Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats
Abstract
Neurons have a high demand for cholesterol to develop and maintain membrane-rich structures like axons, dendrites and synapses, but it remains unclear, whether they can satisfy their need by costly de novo synthesis. To address this, we compared cholesterol synthesis in serum-free cultures of highly purified CNS neurons and glial cells from postnatal rats. We observed marked cell-specific differences: Compared with glial cells, neurons showed different profiles of biosynthetic enzymes, post-squalene precursors and cholesterol metabolites, and they produced cholesterol less efficiently, possibly because of very low levels of lanosterol-converting enzymes. Astrocytes responded to inhibition of cholesterol synthesis with a much stronger up-regulation of biosynthetic enzymes than neurons. Our results support the idea that neurons cannot produce cholesterol efficiently and that they depend on an external source of this lipid.
Abbreviations used
-
- 24OC
-
- 24-hydroxycholesterol (KEGG number C13550)
-
- 7D
-
- 7-dehydrocholesterol (C01164)
-
- 7OC
-
- 7β-hydroxycholesterol
-
- AST
-
- astrocytes
-
- CE
-
- cholesteryl ester
-
- CH
-
- cholesterol
-
- Cyp46a1
-
- cholesterol 24-hydroxylase (EC 1.14.13.98)
-
- Cyp51
-
- cytochrome P450, family 51 (lanosterol 14-demethylase EC 1.14.13.70)
-
- DE
-
- desmosterol (C01802)
-
- Dhcr24
-
- 24-dehydrocholesterol reductase (EC 1.3.1.72)
-
- GCM
-
- glia-conditioned medium
-
- GC-MS
-
- gas-chromatography and mass spectrometry
-
- LA
-
- lanosterol (C01724)
-
- LT
-
- lathosterol (C01189)
-
- MCD
-
- β-methyl-cyclodextrin
-
- MIC
-
- microglial cells
-
- Nsdhl
-
- NAD(P)-dependent steroid dehydrogenase-like (EC 1.1.1.170, part of C4-demethylation enzyme complex)
-
- OC
-
- 24- and 7α-hydroxycholesterol
-
- OLI
-
- oligodendrocytes
-
- PC
-
- phosphatidyl-choline
-
- PE
-
- phosphatidyl-ethanolamine
-
- RGCs
-
- retinal ganglion cells
-
- SM
-
- sphingomyelin
-
- Sqs
-
- squalene synthase (EC 2.5.1.21)
-
- TG
-
- triacylglycerol
-
- TLC
-
- thin-layer chromatography
-
- TM
-
- testis meiosis-activating sterol (T-MAS or 14-Demethyllanosterol C05108)
-
- ZA
-
- zaragozic acid
-
- ZN
-
- zymostenol (C03845)
-
- ZY
-
- zymosterol (C05437)
The brain contains a quarter of all cholesterol in the body, but has no access to cholesterol supplied by synthesis in the liver or by food absorption in the intestine (Dietschy et al. 1993; Bjorkhem and Meaney 2004). Therefore, cells in the brain rely entirely on cholesterol synthesis, a resource-intense process that requires energy, molecular oxygen and 21 enzymes (Bloch 1964; Waterham 2006; Ikonen 2008). Previous studies indicate that neurons have a particularly high demand for cholesterol to form and maintain axons (Heacock et al. 1984; Hayashi et al. 2004), dendrites (Fan et al. 2002; Goritz et al. 2005) and synaptic connections (Mauch et al. 2001; Pfrieger 2003b). It has been estimated that neurons renew 20% of their cholesterol content daily (Dietschy and Turley 2004). So far, it is unknown, how these highly specialized cells meet their need for this essential lipid (Pfrieger 2003a). This question is of clinical importance, as deficits in cholesterol synthesis (Waterham 2006; Valenza et al. 2007) or transport (Corder et al. 1993; Vanier and Millat 2003; Vance et al. 2005) cause neurodegeneration and severe neurological symptoms. Here, we compared cholesterol synthesis in neurons and different types of glial cells. Currently, this topic cannot be addressed in vivo, therefore we used serum-free primary cultures of immunoisolated cells. Our results reveal distinct precursor profiles and less efficient cholesterol synthesis in neurons compared to astrocytes suggesting that neurons delegate this costly metabolic pathway to astrocytes.
