Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress
Abstract
J. Neurochem. (2010) 112, 13–23.
In the last 40 years, especially with the application of new neurochemical and molecular biological techniques, there has been explosive progress in understanding how certain ligands and drugs are transported across the blood-brain barrier and choroid plexus out of brain and CSF. In the CNS, there are several separate efflux transporters with very broad specificity that are responsible for much of the efflux transport. This review focuses on three such transporters: organic acid transporter-3, peptide transporter-2 and P-glycoprotein for which there is substantial new information including ‘knockout’ models in mice and, in one case, dogs. Moreover, the structural biology and transport mechanism of P-glycoprotein at 3.8 angstroms is described. The overall objective is to show how this new knowledge provides a more thorough understanding (e.g., of molecular mechanisms) of efflux transport and in several cases leads to clinically relevant information that allows better treatment of certain CNS disorders (e.g., meningitis and brain cancer).
Abbreviations used:
-
- AA
-
- ascorbic acid
-
- BBB
-
- blood-brain barrier
-
- BCRP
-
- breast cancer resistance protein
-
- BCSFB
-
- blood-CSF barrier
-
- CP
-
- choroid plexus
-
- DPH
-
- diphenhydramine
-
- KO
-
- knockout
-
- OAT-3
-
- organic acid transporter 3
-
- PEPT-2
-
- peptide transporter 2
-
- P-GP
-
- P-glycoprotein
When I began my studies of ligand transport through the blood-brain (BBB) and blood-CSF barrier (BCSFB) in 1971, there was substantial knowledge of the anatomy and histology of the BBB and BCSFB as outlined in Davson’s (1970) magisterial book. Then, as now, the BBB and BCSFB were understood to depend on tight junctions between the cerebral capillaries (BBB), and the choroid plexus (CP) epithelial and arachnoid membrane cells (BCSFB), respectively. Moreover, Davson (1970) artfully explained the theory that ligand passage between blood and brain or CSF depended on lipid solubility, molecular size and degree of ionization. However, Davson pointed out there was newer data that iodide, penicillin, and other organic acids were transported out of CSF faster than expected (Becker 1961; Pappenheimer et al. 1961;Welch 1962; Fishman 1964). Moreover, there was also data that the excised CP incubated in artificial CSF concentrated iodide over 20 times by an energy dependent process (Welch 1962). Was the CP responsible for clearing iodide from CSF in vivo? We now know it is (Davson 1970).
To my eye, at that time, one of the most striking comparisons of steady-state ligand distributions in the CNS was between penicillin G and ascorbic acid (AA) (Spector and Lorenzo 1973, 1974), both water-soluble charged molecules of similar size and shape. In animals and humans, CSF concentrations of penicillin G and AA were ∼ one and four-hundred percent those of the plasma concentrations, respectively. This was obviously not consistent with the then current distribution theories. Moreover, the ratio between brain and plasma for these two molecules was even greater. Further doubts arose (about the then current distribution theories) from inspection of Hammarstrom’s (1966) whole body mouse autoradiographic pictures of (14C) ascorbic acid after intravenous injection. I was surprised to see the CP ‘light up’ first, then the CSF and over time the brain closest to CSF. Finally the whole brain lit up uniformly like a white ball. It certainly looked like the CP was transferring AA from blood into CSF; the AA was then entering brain from the CSF. We now know this occurs in mammals, why and how (Spector and Lorenzo 1973; Spector and Johanson 2006; Spector 2009).
We also know there are multiple (hundreds) of separate transporters (Table 1), many of these in the cerebral capillaries or CP (Hediger et al. 2004). Over the last 40 years, there has been substantial progress in understanding these transporters. In this review we will focus on three efflux transporters in the CNS at the BBB and/or BCSFB (Table 1b). In the past, much of the work in this area has been done using traditional techniques, e.g., in vitro radiochemical studies with isolated CP and brain endothelial cells to study ligand uptake, two chambered studies using tight-junctioned monolayers of forebrain capillary endothelial cells or CP epithelial cells to show directionality of transport between apical (lumen in capillaries and CSF-side in CP) and basolateral sides, ventriculo-cisternal perfusion studies to measure influx or efflux from the CSF compartment, brain efflux studies, intraventricular injection studies and whole animal distribution studies. Moreover, in vitro in freshly removed CP, Bresler et al. (1979) and we (Spector 1980b) were able to observe visually (with fluorescein and riboflavin, respectively, using fluorescence microscopy) ligands going from the medium, then being concentrated in the cytoplasm of CP epithelial cells and finally being concentrated on the basolateral side of the CP. Subsequent studies with confocal fluorescence microscopy confirmed and extended these results (Breen et al. 2002). These studies clearly showed there were two steps: a concentrative transport step at the apical membrane and a second (concentrative) efflux step at the basolateral membrane of the CP (Fig. 1). In other words, there were two systems involved – one at the CP epithelial apical membrane ‘in’ and a second one at the basolateral membrane ‘out’. In later studies it became clear that both riboflavin and fluorescein were transported on the same CP apical system – the organic acid transporter 3 (see Table 1 and below). Unlike AA which is transported into CSF from blood via CP (Spector and Johanson 2006), fluorescein and riboflavin are transported out of CSF into blood through CP by a complex bi-step system. However, the AA system in CP is also a bi-step system with sodium-dependent vitamin C transporter 2 transporting AA into CP from the blood side and by an unknown mechanism releasing AA from the CSF side.
