Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells
Abstract
Alpha-synuclein is a presynaptic protein strongly implicated in Parkinson's disease (PD). Because dopamine neurons are invariably compromised during pathogenesis in PD, we have been exploring the functions of alpha-synuclein with particular relevance to dopaminergic neuronal cells. We previously discovered reduced tyrosine hydroxylase (TH) activity and minimal dopamine synthesis in stably-transfected MN9D cells overexpressing either wild-type or A53T mutant (alanine to threonine at amino acid 53) alpha-synuclein. TH, the rate-limiting enzyme in dopamine synthesis, converts tyrosine to l-dihydroxyphenylalanine (L-DOPA), which is then converted to dopamine by the enzyme, aromatic amino acid decarboxylase (AADC). We confirmed an interaction between alpha-synuclein and AADC in striatum. We then sought to determine whether wild-type or A53T mutant alpha-synuclein might have affected AADC activity in dopaminergic cells. Using HPLC with electrochemical detection, we measured dopamine and related catechols after L-DOPA treatments to bypass the TH step. We discovered that while alpha-synuclein did not reduce AADC protein levels, it significantly reduced AADC activity and phosphorylation in our cells. These novel findings further support a role for alpha-synuclein in dopamine homeostasis and may explain, at least in part, the selective vulnerability of dopamine neurons that occurs in PD.
Abbreviations used
-
- A53T
-
- a-Syn mutant, alanine to threonine at amino acid 53
-
- AADC
-
- aromatic amino acid decarboxylase
-
- a-Syn
-
- alpha-synuclein
-
- DOPAC
-
- dihydroxyphenylacetic acid
-
- L-DOPA
-
- l-dihydroxyphenylalanine
-
- PD
-
- Parkinson's disease
-
- TH
-
- tyrosine hydroxylase
-
- WT
-
- wild-type a-Syn
Parkinson's disease (PD), the second most common neurodegenerative disorder after Alzheimer's disease, is characterized by motor symptoms that occur after nigro-striatal degeneration and loss of dopaminergic neurons (Braak and Braak 2000). Alpha-synuclein (a-Syn) is implicated in the pathogenesis of PD by its abundance in Lewy bodies (Spillantini et al. 1997), by the finding of a-Syn point mutations in familial autosomal dominant PD (A53T, A30P, E46K) (Polymeropoulos et al. 1997; Kruger et al. 1998; Zarranz et al. 2004), and by the identification of multiple copies of wild-type a-Syn in rare families with the disease (Singleton et al. 2003; Chartier-Harlin et al. 2004; Ibanez et al. 2004). The mechanism by which mutations and multiplications of a-Syn result in familial PD is unknown, as are the causes of sporadic PD, yet neuropathological features are similar in most familial and sporadic cases (Bostantjopoulou et al. 2001). Moreover, in animal models, including transgenic mice (Masliah et al. 2000; Giasson et al. 2002; Lee et al. 2002) and Drosophila (Feany and Bender 2000), pathology results from a-Syn overexpression that includes a-Syn inclusions and some neuronal loss, although animal models vary phenotypically (Fernagut and Chesselet 2004).
A role for a-Syn in PD is well established. However, its functions, especially with regard to dopamine neurons, are still unfolding. a-Syn contributes to brain development and synaptic plasticity (Jin and Clayton 1997), binds to and regulates the dopamine transporter (Lee et al. 2001; Wersinger et al. 2003), and affects synaptic vesicles in various models (Murphy et al. 2000; Lotharius et al. 2002; Liu et al. 2004; Yavich et al. 2004). Accumulating data strongly support a chaperone function for a-Syn (Kim et al. 2000), which has homology to the 14-3-3 molecular chaperone proteins (Ostrerova et al. 1999).
