acetyl-CoA. Triple-labeled isotopomers of glutamate, glutamine and aspartate arise if 13C-labeled oxaloacetate condenses with [1,2-13C]acetyl-CoA in the astrocytes, since acetate only accumulates in these cells (Waniewski and Martin 1998). When [U-13C]glutamine is employed as substrate, direct neuronal synthesis of GABA via [U-13C]glutamate leads to [U-13C]GABA whereas synthesis of GABA via the TCA cycle results in [3,4-13C]GABA (i.e. double-labeled) from [1,2,3-13C]glutamate (i.e. triple-labeled; see Fig. 1b).
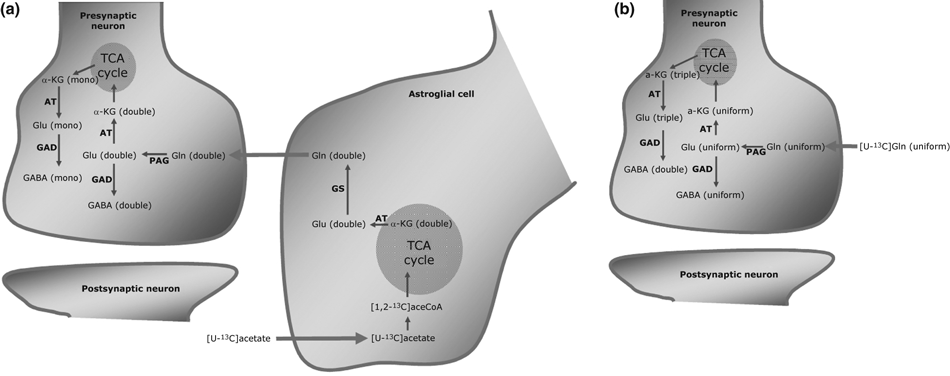
(a) Simplified schematic cartoon showing how mono and double labeling in GABA derived from [U-13C]acetate metabolized in astrocytes is employed to distinguish between GABA synthesized via the TCA cycle or directly from astrocyte-derived glutamine, respectively. (b) Simplified schematic cartoon showing how double and uniformly labeled GABA derived from neuronal metabolism of [U-13C]glutamine is employed to distinguish between GABA synthesized via the TCA cycle or directly from exogenous glutamine, respectively. See text for additional details. Abbreviations: α-KG, α-ketoglutarate; AT, aminotransferase; GAD, glutamate decarboxylase; GDH, glutamate dehydrogenase; Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; TCA, tricarboxylic acid.
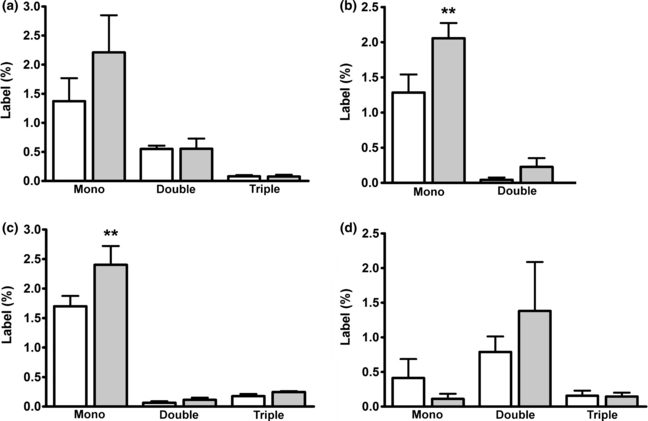
Labeling (%) in glutamate (a), GABA (b), aspartate (c) and glutamine (d) in forebrain extracts from sham operated (n = 6, open bars) and BDL rats (n = 6, grey bars) treated with four injections of [U-13C]acetate (total dose 3.2 μmol/g body weight). Results are presented as averages ± SEM. **p < 0.01 by anova followed by Bonferroni post hoc test when comparing the sham-operated group with the BDL group.