Materials and methods
Cell culture
Seven-day-old Wistar rats (animal facility, l’IFR des Neurosciences, Strasbourg, France) were decapitated according to institutional guidelines. Retinal ganglion cells (RGCs) and cerebellar neurons were purified by sequential immunopanning as described (Barres et al. 1988; Steinmetz et al. 2006). Cultures of astrocytes (AST) and microglial cells (MIC) were obtained by one-step immunopanning from cortical glial cultures, which were also used to prepare glia-conditioned medium (GCM), and cultures of oligodendrocytes (OLI) were obtained from optic nerves (Supporting information). Immunocytochemical staining revealed that the astrocyte preparation contained mainly glial fibrillary acid protein (Gfap)/S100β-positive cells (88 ± 3%; mean ± SD; n = 1300) and only very few O4/CNPase-positive oligodendrocytes (3 ± 2%, n = 1400 cells; n = 3 culture preparations) or CD68-positive microglia (0.3 ± 0.5%, n = 2400 cells; n = 3 culture preparations). The microglia preparation contained 100% CD68-positive cells (n = 1900 cells; n = 3 culture preparations). The oligodendrocyte preparation contained a large fraction of O4/CNPase-positive oligodendrocytes (63 ± 20%, n = 1400) and only very few Gfap/S100ß-positive astrocytes (3 ± 2%, n = 1300) or CD68-positive MIC (2 ± 1%, n = 1200). For neuron-astrocyte cocultures, astrocytes were added to 1-day-old cultures of immunoisolated RGCs at a ratio of 1 : 6. For lipid and protein analysis, neurons and glial cells were plated at 200–300 cells/mm2 on poly-d-lysine-coated tissue culture plates (Ø 35 mm, Falcon). For immunocytochemistry, cells were plated at 80 cells/mm2 on poly-d-lysine-coated glass cover slips (Ø 12 mm; Hecht-Assistent, Sondheim, Germany). All cell types were cultured in the same, chemically defined medium containing Neurobasal (Invitrogen, Cergy-Pontoise, France), B27 (Invitrogen), bovine serum albumin (100 μg/mL; crystalline grade #A4161, Sigma, Saint-Quentin Fallavier, France), brain-derived neurotrophic factor (25 ng/mL; PeproTech, London, UK), ciliary neurotrophic factor (10 ng/mL; PeproTech), forskolin (10 μM; Sigma), glutamine (2 mM; Invitrogen), insulin (5 μg/mL; Sigma), N-acetylcysteine (60 μg/mL; Sigma), penicillin (100 units/mL; Invitrogen), progesterone (62 ng/mL; Sigma), putrescine (16 μg/mL; Sigma), sodium pyruvate (1 mM; Invitrogen), sodium selenite (40 ng/mL; Sigma), streptomycin (100 μg/mL; Invitrogen), transferrin (100 μg/mL; Sigma) and tri-iodothyronine (40 ng/mL; Sigma). For some experiments, RGCs were treated for indicated times with GCM, which was prepared as described (Goritz et al. 2005), with zaragozic acid (ZA) (3 μM, Sigma) or with β-methyl-cyclodextrin (MCD) (5 μM, Sigma) (Supporting information).
Lipid analysis
To analyse newly synthesized lipids, cells were incubated with radioactive precursors, lipids were extracted, separated by thin-layer chromatography (TLC) and quantified by TLC scanners (Supporting information). Sample adjustment and normalization of incorporation rates were based on protein and DNA content determined using fluorescence-based assays (Supporting information). Total sterol content was analysed by gas chromatography coupled to mass spectrometry (Supporting information).
Immunocytochemical staining, immunoblotting
Immunocytochemical staining and immunoblotting were carried out using standard procedures and specific antibodies (Supporting information).
Statistical analysis
Statistical analysis was performed using indicated tests (STATISTICA 8.0, StatSoft Inc., Maison-Alfort, France). Error bars in figures indicate standard deviations. Levels of significance are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
We analysed neuronal cholesterol synthesis in primary cultures of a specific cell type, namely RGCs. These neurons can be highly purified from postnatal rats (Fig. 1a) (Barres et al. 1988) and maintained in the absence of serum (Meyer-Franke et al. 1995), which is a prerequisite to study lipid metabolism. For comparison, we also studied primary cultures of the main glial cell types including astrocytes, oligodendrocytes and microglia, which can also be purified by immunoisolation and grown under the same chemically defined conditions as neurons (Fig. 1a).