Previously, we discovered an interaction between a-Syn and tyrosine hydroxylase (TH; EC 1.14.16.2) that reduced dopamine synthesis, both in dopaminergic MN9D cells transfected with a-Syn and using recombinant a-Syn protein for purely in vitro assays (Perez et al. 2002). This effect occurred, in part, through diminished phosphorylation of TH at key regulatory seryl residues, and was observed in MN9D as well as PC12 cell models (Peng et al. 2005). Importantly, in several rodent models overexpressing a-Syn, reduced TH activity and/or diminished dopamine synthesis is also observed (Masliah et al. 2000; Kirik et al. 2002; Richfield et al. 2002). Furthermore, in a-Syn knockout mice, increased striatal dopamine is available for release at the second stimulus in a paired pulse paradigm (Abeliovich et al. 2000). a-Syn also interacts with additional proteins that contribute to the regulation of dopamine synthesis, including extracellular signal regulated protein kinase (ERK) (Ostrerova et al. 1999; Iwata et al. 2001), protein kinase C (PKC) (Ostrerova et al. 1999) and protein phosphatase 2A (PP2A) (Peng et al. 2005). Together these findings support a key role for a-Syn in the regulation of dopamine synthesis (reviewed in Perez and Hastings 2004).
In our a-Syn overexpressing cell lines, dopamine levels were drastically reduced while steady-state levels of l-dihydroxyphenylalanine (L-DOPA) were not. This suggested that L-DOPA was accumulating rather than being efficiently converted to dopamine. This observation raised the possibility that a-Syn might affect the activity of aromatic amino acid decarboxylase (AADC; EC 4.1.1.28), the enzyme that converts L-DOPA to dopamine (Christenson et al. 1972). AADC activity is regulated in the CNS, but relatively little is known about the regulatory mechanisms. In retina, AADC activity increases following light exposure (Hadjiconstantinou et al. 1988) and in striatum, AADC activation occurs following dopamine receptor activation (Zhu et al. 1992; Hadjiconstantinou et al. 1993). Short-term activation of AADC is independent of protein synthesis (Zhu et al. 1993) or mRNA transcription (Cho et al. 1997) but can be stimulated by the cAMP-dependent protein kinase, PKA, which phosphorylates both striatal and recombinant AADC (Duchemin et al. 2000). Curiously, studies in bovine adrenal chromaffin cells showed no evidence of AADC regulation by phosphorylation (Waymire and Haycock 2002), indicating that CNS AADC might be modulated somewhat differently to peripheral AADC. Indeed, alternate promoters in the AADC gene are utilized in neuronal and non-neuronal tissues in rat (Albert et al. 1992; Jahng et al. 1996) and in human (Le Van Thai et al. 1993). Here, we explored the impact of wild-type (WT) and the A53T (alanine to threonine at amino acid 53) mutant a-Syn on AADC activity, and discovered that both forms of a-Syn significantly lowered AADC activity in multiple dopaminergic clonal cell lines.
Methods
Reagents
Unless otherwise specified, all reagents were obtained from Sigma-Aldrich (St Louis, MO, USA).
Cell lines
MN9D (Choi et al. 1991, 1992) and PC12 cells are widely used dopaminergic cell models (Heller et al. 2000, 2005; Perez et al. 2002; Hermanson et al. 2003; Peng et al. 2005). Stably-transfected clonal lines of MN9D and PC12 cells exhibited normal viability and were cultured as previously described (Perez et al. 2002; Peng et al. 2005). Briefly, cells grown to 70–80% confluence in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 50 U/mL penicillin, 50 µg/mL streptomycin and 200 µg/mL G418, were used 18–24 h after plating.
Dopamine synthesis and AADC activity assays
Intact cells
MN9D cells were treated with 100 µm L-DOPA for 6 h, then resuspended in Dulbecco's phosphate buffered saline (DPBS) at 4°C. Baseline studies confirmed that cells remained viable during L-DOPA treatment (not shown). An aliquot of cells was collected for total protein assay [bicinchoninic acid (BCA); Pierce Biotechnology, Rockford, IL, USA]. Protein in the remaining fraction was precipitated with 1.0 N perchloric acid and then centrifuged. An aliquot of the resulting supernatant fraction was alumina-extracted and analyzed for L-DOPA, dopamine and dihydroxyphenylacetic acid (DOPAC; a metabolite of dopamine) by HPLC with electrochemical detection (Hastings and Zigmond 1994).