| (a) |
| Solute carrier family; SLC (Hediger et al. 2004)a |
| 43 subfamilies |
| > 300 members |
| ATP-binding cassette transporter family; ABC |
| 7 subfamilies |
| > 40 members |
| Nutrient transporters |
| (b) |
| SLC 22 A8 – OAT-3; Organic acid transporter 3 |
| SLC 15 A2 – PEPT-2; Peptide transporter 2 |
| ABCB1 – P-GP; P-glycoprotein; multidrug resistant protein 1, MDR1 |
- aCapital letters, human genes; small letters, animal genes.
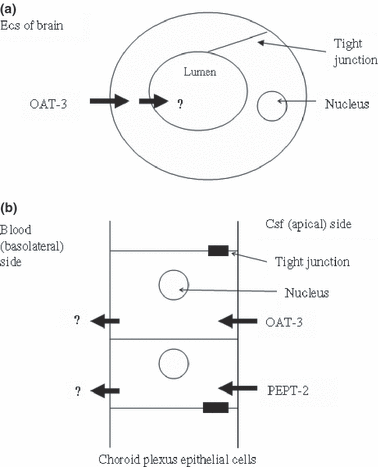
(a) The carrier mechanism for vectorially transporting oat-3 ligands (e.g., penicillin G) across the capillary endothelial cells is displayed. Oat-3 ligands are actively transported from the extracellular space (ECS) of brain across the abluminal membrane (black arrow) into the endothelial cell cytoplasm, and then released into the capillary lumen by an unknown (?) carrier mechanism. (b) Oat-3 and pept-2 ligands are transported vectorially across the choroid plexus from CSF into blood. Shown in (b) are the apical locations for the separate transporters oat-3 and pept-2 (black arrows), which transport ligands ‘uphill’ against a concentration gradient into the CP cytoplasm. The ligands, if not metabolized intracellularly, are then released across the basolateral (blood) side by unknown (?) carrier-mediated mechanisms. In the case of oat-3 the basolateral mechanism is uphill.
From these and other data, specific efflux transporters were postulated at the BBB or BCSFB. However, a substantial amount of confusion arose about transporter specificity, species differences, location and implications for human biology and pharmacology.
During this time, in parallel, clinical neurochemists and others solved certain problems phenomenologically. For example, clinicians established cephalothin was not useful (in fact it was dangerous) for the treatment of bacterial meningitis whereas the larger cephalosporin, ceftriaxone and the related imepenem were useful even though the causative bacteria were equally sensitive to these three antibiotics in vitro (Spector 1990). It was not until many years later that basic scientists explained the neurochemical basis for these results (Spector 1986; Suzuki et al. 1987, 1989: See below). Another example is the use of cancer chemotherapy drugs – many of which are extremely neurotoxic (e.g., vinblastine, taxol) yet could be safely given to patients. It was only with the discovery of the existence and function of P-glycoprotein (P-GP) (Table 1) at the BBB that this mystery was at least partially solved. Finally, it was known for years that collie dogs but not other dogs were exquisitely sensitive to the neurotoxicity of ivermectin, an anti-parasitic drug. Why? Once again it was ultimately established that collie dogs were P-gp knock-outs (See below).
Over the last 15 years, there has been tremendous progress with the application of modern neurochemical, immunological and molecular biological techniques. Although the early workers were sagacious in postulating a large number of specific systems at the BBB or BCSFB – systems that transport therapeutically important or toxic ligands out of brain and CSF, until recently the evidence was mainly indirect (e.g., kinetic.) In this paper we will review some of the newer direct results – focusing exclusively on efflux transporters in the CNS.
But before beginning we should now briefly place the BBB and BCSFB in proper perspective. Then we must explain the criteria necessary to draw correct conclusions in transport biology in the CNS – a complex area – to avoid the mistakes of the past.
We now know the BBB and BCSFB have four general functions; nutrition of the brain, homeostasis, protection of the brain especially from toxins, and bidirectional signaling. This is done by mechanisms shown in Table 2. As noted above, our focus will be on number 3, Table 2.