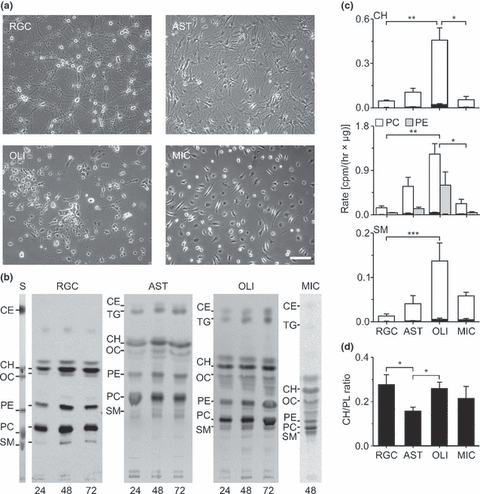
Lipid synthesis in neurons and glial cells. (a) Phase-contrast micrographs of immunoisolated RGCs, astrocytes (AST), oligodendrocytes (OLI) and microglial cells (MIC) that were cultured for 6 days in chemically defined, serum-free medium. Scale bar, 100 μm. (b) Autoradiograms of TLC plates loaded with lipid extracts from indicated cell types. Cells were cultured for 4 days in defined medium and then incubated for indicated durations (in hours) with [1-14C]-acetate. Same amounts of cellular material based on protein content were loaded on corresponding lanes of each cell type. For lipid identification, lipid standards were separated in parallel to cellular material. S, Image of iodine-stained TLC containing selected lipid standards. CE, cholesteryl ester; CH, cholesterol; OC, 24S- and 7α-hydroxycholesterol; PC, phosphatidyl-choline; PE, phosphatidyl-ethanolamine; SM, sphingomyelin; TG, triacylglycerol. (c) Mean rates of [1-14C]-acetate incorporation in cholesterol, phospholipid and sphingomyelin (RGC, AST, OLI: n = 5; MIC: n = 4 indep. preparations) calculated after 48 h incubation with radioactive precursor and normalized to protein content in cell (white, gray) and medium (black) extracts from neuronal and glial cultures. (d) Mean ratios of newly synthesized cholesterol to phospholipids in neurons and glial cells calculated from incorporation rates of radioactive acetate normalized to protein content [RGC, AST, OLI: n = 5; MIC: n = 4 indep. preparations]. Asterisks in (c), (d) indicate statistically significant differences [Kruskal-Wallis ANOVA by ranks test followed by post-hoc comparison of mean ranks].
Lipid synthesis in neurons and glial cells
First, we compared the synthesis of cholesterol and other lipids in neurons and glial cells by incubation with radioactively labeled acetate as precursor. Label was added for increasing durations to 4-day-old cultures, when cells reached stable survival (Meyer-Franke et al. 1995) and showed morphological differentiation (Fig. 1a). Chromatographic analysis of lipid extracts revealed that all cell types formed cholesterol, phospholipids and sphingomyelin in vitro (Fig. 1b). The amount of labeled lipids increased by at least two-fold between 24 and 48 h of labeling (Fig. 1b). In all cell types analysed, a small (<7%) percentage of radioactively labeled lipids was present in the culture medium (Fig. 1c). In neurons, this occurred only in the presence of a medium supplement with a high albumin concentration (data not shown). The normalized rates of acetate incorporation in lipids differed among the cell types analysed with neurons and oligodendrocytes showing the lowest and highest values, respectively (Fig. 1c). Compared with other cells, astrocytes incorporated more radioactive label in phospholipids than in cholesterol (Fig. 1d). The lower incorporation rates in neurons compared with glial cells may have been unrelated to synthesis and caused by slower uptake of acetate, limiting concentrations of radiolabel or stronger dilution of label in endogenous precursor pools in neurons. However, a series of control experiments indicated that this was probably not the case (Supporting information, Fig. S1). In summary, our results revealed that under chemically defined, glia-free conditions, neurons from the postnatal CNS synthesize cholesterol and other lipids, but at lower rates than glial cells.