Cell extracts
AADC activity was directly measured by an in vitro assay (Reinhard et al. 1986) with minor modifications (J. Waymire, University of Texas Houston Medical School, personal communication). Briefly, 1 × 106 cells were collected in DPBS for BCA assay, or in 100 µL ice-cold lysis buffer [phosphate-buffered saline (PBS; pH 7.0), 4-(2-aminoethyl)-benzenesulfonylfluoride (AEBSF; 100 µg/mL), leupeptin (5 µg/mL) and aprotinin (5 µg/mL)], then sonicated and centrifuged at 22 000 g, 4°C. Aliquots of the supernatant fraction were added to assay buffer on ice [PBS pH 7.0, EDTA (125 µm), β-mercaptoethanol (1.25 µm), dithiothreitol (1.25 µm), pyridoxal-5-phosphate (12.5 µm), L-DOPA (250 µm) and pargyline (125 µm, to prevent dopamine breakdown)]. Reactions were begun by mixing lysates with buffer and incubating at 37°C, and halted at 15 min with addition of 1.0 N perchloric acid. Samples were stored at − 80°C until analysis by HPLC. Multiple clonal lines were evaluated.
Immunoblotting and antibodies
Cells were lysed in 1% NP-40 buffer containing protease inhibitors. Total protein was determined by BCA assay (Pierce). The sensitivity and linearity of the AADC antibodies for immunoblots was confirmed in a range from 2.5 to 60 µg total protein. We used 20–40 µg total protein for sodium dodeyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Immunoblots were reacted with antibodies to a-Syn [Syn-1 monoclonal antibody (Mab), 610786: BD Biosciences, San Jose, CA, USA], AADC [polyclonal antibody (Pab) 1569: Chemicon, Temecula, CA, USA; or MAb D0180: Sigma/Aldrich], actin (MAb JLA20: Oncogene Science, Cambridge, MA, USA), phosphoserine (Khundmiri et al. 2004) (rabbit anti-phosphoserine Pab: RDI, Concord, MA, USA), TH phospho-Ser40 (Chemicon AB5935) or Akt Phospho-Ser473 (Cell Signaling 9271S: Beverly, MA, USA). Peroxidase-conjugated secondary antibodies (Calbiochem, San Diego, CA, USA) were applied for 1 h at 22°C. Chemiluminescent signal (Dupont/NEN, Boston, MA, USA) was assessed within a linear range similar to our previous studies (Perez et al. 2002) and quantitated using imagequant 5.1 (Amersham Biosciences/GE Healthcare, Piscataway, NJ, USA). AADC protein levels were normalized to total actin, as a loading control.
Immunoprecipitation
Cells were lysed as above and pre-cleared with Protein G beads (Zymed, San Francisco, CA, USA) and 5% bovine serum albumin (BSA; Sigma/Aldrich), followed by overnight incubation in D0180 AADC antibody using previously described methods (Perez et al. 1996).
Co-immunoprecipitation
All steps were carried out at 4°C as described by Peng et al. (2005), but with minor modifications. Briefly, rat striatal tissues were homogenized in co-immunoprecipitation buffer and supernatant fractions were pre-cleared with protein A and protein G beads prior to incubations in: primary antibody (Syn-1 or D0180), pre-absorbed Syn-1 antibody, or beads alone. Immune complexes were separated on 4–20% Tris-Glycine SDS–PAGE gels (Cambrex, Rockland, ME, USA), transferred to nitrocellulose, and visualized by chemiluminescence from immunoblots reacted in anti-AADC (D0180) or anti-Syn-1 antibody.
Statistics
Data were analyzed using anova or t-test using Instat or Prism software (GraphPad, San Diego, CA, USA). Post hoc tests were carried out using the methods of Bonferroni or Tukey–Kramer for data significant at p < 0.05. Data represent the mean ± SEM from experiments repeated two to five times on duplicate or triplicate samples.