Cerebral metabolism of [U-13C]acetate in BDL animals
No changes in labeling of glutamate was detected in BDL animals (Fig. 2a); however, significant increases were observed in percent mono-labeled GABA and aspartate when compared to the sham-operated animals (Fig. 2b and c; both p < 0.01). This indicates a shift towards indirect synthesis (i.e. via the TCA cycle as described above) as opposed to direct synthesis of GABA. Labeling of glutamine in the BDL animals was not different from the sham-operated animals (Fig. 2d).
Metabolism of 13C-labeled acetate in co-cultures of neurons and astrocytes
In order to obtain direct information about the effects of ammonia on neuronal GABA synthesis, a co-culture system of GABAergic neurons and astrocytes was employed in combination with [U-13C]acetate (3 mM). Incorporation of 13C from acetate in glutamate, GABA, aspartate and glutamine is shown in Fig. 3(a–d). Data are presented as percent MCL (see Materials and Methods and Bak et al. 2006b for details). The presence of ammonium chloride (5 mM) did not affect the MCL for any of the four amino acids analyzed (Fig. 3a–d). Glutamine was extensively labeled (Fig. 3d), which is in accordance with the notion that acetate is selectively taken up and metabolized in astrocytes (Waniewski and Martin 1998). The different isotopomers of glutamate, GABA, aspartate and glutamine from the same experiment are shown in Fig. 4(a–d). As expected, extensive double labeling was observed in glutamine and the cultures exposed to ammonium chloride exhibited a significant increase in the percent of double-labeled glutamine reflecting an increased flux through the glutamine synthetase-catalyzed reaction (Fig. 4d; p < 0.001). Moreover, the percent of mono and triple-labeled glutamine was significantly lower when compared to the control condition (Fig. 4d, p < 0.001). Glutamate and aspartate exhibited a decrease in the mono-labeled isotopomer after exposure to ammonium chloride (Fig. 4a and c; p < 0.01 and p < 0.05, respectively). The co-cultures exposed to ammonium chloride showed a significant decrease in double-labeled GABA when compared to the control cultures (p < 0.05), showing that GABA synthesis directly from glutamine-derived glutamate was diminished (Fig. 4b).
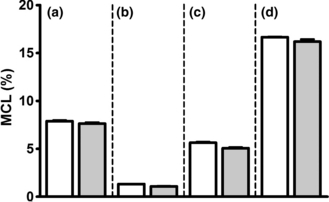
Average incorporation of 13C (MCL, %) in glutamate (a), GABA (b), aspartate (c) and glutamine (d) in co-cultures of cortical neurons and astrocytes incubated in serum-free culture medium containing 3 mM [U-13C]acetate, 2.5 mM glucose and 1 mM lactate in the presence (grey bars) or absence (open bars) of 5 mM ammonium chloride for 2.5 h. The ammonia group was pre-incubated for 1 h in serum-free culture medium with 5 mM ammonium chloride. Results are averages ± SEM of four to five cultures.
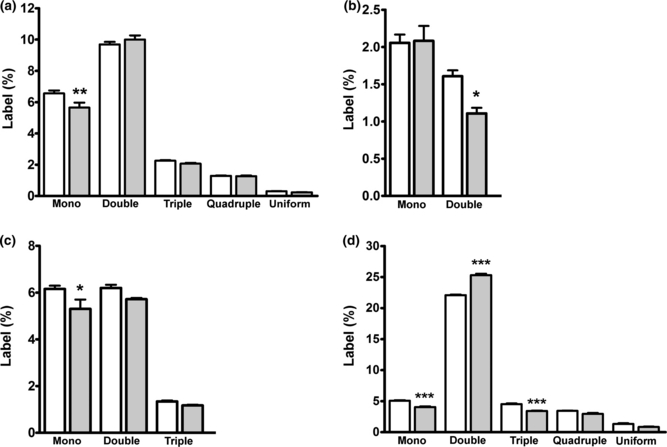
Labeling (%) in glutamate (a), GABA (b), aspartate (c) and glutamine (d) in co-cultures of cortical neurons and astrocytes incubated in serum-free culture medium containing 3 mM [U-13C]acetate, 2.5 mM glucose and 1 mM lactate in the presence (grey bars) or absence (open bars) of 5 mM ammonium chloride for 2.5 h. The ammonia group was pre-incubated for 1 h in a serum-free culture medium with 5 mM ammonium chloride. Results are averages ± SEM of four to five cultures. Statistical analysis was performed by anova followed by Bonferroni post hoc test. *p < 0.05, **p < 0.01 or ***p < 0.001 when comparing the cultures exposed to ammonia with the control cultures.