Cholesterol metabolism in neurons and glial cells
Metabolic labeling with [14C]-acetate revealed that macroglial cells, but not neurons formed large amounts of the storage lipids triacylglycerol and cholesteryl esters (Fig. 1b). Moreover, TLC showed the presence of oxysterols in neurons and glia, which may have been generated by autoxidation or enzymatic activity. To study metabolites of cholesterol directly, we incubated neurons and astrocytes with radioactively labeled cholesterol (Fig. 2). This revealed that added cholesterol was esterified in astrocytes and astrocyte-conditioned medium. RGCs also formed cholesteryl esters, but no radioactive ester was found in neuron-conditioned medium (Fig. 2). Notably, neurons and astrocytes produced distinct profiles of oxysterols. Neurons, but not astrocytes generated, albeit very small, amounts of 24S-hydroxycholesterol (24OC), which effluxed to the culture medium (Fig. 2). The synthesizing enzyme, cholesterol 24-hydroxylase (Cyp46a1; EC 1.14.13.98; Lund et al. 1999) was only present in neurons, but absent from astrocytes (Fig. 2) confirming neuron-specific production of this oxysterol (Lund et al. 1999; Ramirez et al. 2008). Astrocytes produced distinct oxysterols (Fig. 2) as suggested previously (Wong et al. 2007). In summary, macroglial cells formed large amounts of storage lipids like cholesterol esters and triacylglycerol, whereas neurons formed trace amounts of 24OC.

Metabolism of cholesterol. (a) Autoradiograms of TLC plates loaded with medium extracts of RGCs (RGC) and of astrocytes (AST). Before extraction, cells were cultured in defined medium for 1 day and then for three days with [14C]-cholesterol. Subsequently, the medium was replaced and cells were cultured for 3 days in the absence of radioactive cholesterol. As control for autoxidation (CON), [14C]-cholesterol was incubated for 6 days in culture medium in the absence of cells. S, Image of primuline-stained lipid standards loaded on the AST lane of the TLC for lipid identification. RGCs, but not AST produced a low amount of 24OC (arrowhead), whereas only AST esterified cholesterol. (b) Profiles of radioactivity (black, left axis) and primuline fluorescence (gray, right axis) from TLC plates loaded with lipid extracts and lipid standards (7OC and 24OC, arrows) from medium of cell-free control samples (CON, top), RGCs (middle) and AST (bottom). Note small 24OC peak in neurons (arrowhead), but not in AST. (c) Representative immunoblots revealed presence of Cyp46a1 (arrowhead) in neurons, but not in AST. Cells were cultured for 5 days in the absence or presence of cholesterol (CH) and reacted with antibodies against Cyp46a1 and actin as loading control. 24OC, 24S-hydroxycholesterol; 7OC, 7β-hydroxycholesterol.
Sterol synthesis in neurons and glial cells
Next, we studied the post-squalene pathway of cholesterol synthesis by incubating neurons and glial cells with [2-14C]-RS-mevalonolactone, which is a more specific precursor than acetate. To separate structurally similar post-squalene precursors, we developed a high-resolution TLC protocol (Supporting information). Radioactively labeled cholesterol precursors occurred in all cells tested and their levels increased until 72 h of incubation indicating that [2-14C]-RS-mevalonolactone was not depleted from medium (Fig. 3a). Neurons showed a lower normalized rate of sterol labeling compared with glial cells, although this difference did not reach statistical significance (Fig. 3b). The presence of radioactively labeled sterols in neuronal culture medium (Fig. 3b), which was caused by an albumin-rich medium supplement (data not shown), suggested that newly synthesized cholesterol precursors are located in the plasma membrane, as shown recently in other cells (Mutka et al. 2004; Yamauchi et al. 2007). This was confirmed by short-term exposure to MCD, which extracted newly synthesized sterols from neurons and astrocytes at similar rates (Supporting information, Fig. S2).