Results
a-Syn and AADC interact in vivo
To test whether a-Syn and AADC interact in brain, we performed co-immunoprecipitation experiments using rat striatum. Immunoprecipitation with an AADC-specific antibody brought down abundant AADC protein (Fig. 1, lane 2). Immunoprecipitation with the a-Syn antibody revealed co-immunoprecipitation of AADC with a-Syn from rat striatal homogenates (Fig. 1, lane 4), although only 28% as much AADC was co-immunoprecipitated with the a-Syn antibody (n = 2, 28.1 ± 0.4, p < 0.0001). Conversely, AADC antibody immunoprecipitated a-Syn from rat striatum. However, the levels were quite low, perhaps indicating that only a subset of a-Syn molecules interact with AADC at any time (not shown). No AADC was immunoprecipitated by beads alone (Fig. 1, lane 1) or with a pre-absorbed a-Syn antibody (Fig. 1, lane 3). These findings revealed an association between AADC and a-Syn in striatum, with potential physiological relevance.
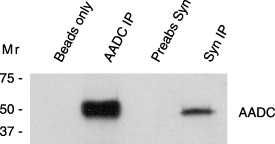
AADC and a-Syn co-immunoprecipitate from rat striatum. A representative immunoblot shows the levels of AADC immunoprecipitated from rat striatum using an AADC-specific antibody, D0180 (lane2) or a-Syn-specific antibody, Syn-1 (lane 4). Samples performed using beads alone (lane 1) or pre-absorbed a-Syn antibody (lane 3) served as negative controls and did not display any AADC signal. Apparent molecular weights as determined from pre-stained standards are indicated on the left.
a-Syn overexpression did not diminish AADC protein levels in dopaminergic cells
Previously, we showed that TH activity is dramatically inhibited by a-Syn overexpression in MN9D and PC12 cells, although a-Syn did not significantly reduce TH protein levels (Perez et al. 2002; Peng et al. 2005). To explore the potential impact of a-Syn on AADC in these same cell lines, we measured AADC protein levels by immunoblots and found equivalent AADC levels in plasmid-transfected control cells, WT a-Syn, and A53T a-Syn MN9D cells (Fig. 2a). When blots were re-probed for actin, those levels also were not significantly different between samples (Fig. 2b). As previously demonstrated, a-Syn levels were increased several fold in a-Syn lines compared with endogenous a-Syn levels seen in plasmid-transfected MN9D control cells (Perez et al. 2002) (2, 5).
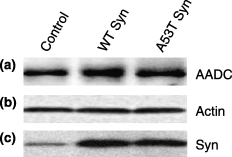
AADC, actin and a-Syn levels in stably-transfected MN9D cells. (a) Representative immunoblot showing AADC levels in plasmid-transfected control MN9D cells, WT a-Syn MN9D cells and A53T a-Syn MN9D cells. (b) Actin levels, shown as a loading control, from the same immunoblot in (a), which was stripped and reprobed. (c) Parallel blot showing a-Syn levels from the same cells as in (a) and (b).
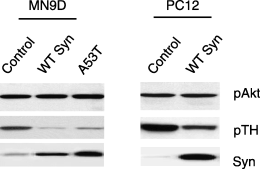
Phosphorylation of Akt is similar in control and a-Syn overexpressing MN9D and PC12 cells. Representative immunoblots showing levels of (a) Akt phosphorylated on Ser473, which is equivalent in all cells; (b) TH phosphorylated on Ser40, which is reduced only in a-Syn overexpressing cell lines; and (c) total a-Syn levels, which are low in control MN9D and PC12 cells but increased in a-Syn MN9D and PC12 cell lines.
a-Syn reduced AADC activity
To assess the impact of a-Syn on AADC activity in stably-transfected MN9D and PC12 cells, we measured dopamine synthesis following L-DOPA treatment to bypass the TH step. To confirm that a-Syn overexpression had not affected L-DOPA uptake, intracellular L-DOPA levels were measured after treatments with 100 µm L-DOPA. Intracellular L-DOPA uptake was similar in control, WT a-Syn and A53T a-Syn MN9D cells (Table 1). An equivalent uptake of L-DOPA was also observed in plasmid-transfected control PC12 cells and in WT a-Syn overexpressing PC12 cells, revealing that a-Syn overexpression did not alter L-DOPA uptake in either MN9D or PC12 cells (Table 1).