| (1) Anatomical barriers |
| (2) Specific nutrient/homeostatic systems for macronutrients (e.g., glucose), micronutrients (e.g., vitamins) and ions at both BBB and CP |
| (3) Multiple broadly promiscuous efflux systems |
| (4) Enzymatic protection at BBB and CP (e.g., dopa decarboxylase; CYP450) |
| (5) Bidirectional signaling transporters (e.g., for leptin) |
| (6) Protein synthesis (e.g., transthyretin in CP) |
| (7) CSF production by CP for transfer of ligands in/out of CSF, brain buoyancy, sink effect, and many other functions |
Efflux transporters: criteria for functionality in vivo
What are the criteria for identifying and characterizing a protein as an efflux transporter at the BBB and/or the BCSFB? We and others have suggested the methodological criteria outlined in Table 3 (Spector 2000; Pritchard and Miller 2005). These criteria are stringent and burdensome but there are reasons for this. For example, unlike the nutrient (influx) carriers, which have fairly strict ligand preferences (e.g., the CP AA transporter basically transports only AA; its stereoisomer has only 10% the affinity for the transporter – sodium-dependent vitamin C transporter 2) (Spector and Johanson 2006), some of the efflux transporters are almost unbelievably promiscuous (See Table 4) and there is overlapping specificity in some cases as discussed below. Moreover, in a few cases, even inserting the same transporter gene into Xenopus eggs or alternatively mammalian cells, one obtains not just quantitative kinetic differences, but qualitative differences (Pritchard and Miller 2005) making interpretation problematic. Hence, to be certain of conclusions, one needs to have all the criteria derived from the methods and techniques in Table 3– consistent and coherent.
| (1) Gene isolation |
| (2) Messenger RNA – presence in capillaries and/or CP epithelial cells, and quantitation |
| (3) Transport proteins |
| Characterization including purification |
| Post-translational changes e.g., glycosylation |
| Quantitation and mechanism of action |
| (4) Location of transporter protein in vivo |
| Capillaries – luminal or abluminal side or both |
| CP – apical (CSF) or basolateral side or both |
| (5) In vitro uptake studies with fresh CP or capillaries, or monolayers |
| With radiochemicals or |
| Fluorescence |
| (6) Expression of transporter in mammalian cells or Xenopus eggs with: |
| Kinetic studies – direct or inhibition type |
| (7) In vivo studies with radioligands |
| Whole animal |
| Ventriculocisternal perfusions |
| Influx through BBB |
| Efflux out of brain |
| Intraventricular injections |
| (8) Knockout (mice) or other species without transporter |
| In vitro in capillaries and/or isolated CP |
| In vivo– ligand distribution versus ‘wild’ type; other studies in 7 above |
| (9) Coherent and consistent kinetics in 5, 6, 7, 8 above |
| Direct studies |
| Inhibition studies |
| Specificity and mechanism of action |
| (10) Human studies |
| Human gene |
| As many of 2 through 9 as possible |
| Oat-3 | Pept-2 | P-gp |
|---|---|---|
| Penicillin G (330)a | Tripeptides (∼300) | Ivermectin (∼865) |
| Cimetidine (252) | Aminolevulinic acid (131) | Digoxin (780) |
| Riboflavin (376) | Dipeptides (∼200) | Loperamide (477) |
| Lumichrome (242) | Cefadroxil (381) | Vinblastine (811) |
| Cephalothin (396) | Carnosine(226) | Domperidone (425) |
| Cyclosporin (1190) |
- aMolecular weights in parentheses.
The reasons for focusing on organic acid transporter 3 (oat-3; non-human), P-gp and peptide transporter-2 (pept-2) (Table 1) are four-fold. First, beside different specificities, they have different mechanisms. Second, they are located in different places: oat-3 is located on both the CP apical and abluminal brain capillary membrane; pept-2 is located on the apical choroid plexus membrane but not in or on cerebral capillaries; P-gp is located at the luminal brain capillaries wall. Third, all three transporters can meet most of the criteria in Table 3 [e.g., there are healthy knockout (KO) mice for all three]. Moreover, there is more convincing data for these three, in my view, than for most of the other CNS efflux transporters, and individual compounds can be found specific for these transporters (e.g., penicillin for oat-3; cefodroxil for pept-2 and ivermectin for P-pg) (Table 4). Finally, the unraveling of the transport mysteries of these three brought extremely important practical medical and pharmacological data.
In what follows, I will not follow the historical patterns of discovery but will review data that are relevant to these transporters today. At the end in the general discussion, I will briefly review the ontogeny (development) of these transporters, the speculation for the existence of these transporters (e.g., from an evolutionary point of view) as well as fit those transporters into modern life – especially with regard to medicine and toxicology. I will also make comments on our ability to predict the fate of exogenous compounds vis a vis the systems in Table 4, and our ability to use the techniques involved in the characterization of those transporters as pharmaceutical screening methods.
Organic acid transporter 3 (non-human)
The evidence is coherent and overwhelming that oat-3 is a powerful promiscuous active efflux transporter (secondary type) in rodent and human CNS (Table 5) (Deguchi et al. 2006;Kikuchi et al. 2003; Kusuhara and Sugiyama 2005; Miller 2004; Nagata et al. 2002; Ogawa et al. 1994; Sykes et al. 2004; Sweet et al. 2002, 2003). However, unlike P-gp (discussed below) which has been purified, crystallized and carefully studied (see below), we have little idea how oat-3 works on a molecular level. Although we have excellent kinetic and high resolution visual (fluorescence microscopy) data of the first uphill step into CP epithelial or capillary endothelial cells, we do not know how oat-3 ligands are then transported uphill across the CP basolateral or capillary luminal barriers, respectively (Table 5; Fig. 1).