Sterol synthesis in neurons and glial cells. (a) Autoradiograms of TLC plates loaded with lipid extracts from indicated cell types (COC, neuronal-astrocyte cocultures). Cells were cultured for 4 days in defined medium and then incubated for indicated durations (in hours) with [2-14C]-RS-mevalonolactone. Sterol biosynthetic intermediates were separated by a special TLC protocol and identified by co-migrating standards. Bands below 7D correspond to [2-14C]-RS-mevalonolactone. S, Primuline-stained TLC containing indicated sterol standards. (b) Mean rates of sterol synthesis (RGC: n = 5; AST, OLI, MIC: n = 4, COC: n = 1 indep. preps.) normalized to protein content in indicated culture preparations extracted from cells (white) and medium (black). Asterisks indicate statistically significant differences (Kruskal–Wallis anova by ranks test followed by post-hoc comparison of mean ranks). Tests were performed on combined activity in cell and medium extracts. (c) Mean contribution of cholesterol and indicated precursors to total activity (cellular and effluxed) in indicated culture preparations. ND, not determined, pooled sterol bands that could not be identified. (d) Phase-contrast micrographs of RGCs cultured for indicated periods (in days) under defined conditions. Scale bar, 20 μm. (e) Average rates of sterol synthesis (n = 3 indep. preps.) normalized to protein content (gray columns; left axis) and mean contribution of individual newly synthesized sterols to total activity (stacked columns, right axis) at indicated culture periods (in days). RGCs were cultured for indicated periods, incubated during the last 36 h with [2-14C]-mevalonolactone and then processed for sterol extraction. (f) Diagram illustrating selected enzymatic steps and precursors in the biosynthesis of cholesterol that are relevant to this study. Precursors on the left and right are part of the Bloch and the Kandutsch–Russell pathway, respectively. Enzymes: 1, Sqs; 2, Dhcr24; 3, Cyp51; 4, Nsdhl. Precursors: 7D, 7-dehydro-cholesterol; CH, cholesterol; DE, desmosterol; LA, lanosterol; LT, lathosterol; TM, T-MAS; ZN, zymostenol; ZY, zymosterol.
Notably, in neurons about 40% of the radioactivity were incorporated in lanosterol (LA), whereas in macroglial cells the major fraction was present in cholesterol (Fig. 3). To determine, whether this phenomenon occurred in other CNS neurons, we analysed sterol synthesis in primary cultures of immunoisolated cerebellar neurons (Steinmetz et al. 2006). In this neuronal preparation, radioactivity accumulated in LA as well (data not shown) indicating that this was not specific to RGCs.
We next studied, whether the rate and pattern of mevalonate incorporation in sterols changed during culture, possibly due to neuronal differentiation (Fig. 3d). To address this, we incubated neurons after two culture periods with [2-14C]-RS-mevalonolactone. The apparent rate of radioactive labeling increased during culture (Fig. 3e). Interestingly, already after 1.5 days most radioactivity was contained in LA and lathosterol (LT) (Fig. 3e and f) and the labeling of precursors at the expense of cholesterol increased during the culture period (Fig. 3e).
In cocultures of neurons and astrocytes, the rate of mevalonate incorporation in sterols and the pattern of precursor labeling resembled those of astrocytes (Fig. 3) suggesting that cholesterol synthesis occurred predominantly in astrocytes. To test this, we performed immunocytochemical staining for squalene synthase (Sqs; EC 2.5.1.21), which catalyses the first reaction that is specific for cholesterol synthesis (Fig. 3). In glia-free cultures, Sqs was present in neurons (Fig. 4). In cocultures, however, the enzyme was absent from neurons growing in contact with astrocytes and present at low levels in neurons growing without contact to glia (Fig. 4). GCM treatment also lowered neuronal Sqs levels (Fig. 4) (Goritz et al. 2007) and reduced cholesterol synthesis by 83 ± 6% (mean ± SD, n = 3 preparations).
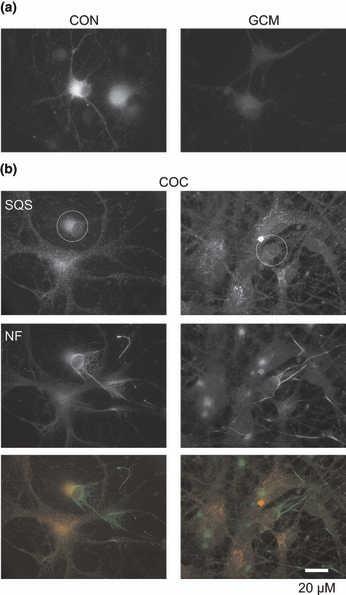
Glia-induced down-regulation of Sqs in neurons. (a) Fluorescence micrographs of RGCs cultured for 4 days in defined medium and then for 3 days in defined medium (CON) or in the presence of GCM (GCM). (b) Fluorescence micrographs of RGCs that were cultured for 6 days in the presence of glial cells. Circles mark neuronal somata growing without (left) or with (right) contact to glial cells. Cells were immunostained with antibodies against Sqs [(a) and (b), top] and neurofilament [NF; (b) middle]. Bottom, merged images from top (Sqs, red) and middle (NF, green).