| L-DOPA (pmol/µg protein) | DOPAC (pmol/µg protein) | |||
|---|---|---|---|---|
| Untreateda | L-DOPAb | Untreateda | L-DOPAb | |
| MN9D cells | ||||
| Control | 0.213 ± 0.033 | 1.803 ± 0.489 | 0.025 ± 0.006 | 0.096 ± 0.026*** |
| WT a-Syn | 0.241 ± 0.202 | 1.676 ± 0.725 | 0.003 ± 0.002* | 0.003 ± 0.002 |
| A53T a-Syn | 0.280 ± 0.081 | 2.171 ± 0.898 | 0.009 ± 0.001* | 0.008 ± 0.003 |
| PC12 cells | ||||
| Control | 0.249 ± 0.059 | 1.569 ± 0.350 | 0.061 ± 0.013 | 0.327 ± 0.055*** |
| WT a-Syn | 0.297 ± 0.117 | 1.506 ± 0.441 | 0.022 ± 0.004 | 0.191 ± 0.065 |
- a 0.0 µ m L-DOPA, b100 µm L-DOPA for 6 h. Mean ± SEM performed on duplicate samples from 2 to 3 independent experiments. *p < 0.05 within treatments. ***p < 0.001 between treatments.
Dopamine content following L-DOPA treatment was significantly increased in control MN9D cells (Fig. 3a). Surprisingly, in WT a-Syn or in A53T a-Syn MN9D cells no statistically significant differences in dopamine content were observed following L-DOPA treatment (Fig. 3a), although we noticed that both cells lines showed a trend towards a small increase over multiple experiments. It is noteworthy that dopamine levels in a-Syn overexpressing cells, even after L-DOPA treatments, were fourfold lower than dopamine levels in untreated control cells at baseline. Similarly, dopamine levels did not significantly increase in WT a-Syn overexpressing PC12 cells after L-DOPA treatments, although statistically significant increases were measured in control PC12 cells (Fig. 3b). DOPAC levels were also quantified after L-DOPA treatments to assess dopamine breakdown. Neither MN9D nor PC12 cells overexpressing a-Syn significantly increased DOPAC levels following L-DOPA treatments (Table 1), whereas control cells exhibited significantly increased DOPAC levels in parallel with their increased levels of dopamine synthesis (Table 1, Fig. 3a). Considering that all cells had equivalent intracellular L-DOPA uptake and equivalent AADC protein levels, these data suggest that a-Syn contributes to inhibition of AADC activity.
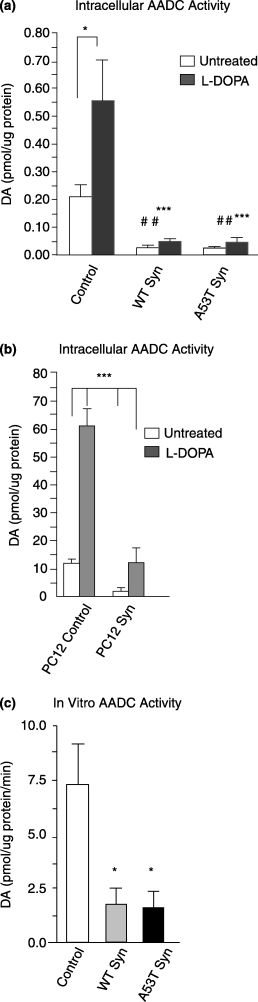
a-Syn effects on AADC activity in MN9D and PC12 cells. (a) MN9D cells treated with 100 µm L-DOPA for 6 h were analyzed for DA content by HPLC. DA levels were significantly increased by L-DOPA treatment only in control MN9D cells. WT and A53T a-Syn cells had significantly less DA, both at baseline and after L-DOPA, compared with control MN9D cells. Data represent the mean ± SEM from duplicate samples in two independent experiments; *p < 0.05 untreated control MN9D compared with L-DOPA-treated control MN9D; ##p < 0.01 untreated WT and A53T a-Syn compared with untreated control MN9D; ***p < 0.001 WT and A53T a-Syn cells compared with L-DOPA-treated control MN9D cells. (b) PC12 cells treated with 100 µm L-DOPA for 6 h were analyzed for DA content by HPLC. Data represent the mean ± SEM from duplicate or triplicate samples in three independent experiments; ***p < 0.001 for control PC12 treated with 100 µm L-DOPA compared with other conditions. (c) In vitro assay of AADC activity using cell lysates after the addition of 250 µm L-DOPA and all the necessary co-factors for dopamine synthesis, plus pargyline to block dopamine degradation. Dopamine levels were measured after 15 min reactions as described in methods. Data represent the mean ± SEM for triplicate samples from three independent experiments; *p < 0.05.