| (1) Gene – rat cDNA 536 amino acids with molecular mass of 92 kDa |
| (2) Messenger RNA – localized in CP epithelial cells and brain capillaries |
| (3) Transport protein – glycosylated; hydropathy analysis 12 transmembrane domains – putative physiological mechanism: OAT3 transporter is an exchanger driven by outward concentration gradient of dicarboxylates coupled to the sodium gradient. Molecular mechanism unknown |
| (4) Transporter location – apical (CSF) side of CP; abluminal side of cerebral capillary |
| (5) In vitro studies with CP – show concentration by apical membrane and further concentration across basolateral membrane; monolayers of porcine CP epithelial cells consistent, See text |
| (6) Transporter expressed in mammalian cells and Xenopus oocytes |
| (7) In vivo studies (using techniques 7 a → e; Table 3) show oat-3 ligands (Table 4) predominantly transported out of CSF and brain |
| (8) KO studies show normal mice with loss of oat-3 activity in CP; in vivo, probenecid and penicillin block oat-3 ligand transport out of CSF |
| (9) Coherent and consistent kinetics in: |
| Direct studies (See text) |
| Inhibition studies in 5→8 above |
| Specificity very broad (Table 4) |
| (10) Human OAT3 gene cloned – 543 amino acids with putatively 12 transmembrane domains; kinetics and in vivo behavior in humans (where available) similar to rodent. Human OAT3 present in human CP and endothelial cells |
In the following discussion, I will focus on penicillin G, cimetidine, riboflavin and fluorescein because (with the exceptions documented below) these ligands seem to be mainly transported out of the CNS by oat-3. They all have a KT (one half saturation concentration) of between 10 and 100 μM for oat-3 in vitro and in vivo, and where studied, mutually inhibit each others transport competitively as expected (Table 6). The data in Table 6 are from rabbits but comparable data exists in rats and pigs. Sodium iodide which has a powerful transport system in rabbit CP has no effect on penicillin or riboflavin transport (Table 6) (Spector 1980a).
| Riboflavin | Penicillin | |
|---|---|---|
| K T a | 78 μM | 42 μM |
| K I a | 77 μM (penicillin) | 45 μM (riboflavin) |
| Inhibitor ligand (μM) | (14C) riboflavin (0.7 μM)% controlb | (14C) penicillin (0.1 μM) % controlb |
| Control | 100 | 100 |
| Fluorescein (10) | 54* | 52* |
| Sodium iodide (103) | 136 | 128 |
| Lumichrome (100) | 25* | – |
| Probenecid (100) | 44* | 22* |
- a K T = one-half saturation concentration determined directly.
- a K I = one-half saturation concentration determined indirectly.
- bRabbit choroid plexuses were incubated for 15 min (riboflavin) or 5 min (penicillin) in artificial CSF containing either 0.7 μM riboflavin or 0.1 μM (14C) penicillin under 95% O2 : 5% CO2 in a metabolic shaker. At the end of the incubation the ratio of (14C) in the tissue to medium was determined.
- *p < 0.01.
In the case of penicillin G which at steady state in normal animals or humans achieves a CSF concentration of 1–2% that of plasma (and this is not because of plasma protein binding), the outward clearance of penicillin G from the rabbit CSF compartment divided by the inward clearance is ∼14 times consistent with the in vivo distribution data (Spector and Lorenzo 1973). After intracerebral injection, (14C) penicillin is also cleared from brain by oat-3. Suzuki et al. (1997) have estimated that ∼ three-quarters of penicillin cleared from the CNS is by oat-3 in the CP; the remainder by oat-3 at the BBB or by CSF bulk flow. In vivo, probenecid, a blocker of oat-3 function, increases the distribution of CSF to plasma penicillin (rabbit) (Spector and Lorenzo 1973) and cimetidine (rat) (Nagata et al. 2004) (Table 4) by three and four times, respectively.
In oat-3 KO mice, fluorescein uptake by the isolated CP is less than one-quarter that of ‘wild’ mice, thus convincingly showing the role of CP in fluorescein uptake in rodents (Sweet et al. 2003). These studies nicely complement the in vitro uptake studies with fluorescein and riboflavin in CP and the in vivo fluorescence studies described above.
It should be noted that for both penicillin G (Suzuki et al. 1997) and riboflavin (Spector 1980a) there are separate inward transport systems at the BBB that have high affinity constants (KT ∼ 10 μM and 0.1 μM, respectively) and low capacity. The molecular nature of these systems is unknown. In the case of penicillin, this system is overwhelmed by the oat-3 efflux systems in CP and brain capillaries. For riboflavin, see below.