In summary, our results indicate a lower rate of sterol synthesis in neurons compared with glial cells. In neurons, radioactive label was mainly found in the precursor LA, whereas in glial cells the largest fraction was contained in cholesterol. In the presence of glia, neuronal cholesterol synthesis was strongly reduced.
Total sterol composition of neurons and astrocytes
Next, we addressed, whether the differential radioactive labeling of cholesterol precursors in neurons and astrocytes manifested itself in their total sterol content using gas-chromatography and mass spectrometry (GC-MS) (Fig. 5). In neurons and astrocytes cultured for 1 week, cholesterol precursors represented a large portion of the sterol content (Fig. 5). LA was only detected in the total sterol mass of neurons, but not of astrocytes, and this occurred already after 1.5 days in vitro (Fig. 5). Notably, neurons contained mainly precursors of the Kandutsch–Russel pathway including 7-dehydrocholesterol (7D) and LT, whereas astrocytes contained precursors of the Bloch-pathway, namely desmosterol (DE) (3, 5). These data confirmed that LA accumulates specifically in RGCs and suggested that neurons and astrocytes employ different pathways to synthesize cholesterol.

Distinct precursor profiles in neurons and astrocytes. (a) Elution profiles and selected mass spectra of lipid extracts separated and identified by GC-MS from RGCs (top) and from astrocytes (bottom) cultured for 1 week in defined medium. All sterol-containing peaks are indicated with the respective component. Inserts, mass spectra of indicated peaks (as 3β-hydroxy sterols) revealed that neurons contained 7D, whereas astrocytes contained DE. Authentic sterol standards run in parallel confirmed respective chromatographic properties and mobility and mass spectra. Prominent ion fragments in each mass spectrum at m/z ratios 351 (M+-H2O-Me); 271 (M+-SC). M+, molecular ion; Me, methyl group; SC, side chain. (b) Mean contributions of cholesterol and indicated precursors to total content of sterols that could be detected and quantified by GC-MS in neurons and astrocytes cultured in defined medium (RGCs: as indicated; n = 3, for AST: 8 days; n = 2).
Efficacy of post-squalene precursor conversion in neurons
The predominant labeling of LA and its presence in the total sterol mass raised the question, whether the conversion of this and subsequent cholesterol precursors was inefficient (Fig. 3). To study downstream processing of LA in neurons, we performed pulse-chase labeling using [2-14C]-RS-mevalonolactone. As shown in Fig. 6, the amount of labeled LA decreased during the chase period of 48 h. However, radioactive label was not incorporated in cholesterol, but rather accumulated in intermediate precursors, namely LT and 7D (Fig. 6). These results indicated an inefficient conversion of post-squalene precursors and confirmed the presence of the Kandutsch–Russel pathway in neurons.

Turnover of cholesterol precursors in neurons. (a) Autoradiograms of TLC plates loaded with lipid extracts from RGCs that were cultured for 4 days in defined medium, incubated for 48 h with [14C]-mevalonolactone (pulse) and then incubated for 48 h with non-radioactive mevalonolactone (chase). Lipids extracted after the pulse (P) and the chase (C) period were analysed. (b) Mean percentual changes in individual precursors from pulse to chase (n = 3 indep. preparations).
Different levels of cholesterol synthesizing enzymes in neurons and astrocytes
The slow conversion of LA in neurons may have been caused by low levels of the enzymes that process this precursor, namely 24-dehydrocholesterol reductase (Dhcr24; EC 1.3.1.72) and cytochrome P450, family 51 (Cyp51; EC 1.14.13.70) (Fig. 3). To address this, we compared the levels of selected cholesterol synthesizing enzymes in neurons and astrocytes by immunoblotting. Cyp51 showed very low levels in neurons and astrocytes, whereas Dhcr24 was not detectable in either cell type (Fig. 7). On the other hand, normalized levels of Sqs and NAD(P)-dependent steroid dehydrogenase-like (Nsdhl; EC 1.1.1.170) acting upstream or downstream of LA, respectively (Fig. 3), were significantly higher in neurons than in astrocytes (Fig. 7). We next studied how the enzyme levels in neurons and astrocytes changed, when the cellular cholesterol was reduced by treatment with the Sqs inhibitor ZA. ZA enhanced enzyme levels in both cell types, but the increases were much stronger in astrocytes compared with neurons (Fig. 7). Notably, ZA induced the presence of Dhcr24 and a caspase-generated fragment (Greeve et al. 2000) in astrocytes, whereas both LA-converting enzymes remained undetectable in neurons (Fig. 7).