To bypass any potential effect on vesicular dopamine packaging or release in our a-Syn cells, we used a well characterized in vitro assay to measure directly the ability of AADC to convert L-DOPA to dopamine. We added 250 µm L-DOPA to cell extracts prepared from equal numbers of cells, then measured dopamine synthesis by HPLC after 15 min reactions (see Materials and methods for details). Similar to what we noted for intact cells treated with L-DOPA, AADC in cell extracts from control MN9D cells produced significantly more dopamine than WT a-Syn or A53T a-Syn MN9D AADC (Fig. 3c), further supporting an inhibitory effect of a-Syn on AADC activity.
MN9D cells are a fusion of embryonic day 14 midbrain dopamine neurons to N18TG2 neuroblastoma cells and, several years ago, they were confirmed to synthesize primarily the neurotransmitter, dopamine (Choi et al. 1991). However, we noted very low but measurable levels of serotonin in our MN9D cells by HPLC. Because AADC mediates the conversion of 5-hydroxytryptophan to serotonin as well as of L-DOPA to dopamine, we exploited the finding of serotonin in our cells as an independent measure of AADC activity. Serotonin levels from control MN9D, WT a-Syn MN9D and A53T a-Syn MN9D cells were measured in parallel with dopamine in a subset of experiments. We noted 40% lower serotonin levels in a-Syn overexpressing MN9D cells compared with control MN9D cells (p < 0.05), suggesting that reduced AADC activity towards 5-hydroxytryptophan also occurred in cells overexpressing a-Syn.
a-Syn overexpression reduced serine phosphorylation on AADC
One mechanism by which a-Syn might affect AADC activity is by binding to AADC in a manner such as to affect its phosphorylation. Regulation of AADC activity by phosphorylation has been described by others (Duchemin et al. 2000). Therefore, we explored the phosphorylation state of the AADC from control and WT a-Syn overexpressing MN9D cells. We immunoprecipitated AADC from cells, then assessed its phosphorylation state on immunoblots using a well characterized phospho-serine-specific antibody. We found that although equal amounts of total AADC could be immunoprecipitated from both control and WT a-Syn cells (Fig. 4a), the AADC from control cells was approximately 65% more phosphorylated than that from a-Syn overexpressing cells (Fig. 4b; n = 3, 66.33 ± 4.37, p < 0.01). These data are suggestive of an effect of a-Syn on AADC phosphorylation. However, additional experiments, such as proteomic analyses, are required to delineate firmly the phosphorylation status of AADC, and to establish firmly which seryl residues may be phosphorylated.
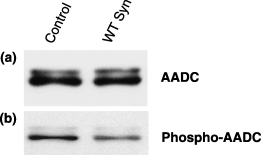
Reduced phosphorylation of serine residues on AADC in a-Syn MN9D cells. AADC was immunoprecipitated from control MN9D cells and WT a-Syn MN9D cells. A representative immunoblot reveals (a) total AADC immunoprecipitated from cells and (b) the phosphorylation state of the immunoprecipitated AADC as determined using a phospho-serine-specific antibody.
One mechanism by which serine phosphorylation can be reduced is by dephosphorylation of serine by an active PP2A phosphatase. We previously demonstrated that both MN9D and PC12 a-Syn overexpressing cells have a doubling of PP2A activity (Peng et al. 2005). To evaluate whether PP2A activation in our a-Syn cells generalized to other substrates, we also measured the phosphorylation state of the Akt kinase, a well characterized PP2A substrate that, when phosphorylated, is anti-apoptotic (Brunet et al. 1999). We found equivalent levels of both total Akt (not shown) and phosphorylated Akt in MN9D and PC12 cells (Fig. 5a), even when a-Syn was overexpressed (Fig. 5c). As a control, we reconfirmed a reduction in TH phosphorylation in our a-Syn cells (Fig. 5b). Our findings, from multiple experiments, reveal that reduced phosphorylation occurs on some, but not all, PP2A substrates in our a-Syn overexpressing cell lines.