Finally, to study the directionality and kinetics of ligand transfer through the CP, Galla and associates (Hakvoort et al. 1998; Kusuhara and Sugiyama 2004) grew tight-junctioned porcine CP epithelial cell monolayers on porous supports. They measured the directionality and kinetics of ligand transport. Their results are summarized in Table 7. Penicillin, riboflavin and fluorescein were transported as expected – apical to basal against a concentration gradient; penicillin G competitively inhibited fluorescein and riboflavin transport with kinetics similar to in vitro kinetics in the isolated intact CP. These studies (taken as a whole) leave little doubt of the role of oat-3 in animals and, as far as the evidence goes, in humans.
| Substrate | Transport direction | K T (μM) | V max (nmol/cm2 × h) |
|---|---|---|---|
| Ascorbic acid (SVCT2) | b → a | 67 ± 12 | 3.9 ± 0.3 |
| Penicillin G | a → b | 107 ± 8 | 1.8 ± 0.1 |
| Fluorescein | a → b | 22 ± 2 | 1.9 ± 0.1 |
| +400 μM | 108 ± 21 | 1.8 ± 0.1 | |
| Penicillin G | |||
| Riboflavin | a → b | 78 ± 4 | 1.8 ± 0.1 |
| +400 μM | 217 ± 22 | 2.2 ± 0.3 | |
| Penicillin G |
- K T, one-half saturation concentration; Vmax, maximum transport rate; SVCT2, sodium-dependent vitamin C transporter 2; b, basal; a, apical (CSF side).
The situation with riboflavin in the CNS is reminiscent of riboflavin handling by the kidney (Spector 1982). In kidney riboflavin is normally almost totally reabsorbed (by a specific uncharacterized system in the tubular cells) from the glomerular filtrate to conserve riboflavin. (The normal plasma riboflavin is ∼ 0.2 μM). Above 0.5 μM plasma riboflavin, like penicillin G, is secreted into urine by oat-3 on the abluminal tubular membrane and by an unknown mechanism at the tubular luminal membrane.
In vivo, in the CNS, riboflavin is transported into brain by a high affinity system (0.1 μM) at the BBB not sensitive to inhibition by penicillin or probenecid (Spector 1980a). From the CSF, riboflavin is very rapidly cleared into blood by a system in CP; the reason for riboflavin clearance by oat-3 in CP is obscure (Spector 1980a).
The oat-3 and pept-2 systems (discussed below) in the CNS have very important implications for clinical neurochemistry. For example, as noted above, some penicillins, cephalosporins and penems have great utility in the treatment of meningitis; others none even though the causative bacterial organisms are sensitive. We now understand the reasons and they are principally kinetic, because of oat-3 and/or pept-2 (Table 8) (Spector 1986; Suzuki et al. 1987, 1989). Antibiotics that are transported out of CSF by oat-3 (e.g., penicillin G and cephalothin) or by pept-2 (e.g., cefadroxil; see below) do not achieve consistent high CSF concentrations even in meningitis. It is the degree and time above the MBC (minimum bacteriocidal concentration) in CSF that counts. As noted in Table 8, the two antibiotics that have minimal affinity for oat-3 or pept-2 are useful in patients with meningitis. In humans, the half-life of ceftriaxone in CSF is 17 h; of penicillin G and cephalothin, if measurable, just a few hours (Lutsar et al. 1998).
| Transporter | Ligand |
|---|---|
| Oat-3 | Penicillin G; cephalothin |
| pept-2 | Cefadroxil |
| Neither | Ceftriaxone; imepenem |
Peptide transporter 2
The characteristics of rodent pept-2 are shown in Table 9 (Shu et al. 2002; Ocheltree et al. 2004, 2005; Smith et al. 2004; Hu et al. 2007; Shen et al. 2007; Kamal et al. 2008). In vitro, pept-2 from animals and humans transports a large number of dipeptides including carnosine and tripeptides, and the antibiotics cefadroxil and cephalexin – the later two having an α-amino group (Table 7). However, cephalothin and cephaloridine, which do not have an α-amino group, are not transported by pept-2 but by oat-3 (Shu et al. 2002). Para-aminohippuric acid (a ligand for oat-3) at 1 and even 5 mM does not inhibit cefadroxil transport by isolated CP, thus excluding oat-3 from the uptake of cefadroxil (Ocheltree et al. 2004). Finally, it is worth noting that the sartans (e.g., losartan) are the most potent inhibitors of human PEPT-2 in vitro (Brandsch 2009). However, sartans are not transported by human PEPT-2 (Brandsch 2009). The implication of this finding in humans needs exploration in vivo.
| (1) Gene – cDNA 729 amino acids with molecular mass of 85 kDa |
| (2) Messenger RNA – localized in CP epithelial cells but not brain capillaries |
| (3) Transport protein – glycosylated; hydropathy analysis suggests 12 transmembrane domains – putative physiological mechanism: inwardly directed proton gradient and negative membrane potential |
| (4) Transporter location: apical side of CP |
| (5) In vitro studies with CP – show concentration by apical membrane; monolayers of CP epithelial cells in vitro show apical → basal transport. How unmetabolized ligands efflux from CP uncertain |
| (6) Rodent transporter expressed in mammalian cells and Xenopus oocytes |
| (7) In vivo techniques show pept-2 ligands (Table 4) transported out of CSF |
| (8) KO mice with loss of PEPT2 activity are normal; in vivo, distribution studies show five and eight times as much cefadroxil and aminolevulinic acid in CSF respectively in KO mice |
| (9) Coherent and consistent kinetics in: |
| Direct studies in vivo and in vitro |
| Inhibition studies |
| Specificity – peptide backbone of 2 or 3 amino acid residues with both free amino and carboxyl group – preference for l-amino acids |
| (10) Human PEPT-2 cloned – 80% homology with mouse gene: expressed in mammalian cells with similar kinetics; cefadroxil not useful in meningitis |
The discovery and characterization of pept-2 was a major scientific and clinical advance because we can now classify most penicillins, cephalosporins and penems into ligands for oat-3 and/or pept-2 or neither. This way of looking at these drugs allows an explanation of what drugs are useful in meningitis or other bacterial CNS infections, i.e., drugs with no or minimal affinity for organic acid transporter 3 (OAT-3; human) or PEPT-2 are more likely to be useful in meningitis – e.g., ceftriaxone, cefotaxime and imipenem.