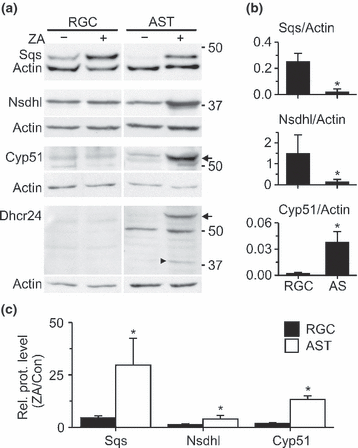
Levels of selected cholesterol synthesizing enzymes in neurons and astrocytes. (a) Immunoblots of lysates from 6-day-old cultures of RGCs and AST that were cultured for the last 4 days in the absence or presence of ZA. Blots were reacted with antibodies against indicated cholesterol biosynthetic enzymes (Cyp51, Dhcr24, Nsdhl, Sqs) and against actin as loading control. Arrows indicate bands representing Cyp51 and Dhcr24 and the arrowhead indicates a proteolytic fragment of Dhcr24. (b) Mean enzyme levels normalized to actin in RGCs (n = 6 indep. preparations) and AST (n = 4). (c) ZA-induced increase in normalized enzyme levels in RGCs (n = 6) and AST (n = 4) compared with untreated controls. Asterisks in (b) and (c) indicate statistically significant changes in RGCs compared with AST (Mann–Whitney U-test).
Together, these results revealed cell-specific profiles of cholesterol synthesizing enzymes and a higher capacity of astrocytes to compensate for cholesterol deficit than neurons.
Discussion
Our study revealed marked differences in cholesterol biosynthesis between brain cells in vitro. Neurons and glial cells showed distinct profiles of biosynthetic enzymes, post-squalene precursors and cholesterol metabolites. Neurons produced cholesterol inefficiently and showed a much lower capacity to up-regulate the synthesis pathway than astrocytes.
Cholesterol synthesis has been studied previously in cultured neurons and glia from embryonic animals (Volpe et al. 1985; Saito et al. 1987; Tabernero et al. 1993; Vance et al. 1994; Fan et al. 2002; Suzuki et al. 2007; Hascalovici et al. 2009). However, only few studies performed a side-by-side comparison using labeled acetate (Saito et al. 1987) or lactate (Tabernero et al. 1993) or tritiated water (Lopes-Cardozo et al. 1986) as precursor and they reported conflicting results. Our use of acetate and mevalonate showed lower rates of lipid synthesis in postnatal neurons than in glia. Our control experiments to address the confounding influence of precursor uptake, depletion or endogenous pool size (Dietschy and Turley 2004) confirmed this result and suggest that the rate of cholesterol synthesis is underestimated in astrocytes compared with neurons.
Our analysis of post-squalene precursors suggests that the neuronal cholesterol synthesis is inefficient. This was indicated by the preferential metabolic labeling of LA and the slow conversion of subsequent precursors in pulse-chase experiments. Preferential labeling of LA was also reported in cultured neurons from rat cortex (Tabernero et al. 1993) and hippocampus (Suzuki et al. 2007), but not studied further. Notably, the presence of LA in the total sterol content of neurons may further suppress neuronal cholesterol synthesis. It was shown in vitro that only LA, but not other precursors, stimulates the degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Song et al. 2005).