Metabolism of 13C labeled glutamine in co-cultures of neurons and astrocytes
The effect of ammonia on GABA synthesis via the indirect pathway involving the neuronal TCA cycle could theoretically reflect the increased availability of extracellular glutamine under this condition. To test this, metabolism of [U-13C]glutamine at three different concentrations (0.1, 0.3 and 0.5 mM) was investigated in the co-culture system. The data from the control experiments (i.e. no ammonium chloride present) has been published previously (Leke et al. 2008). As shown in Fig. 5, the MCL of glutamate, GABA, aspartate and glutamine (Fig. 5a–d) was significantly increased as a function of the [U-13C]glutamine concentration (open bars, p < 0.001). Moreover, the co-cultures incubated with ammonium chloride exhibited a decrease in MCL in all four amino acids, regardless of the external glutamine concentration (grey bars; p < 0.01). This may reflect an increase in de novo synthesis of non-labeled glutamine from glucose.
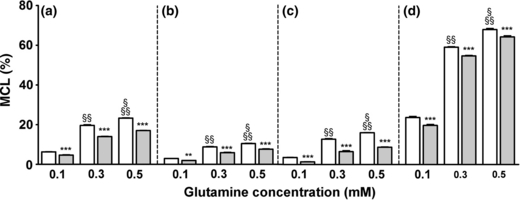
Average incorporation of 13C (MCL, %) in glutamate (a), GABA (b), aspartate (c) and glutamine (d) in co-cultures of cortical neurons and astrocytes incubated with three different concentrations of glutamine (0.1, 0.3 and 0.5 mM) in the presence (grey bars) or absence (open bars) of 5 mM ammonium chloride for 2.5 h. The ammonia group was pre-incubated for 1 h in serum-free culture medium with 5 mM ammonium chloride. Results are averages ± SEM of four to five cultures. Statistical analysis was performed by two-way anova followed by Bonferroni post hoc test. ***p < 0.001 or **p < 0.01 when comparing the cultures exposed to ammonia (gray bars) with the respective control condition (open bars). §p < 0.001 when comparing 0.5–0.3 mM glutamine control group. §§p < 0.001 when comparing the 0.5 mM and the 0.3 mM to the 0.1 mM glutamine control group.
In order to evaluate to what extent synthesis of GABA via the TCA cycle at the different glutamine concentrations was affected by ammonium chloride, uniform labeling in glutamate and GABA, representing direct synthesis without TCA cycle involvement, was compared to the sum of mono, double (only GABA) and triple (only glutamate) labeling representing synthesis via the TCA cycle. It should be mentioned that unlabeled astrocyte-derived glutamine might also be metabolized in the neurons under these conditions thereby promoting the entry of acetyl-CoA by providing TCA cycle intermediates. Figure 6(a and b) shows these results calculated as ratios; thus, a ratio below 1 indicates preferential synthesis via the TCA cycle and vice versa. For glutamate (Fig. 6a) the ratio was below 1.0 at all glutamine concentrations and a significant concentration dependency was observed as the ratio at 0.1 mM glutamine was significantly lower than that found at 0.3 and 0.5 mM glutamine (open bars; p < 0.001). Exposure to ammonium chloride significantly reduced the ratios at all levels of glutamine (grey bars; p < 0.05). Contrary to what was observed for glutamate synthesis, the pathway for GABA synthesis involved direct synthesis to a larger extent than the indirect pathway via the TCA cycle, a finding independent of the glutamine concentration (Fig. 6b). This indicates that endogenous (i.e. from astrocytes) and exogenous glutamine are metabolized differently, that is, heterogeneity of the neuronal glutamate pools. Exposure to ammonium chloride had no effect on the pathway for GABA synthesis regardless of the external glutamine concentration (Fig. 6b).