There are two potential problems with our interpretation of these data. First, in the human central nervous system, there are relatively few or no data. For example, in human CP we do not know for certain that PEPT-2 is present on the apical cell membrane. There is however indirect data that like in mice, KO of the PEPT-2 gene exists. In a public data base from living humans, one such (non-functional) gene was found (Terada et al. 2004). It is also noteworthy that homozygous KO pept-2 mice are born normal and develop normally (Shen et al. 2003).
Finally, there is one cephalosporin, cefadizime that does not fit into the above paradigm (Matsushita et al. 1991). Unlike penicillin G and ceftriaxone, which have a low capacity saturable influx system (barely detectable) at the BBB (Matsushita et al. 1991), cefodizime readily penetrates into brain and CSF from blood by a saturable system sensitive to penicillin G inhibition. The nature of this influx system remains unknown. It should also be noted that cefodizime (as expected) is useful for the treatment of meningitis in humans.
P-glycoprotein
The characteristics of P-gp in cerebral capillaries are shown in Table 10 (Schinkel et al. 1994; Jonker et al. 1999; Sadeque et al. 2000; Sasongko et al. 2005; Hsiao et al. 2006; Daood et al. 2008; Geyer et al. 2008; Aller et al. 2009;Liow et al. 2009). In humans, P-GP is detectable in cerebral capillaries at ∼ 22 weeks of gestation (Daood et al. 2008). One of the most striking characteristics is the extreme promiscuity of this transporter (Table 4) (Tang et al. 2009). It transports molecules from molecular weight of 400 to ∼ 4000 out of brain. It is interesting that P-gp is ∼ twice as large as oat-3 and pept-2 consistent with it transporting much larger molecules (Table 4). P-gp also lowers ligand concentration to a lesser extent in CSF (Tang et al. 2009). This degree of promiscuity is unusual in nature since most enzymes, receptors and transporters are more specific – some only processing one ligand. Both the human and mouse transporter tend to favor more lipid soluble molecules. There is some evidence that the mouse transporter is more ‘powerful’in vivo; there is also ∼ twice as much p-gp transporter RNA in mouse brain capillaries (Syvänen et al. 2009; Warren et al. 2009).
| (1) Rodent genes – Expressed as protein 140–170 kDa |
| (2) Messenger RNA – localized in brain capillaries – not CP |
| (3) Transport protein – hydropathy analysis suggests 12 transmembrane domains in two 6 domain ‘halfs’– putative structure and mechanism – see text and Fig. 2 |
| (4) Transporter location: luminal side of brain capillary |
| (5) In vitro studies |
| Monolayers of capillary endothelial cell show clear abluminal to luminal transport |
| (6) Rodent transporter expressed in mammalian cells and Xenopus oocytes |
| (7) In vivo techniques show P-gp ligands excluded from brain; inhibitors of P-gp increase brain ligand levels |
| (8) KO studies in mice (and collie dogs) show loss of capillary P-gp; in vivo ivermectin mouse brain levels in KO mice are 30 times (relative to plasma levels) higher than in wild type mice. CSF levels of P-gp ligands in KO mice also increase although not as much as brain |
| (9) Coherent and consistent studies with P-GP in: |
| Direct studies in vivo and in vitro |
| Inhibition studies |
| Specificity and mechanism partially defined – see text |
| (10) Human studies consistent with animal data: 87% sequence homology mouse to human; P-GP located in human cerebral capillaries; similar kinetics to mouse; but some evidence exists that P-GP in humans not as active as in mice (see text) |
In vivo, in both animals and humans, P-GP serves a protective role – keeping potentially very toxic substances and drugs out of brain (e.g., ivermectin, loperamide and domperidone, a D-2 blocker; Table 4). A related D-2 blocker, metaclopromide more freely enters brain and has long term severe chronic toxicity with a black box USA Food and Drug Administration (FDA) warning. Also in cancer chemotherapy, many chemotherapeutic drugs are kept out of the brain by P-GP – this may either be helpful or harmful depending on the clinical situation.
Although educated guesses could be made to predict P-gp ligands, until Aller et al. 2009, the mechanism for P-gp activity was only known in rough outline. If an investigator really wished to know, that person would have to study the compound. (See discussion.)