Why does LA accumulate in neurons? Our results point to very low levels of the LA-converting enzymes, Dhcr24 and Cyp51. Dhcr24 may indeed limit cholesterol biosynthesis, as its over-expression in neuroblastoma cells enhances their cholesterol content (Crameri et al. 2006). The low neuronal levels of Dhcr24 and Cyp51 may be due to transcriptional regulation. Dhcr24 transcripts are reduced during neuronal differentiation of human mesenchymal stem cells in vitro (Benvenuti et al. 2006), although changes in other biosynthetic enzymes were not studied. Transcription of Cyp51, which demethylates LA, is not only regulated by the SREBP pathway, but also influenced by cAMP-dependent signaling (Rozman et al. 1999). Therefore, factors other than the cholesterol content may control the level of this enzyme. So far, it is unknown, whether the observed differences in neuronal and glial levels of Cyp51 and other enzymes are also present in vivo, as their cellular distribution in the CNS has not been defined except for Dhcr7. This enzyme was detected by double in situ hybridization in cholinergic neurons of adult mice (Korade et al. 2007).
The low efficacy of cholesterol synthesis indicated by our experiments supports the hypothesis that neurons abandon costly cholesterol synthesis and rely on less expensive delivery by astrocytes (Pfrieger 2003a). This is further indicated by the massive down-regulation of neuronal Sqs levels in co-culture with glial cells. A recent study confirmed the absence of Sqs from cerebellar granule cells in adult mice in vivo (Funfschilling et al. 2007). On the other hand, our results add evidence that astrocytes are net producers of cholesterol. They showed higher rates of cholesterol synthesis than neurons and they incorporated radioactive label predominantly in cholesterol. Moreover, astrocytes synthesized storage lipids like triacylglycerol and cholesteryl esters (Hirsch-Reinshagen et al. 2008) and showed much stronger up-regulation of cholesterol synthesis in response to ZA than neurons. The high ratio of newly synthesized phospolipids to sterols may enable astrocytes to release lipids via lipoproteins (LaDu et al. 1998).
The observed cell-specific profiles of post-squalene cholesterol precursors indicate that neurons and astrocytes use the Kandutsch–Russel and the Bloch pathway, respectively, to synthesize cholesterol (Fig. 3). So far, there are only very few indications on a cell-specific implementation of these pathways. Sperm contains components of the Kandutsch–Russell pathway including DE (Lin et al. 1993) and meiosis-activating sterols (Rozman et al. 2002), whereas keratinocytes contain large amounts of LT (Okamoto et al. 1984). Outside the animal kingdom, plant cells have highly specific sterol contents that probably enable cell-specific functions (Schaeffer et al. 2001; Babiychuk et al. 2008). At present, the functional relevance of distinct precursors in neurons and glia remains unclear. DE, whose presence in astrocytes has been reported in vitro (Mutka et al. 2004), may promote release of cholesterol and other lipids by activating liver X receptors (Yang et al. 2006; Wang et al. 2008). Neuron-specific precursors like LT and 7D may affect the stability of – still elusive (Jacobson et al. 2007) – membrane lipid rafts (Megha and London 2006; Vainio et al. 2006) and thereby influence key neuronal features like synapses (Pfrieger 2003b; Lang 2007). Our results further suggest that previously observed differences in levels of specific cholesterol precursors during development (Lutjohann et al. 2002) or in transgenic mice (Valenza et al. 2007; Burgess et al. 2008; Tamboli et al. 2008; Wang et al. 2008) reflect neuronal or glial contributions to cholesterol metabolism.
Taken together, our results add further evidence for the metabolic specialization of neurons and glial cells and underline the importance of neuron-glia interactions for cholesterol metabolism in the brain.
Acknowledgments
The authors thank Dominique Dalencon (INCI, Strasbourg) for excellent administrative help, Pierrette Bouvier-Navé (Institute of Plant Molecular Biology, Strasbourg) for kind advice on TLC techniques, Dimitri Heintz (Institute of Plant Molecular Biology, Strasbourg) for LC-MS analysis, Gaby Ullrich (INCI, Strasbourg) for help with radioactivity regulations and Alex Tsafriri (Weizmann Institute of Science, Rehovot, Israel) and Masato Ohashi (Okazaki Institute for Integrative Bioscience, Okazaki, Japan) for antibodies. This work was supported by stipends and grants from Centre National de la Recherche Scientifique (FWP, HS), Deutsche Forschungsgemeinschaft (Priority Program 1085: “Cellular mechanisms of Alzheimer’s disease”) (KN, FWP), European Commission (ENINET project, Contract No. LSHM-CT-2005-019063) (FWP), Max-Planck Gesellschaft (KN, FWP), Sanofi-Aventis (KN, FWP) and Region Alsace (KN, FWP).