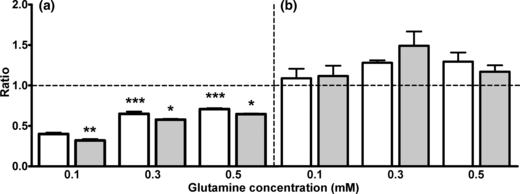
Ratios of uniform labeling in glutamate (a) or GABA (b) divided by the sum of mono, double (only GABA) and triple (only glutamate) in co-cultures cortical neurons and astrocytes incubated with three different concentrations of glutamine (0.1, 0.3 and 0.5 mM) in the presence (grey bars) or absence (open bars) of 5 mM ammonium chloride for 2.5 h. The ammonia group was subjected to a pre-incubation of 1h in serum-free culture medium with 5 mM ammonium chloride. Results are averages ± SEM of four to five cultures. Statistical analysis was performed by anova followed by Bonferroni post hoc test. ***p < 0.001 when comparing the cultures incubated 0.3 and 0.5 mM glutamine with the 0.1 mM glutamine group. *p < 0.05 or **p < 0.01 when comparing the cultures exposed to ammonia with the control condition.
Discussion
Synthesis of GABA in hyperammonemia
The results of present study suggests that GABA synthesis from astrocytic glutamine via the indirect pathway (i.e. involving the TCA cycle, an energy-generating pathway) was enhanced in the BDL rat model of chronic HE compared to sham-operated animals. These findings were supported by data from co-cultures of GABAergic neurons and astrocytes which showed a decrease in direct synthesis of GABA when subjected to ammonium chloride. Thus, the overall result is that GABA synthesis via the indirect pathway was stimulated by hyperammonemia. It has been shown both in vivo and in different in vitro model systems that GABA synthesis to a large extent involves glutamine metabolism through the TCA cycle and that this pool of GABA is associated with the vesicular neurotransmitter pool (Walls et al. 2010, 2011; Waagepetersen et al. 2001; Hassel et al. 1998; Westergaard et al. 1995). In agreement with the presence of distinct pools of GABA (i.e. vesicular and cytoplasmic), GAD is expressed as two different isoforms in the brain, GAD67 and GAD65. GAD67 is found widespread throughout neuronal cell bodies whereas GAD65 is primarily localized in axon terminals (Stone et al. 1999). Furthermore, it is likely that GAD65 is related to synthesis of vesicular GABA, while GAD67 is fundamental for cytoplasmic GABA as well as synthesis of vesicular GABA (Tian et al. 1999; Waagepetersen et al. 2001). Altogether, the present findings may indicate that hyperammonemia results in an increased availability of vesicular GABA that is consistent with an enhanced GABAergic tone in HE, as discussed below. Experiments performed employing rabbits with fulminant liver failure as well as studies of other animal models of chronic and acute HE have indicated that GABAergic neurotransmission may be enhanced albeit overall levels of GABA in the brain and cerebrospinal fluid in the experimental animals and patients with HE seemed normal (Jones and Basile 1998; Ahboucha and Butterworth 2004). It should, however, be noted that both GABA transporter activity and GABAB receptor expression have been shown to be reduced (Wysmyk et al. 1992; Oja et al. 1993; Bender and Norenberg 2000) which may result in an increased synaptic availability of GABA to activate GABAA receptors. This together with studies demonstrating that GABAA receptor expression is influenced by hyperammonemia and that ammonia directly or via an increased production of neurosteroids potentiates GABA neurotransmission (Takahashi et al. 1993; Ha and Basile 1996; Li et al. 2005; Ahboucha and Butterworth 2007; Cauli et al. 2009) supports the notion that HE is associated with an increased GABAergic tone.
Is a high level of extracellular glutamine affecting the biosynthetic pathways for GABA?