A recent surprising finding in vivo is the synergistic interactions between P-gp and breast cancer resistance protein (bcrp or BCRP; ABCG2) at the BBB (Zhou et al. 2009). Both proteins are present in the luminal capillary membranes of mice and humans, and the ligand specificities of P-gp and bcrp overlap. An example of synergy was documented in vivo in mice (Zhou et al. 2009). In bcrp KO mice, there is little effect on imatinib distribution in brain compared to wild type mice whereas in P-pg KO mice, they have four times (relative to plasma) more drug in brain than wild type mice. However, in double KO mice, i.e., those without P-gp and bcrp, there is 28 times more drug in brain relative to wild type mice. BCRP is a ‘half’ transporter with only six trans-membrane domains and one ATP binding site, but as described above can synergistically interact with P-gp by an unknown mechanism (Zhou et al. 2009). This complicates the interpretation of single gene P-gp KO mice.
P-glycoprotein also plays a role in non-sedating anti-histamine H1 blocker pharmacology in the human CNS (Chen et al. 2003). The original histamine H1 blockers, e.g., diphenhydramine (DPH), were sedating (Goldberg et al. 1987; Tsuji 2005). In fact, although a nuisance to allergy sufferers, this property is employed to this day in over-the-counter sleep aids. DPH, although a charged tertiary amine at pH 7.4, is rapidly cleared from blood into brain by a saturable mechanism at the BBB (Goldberg et al. 1987; Tsuji 2005). DPH is not a ligand for P-gp. However, the non-sedating anti-histamine H1 blockers are ligands for P-gp (Chen et al. 2003), and thus somewhat excluded from brain. This property partially explains their non-sedating properties.
Finally, the molecular mechanisms of action of P-gp have been recently clarified by Aller et al. (2009) with their unraveling of the x-ray structure of apo-P-gp at 3.8 angstroms. P-gp shows an internal cavity of 6000 angstroms cubed with one ATP binding site on each half of the molecule (See Fig. 2). Inside the main cavity there are smaller distinct binding sites with definite stereo-selectivity. Ligands enter from the inner leaflet of the lipid bilayer and are then ejected into the blood (Fig. 2). The process is driven by ATP hydrolysis. The reader is encouraged to read and view the elegant figures in the text and supplementary material of this triumph of structural biology (Aller et al. 2009). With this work Aller et al. (2009) have helped explain the broad specificity and location of this carrier at the capillary lumen. Similar studies also need to be done with oat-3 and pept-2 to understand their broad specificity.
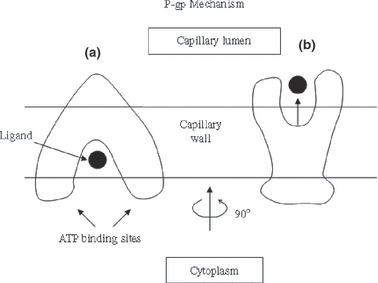
Model of ligand transport by P-gp (See Aller et al. 2009). (a) Ligand (large filled-in black circle) enters ligand binding pocket. (b) When ATP binds to both binding sites (and is ultimately hydrolyzed), this causes ∼ a 90o rotation and a large conformational change in the P-gp with ejection of the ligand into the capillary lumen.
Discussion
In discussing brain and CSF concentrations of substances (i.e., the distribution of exogenous ligands in brain and CSF relative to blood, especially at steady state), the rate of ligand entry, exit, tissue binding, and intra-brain and CP metabolism are the four key factors. In this paper we have mainly discussed ligands where CNS metabolism and tissue binding are not important factors (with a few exceptions like ceftriaxone which is heavily bound to plasma proteins). Our focus has been on BBB and BCSFB efflux transporters which, as described above, can radically alter brain and/or CSF concentrations of myriad ligands, in some cases by a factor of 10–30 times.
As noted in the text and Tables 3, 5, 9, and 10, the scientific transport data supporting our interpretations of oat-3, pept-2 and P-gp in animals and humans are strong, plentiful and coherent. There is no doubt these three separate transporters are present in mammalian species at the BBB and/or BCSFB and have important functions.
It is also clear that lipid solubility, molecular size and charge frequently do not determine ligand distribution in brain. Hundreds if not thousands of compounds are exceptions. For example, in normal mice and dogs, because of P-gp, the brain concentration of ivermectin is ∼ 30 times lower (relative to plasma) than in KO animals (Schinkel et al. 1994). For certain ligands in the CNS like ivermectin for P-gp, cefadroxil for pept-2 and penicillin G for oat-3, the effects are large and important.
With regard to protective transport functions of oat-3, pept-2 and P-gp in the CNS, it is interesting to compare the kidney tubules, and the brain capillaries and CP epithelial cells; the former protect the body as a whole and the later two, the brain. In the tubules, there are substantial concentrations of oat-3, pept-2 and P-gp; oat-3 on blood side secretes ligands (e.g., riboflavin, penicillin) into tubular cells and then the ligands are transferred into urine by an unknown mechanism (Spector 1982); pept-2 reabsorbs di- and tripeptides from urine into tubular cells for intracellular hydrolysis to amino acids (conservation), and P-gp ejects many ligands back into urine. Thus, the polarity is different in the kidneys but the functions are analogous to those in cerebral capillaries and CP epithelial cells. In a metaphorical sense, the choroid plexus could be conceived of as a kidney tubule – like structure in the brain.
What is somewhat surprising is that KO mice without oat-3, pept-2, and P-gp and collie dogs without P-pg are normal at birth, remain normal and reproduce normally unless challenged with certain ligands. This suggests that these three transporters are not essential to life, or possibly other mechanisms take over when these are absent. As noted above, all three of these transporters are normally present at birth. There is also one piece of data (as noted above) that at least one living human has a non-functional PEPT-2 transporter (Terada et al. 2004).
If KO animals are generally normal, why do these systems with their very broad specificity exist at the BBB and BCSFB? There are two schools of thought: one is that these systems were originally involved in physiological functions (e.g., in both kidney and CP). For example, when the concentration of riboflavin in blood or CSF rose above a certain limit, it would be secreted by OAT-3 into the urine or blood by OAT-3 in the tubules or CP epithelial cells, respectively. Gradually, the specificity broadened as animals with more protective transporter functions survived the toxins in the environment – particularly in food. This theory was originally proposed by Levy (Spector 1982). The second theory is that from the start these systems provided both ‘house cleaning’ of endogenous waste products like metabolites of serotonin and catechol amines, and also performed protective functions from exogenous agents/toxins (Deguchi et al. 2006).
However the extremely broad specificity of these transporters arose, it is remarkable. One would never anticipate that penicillin, riboflavin and cimetidine would travel on the same transporter (Table 4). But now knowing this, what are the consequences? For therapy? For toxicology? For drug interactions?
For therapy, these efflux systems are very useful if one is using agents that are brain-toxic and the therapist does not want the agent in the brain (e.g., ivermectin; loperamide; cimetidine). On the other hand, if one is attempting to treat brain disorders (e.g., brain cancer; meningitis), since many effective chemotherapeutic agents are transported out of the CNS by these transporters, they interfere with therapy.
In toxicology, these transporters (Table 4) without question protect the brain from many potential toxins. Even penicillin G is neurotoxic if the plasma levels are too high. For animals and presumably primitive man who ingested many toxins, one can imagine the great utility of these systems at the BBB and BCSFB.
Two related questions that were frequently raised in the past can now be answered. First, although there are clearly ligand interactions in vivo with oat-3, pept-2 and P-gp in animals, are these present and important in humans, especially drug interactions? A related question is: are the levels and functions of these three transporters comparable in people and animals.
In terms of drug interactions, in both rodents and humans cyclosporin (at a clinically tolerable concentration) increases the concentration of verapamil (a P-GP, ligand) in brain comparably (∼ 2 times) (Sasongko et al. 2005; Hsiao et al. 2006). Similarly, quinidine depressed breathing in humans given loperamide, a narcotic agent normally excluded from the brain by P-GP (Table 4) (Sadeque et al. 2000). Thus there is the potential for drug interactions in humans – especially with P-gp. Whether such drug interactions can be useful therapeutically remains to be seen.
In terms of quantitative similarity of these efflux transporters in animals and humans, with OAT-3, there is substantial human evidence that penicillin G distributes itself in CSF as it does in animals, strongly supporting a comparable OAT-3 mechanism. Also, when measured directly the concentration of mRNA for P-GP in human brain capillaries is about one-half that of rodents (Warren et al. 2009). However, there are some data using positron emission tomography scanning that shows some P-GP ligands are better kept out of rodent than human brain – thus implying more P-GP ‘activity’in vivo in rodent CNS (Syvänen et al. 2009).
For an individual compound, can one predict whether it might be transported out of the CNS by OAT-3, PEPT-2 or P-GP? As discussed above, there are broad rules about the specificity of these transporters, but to obtain quantitative, reliable data, simple in vitro techniques can be employed. For example, for oat-3 and pept-2 one can see whether the labeled ligand is accumulated by the isolated CP in vitro. If so, one can perform ligand competition experiments or can study monolayers of CP epithelial cells expressing either pept-2 or oat-3 on the apical side. Similarly, in vitro studies with monolayers of cerebral endothelial cells expressing P-gp can be used as described above. In vivo, there are multiple, sophisticated techniques (Table 3).
Also, there are some data that the concentration, location (on the membrane or intracellularly) and activity of these transporters might be altered in disease states and/or by immunological mediators. The reader is referred to Miller et al. (2008) for a discussion of this literature.
In summary, the reader can see the fantastic progress made over the last 40 years (e.g., from simple notions that penicillin and iodide are cleared from the CSF too fast by an unknown mechanism). Our current knowledge base has expanded remarkably. In some cases, (e.g., riboflavin and fluorescein) one can actually watch the ligand crossing the CP epithelial cells from one side to the other, and then out. This is especially striking with riboflavin (Spector 1980b). There are, however, still several glaring holes in the fabric of our knowledge. One persistent hole is how unmetabolized ligands of oat-3 and pept-2 efflux the other side of the CP or, in the case of oat-3, brain capillary endothelial cells. There are many candidates but none have been unequivocally established. Another is a lack of understanding on a molecular basis how oat-3 and pept-2 work. These problems are solvable with more work. The future of basic and clinical neurochemistry in this area is bright indeed. Moreover, the practical consequences and applications are and will be even more important.
Acknowledgement
The author thanks Michiko and Regine Spector for their aid in preparation of the manuscript and figures.