One mechanism that could explain the favored GABA synthesis via the TCA cycle during elevated ammonia is the ammonia-induced increase in glutamine synthesis and release by the astrocytes as has been shown in different model systems (Butterworth 2002; Zwingmann and Butterworth 2005; Zwingmann and Leibfritz 2005; Leke et al. 2011). To investigate this possible mechanism, the co-cultures were incubated with three different concentrations of [U-13C]glutamine. While the incorporation of 13C in the amino acids glutamate, GABA, aspartate and glutamine was dependent on the exogenous [U-13C]glutamine concentration, exposure to ammonia decreased the 13C incorporation in all amino acids, including GABA regardless of the glutamine concentration. This may be explained by increased de novo synthesis of unlabeled glutamine because of pyruvate carboxylase activity since ammonia has been shown to increase pyruvate carboxylase activity and glutamine synthesis in different models of HE (Kanamatsu and Tsukada 1999; Sibson et al. 2001; Zwingmann et al. 2003). Furthermore, glutamate synthesis from glutamine occurred preferentially via the TCA cycle while that of GABA was mediated both via the TCA cycle and via direct synthesis. These results indicate the presence of a compartmentalized glutamate pool, a phenomenon that has been described previously (Westergaard et al. 1995; Leke et al. 2008; Waagepetersen et al. 2008). This compartmentation was only marginally affected by ammonia.
Synthesis of GABA is coupled to increased TCA cycle activity
The results described here indicate that GABA synthesis via the TCA cycle, an energy-generating pathway, is enhanced during hyperammonemia and that this is independent of the extracellular glutamine concentration. This may be related to the previous finding that hyperammonemia increased glycolysis and TCA cycle activity in cultures of GABAergic cortical neurons (Leke et al. 2011) as well as in glutamatergic cerebellar neurons (Johansen et al. 2007). In addition, increased activity of a number of TCA cycle enzymes has been reported in brain homogenates derived from rats treated with an acute dose of ammonium acetate (Ratnakumari et al. 1986). However, to what extent this TCA cycle enhancement is the only mechanism responsible for the increased GABA synthesis via the TCA cycle is still unclear. It should also be considered that ammonia may have an effect on the enzymes regulating GABA metabolism. Previous findings showed unchanged activity of GAD in patients and animal models of liver failure (Ahboucha and Butterworth 2004). However, total GAD activity might not represent the GABA synthesis in close connection with the vesicular pool. Further experiments are necessary to better clarify the effects of HE and ammonia on the specific isoforms of GAD and other enzymes which regulate GABA metabolism. To what extent the increase in the GABA synthetic pathway connected with the vesicular GABA pool is important for an enhancement in the GABAergic tone awaits further studies. However, the increase in cerebral GABA content in BDL compared to sham-operated animals indicate that the observed effects on the GABA synthetic machinery are important in the pathology of HE. Moreover, the changes observed in GABA synthesis described here might also be important for understanding behavioral impairments associated with HE.
Summary
In summary, we demonstrate that GABA synthesis occurs preferentially via the indirect pathway (i.e. via the TCA cycle) and that this pathway is stimulated by ammonia in cell cultures and in BDL animals. This might be a consequence of increased TCA cycle activity and thus links the ammonia-induced increase in GABA synthesis via the indirect pathway to neuronal energy metabolism. Since the indirect pathway is associated with synthesis of vesicular GABA, we propose that this explains the increased GABAergic tone associated with HE.
Acknowledgement
The skilful technical assistance of Ms Lene Vigh and Ms Mette Simonsen as well as the expert secretarial assistance of Ms Hanne Danø are highly appreciated. Professor Stephen J.H. Dutoit, Department of Pathology, Aarhus University Hospital, is cordially acknowledged for assisting in the histological evaluation of liver from BDL animals. A travel grant to RL from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Capes is cordially acknowledged. The experimental work was supported by grants from the Danish Medical Research Council (09-063399 and 09-065565). The Carlsberg Foundation is acknowledged for a post doc. grant to LKB.