Discussion
Dopamine biosynthesis from a tyrosine substrate requires the activity of two separate dopamine biosynthetic enzymes, TH and AADC. We previously identified a role for a-Syn as an inhibitor of dopamine synthesis by binding to and reducing the activity of TH in both cells and in vitro assays (Perez et al. 2002). Here, we explored the impact of a-Syn on AADC activity. Using co-immunoprecipitation from striatal tissues, we found that a-Syn interacts with AADC in brain. Furthermore, this interaction appears to be physiologically relevant, serving to alter AADC activity as detailed below.
We previously saw a dramatic reduction in dopamine synthesis at baseline in both MN9D (Perez et al. 2002) and PC12 dopaminergic cells that overexpressed a-Syn (Peng et al. 2005). In our current studies, we saw significantly reduced dopamine synthesis in cells overexpressing a-Syn, even after L-DOPA treatments that bypassed any contribution of TH. Control MN9D and PC12 cells had much higher dopamine levels at baseline compared with a-Syn cells, and their dopamine levels increased dramatically after exogenous L-DOPA treatments. In contrast to control cells, cells overexpressing WT a-Syn or A53T a-Syn had extremely low baseline dopamine levels that increased slightly after L-DOPA, although not significantly so as determined by anova. In fact, treatment of a-Syn overexpressing cells with L-DOPA increased dopamine but to such a small extent that those levels were still fourfold less than control cell baseline dopamine levels, and 10-fold less than dopamine levels noted in L-DOPA-treated control cells. We demonstrated that the effect on AADC by WT a-Syn and A53T a-Syn was not due to reduced AADC protein levels but rather, was associated with reduced AADC activity as confirmed in both cellular and in in vitro assays. Because levels were significantly reduced by both WT a-Syn and A53T a-Syn overexpression, both WT and mutant a-Syn appear to be equally able to diminish AADC activity, suggesting no loss of function for the A53T mutation in a-Syn regarding dopamine synthesis.
Because others have seen effects of a-Syn on synaptic vesicles (Murphy et al. 2000; Lotharius and Brundin 2002; Liu et al. 2004; Yavich et al. 2004), we also tested AADC activity, using a purely in vitro assay, to eliminate any effect of a-Syn on vesicles and to confirm our intracellular AADC data. For these in vitro studies, cell extracts were supplemented with all the factors necessary for dopamine synthesis and with a monoamine oxidase inhibitor to prevent dopamine breakdown. We found equivalent reductions in AADC activity from WT a-Syn and A53T a-Syn cell extracts in vitro, providing compelling evidence that the activity of AADC is significantly reduced by an interaction of the enzyme with either WT or A53T a-Syn.
Considerable evidence supports AADC regulation in CNS neurons (Hadjiconstantinou et al. 1988, 1993; Naoi et al. 1988a,b; Zhu et al. 1992, 1993; Duchemin et al. 2000), although AADC activity does not appear to be regulated in bovine adrenal chromaffin cells (Waymire and Haycock 2002). In the CNS, AADC is regulated, at least in part by phosphorylation (Duchemin et al. 2000). In our cells, we previously noted inhibition of TH activity by reduced phosphorylation of Ser40 (Peng et al. 2005), a residue in the TH regulatory domain that stimulates both TH activation and dopamine synthesis (McCulloch et al. 2001). The major kinase that phosphorylates TH at Ser40 is PKA (Campbell et al. 1986), which also stimulates AADC activity and phosphorylates striatal AADC protein (Zhu et al. 1992, 1993; Young et al. 1993; Duchemin et al. 2000). We previously confirmed that our a-Syn overexpressing MN9D and PC12 cells maintain normal PKA levels and PKA activity (Peng et al. 2005), suggesting that the diminished phosphorylation of AADC in our cells was not due to changes in PKA activity. Interestingly, it has been demonstrated that dopamine receptor antagonists stimulate PKA and increase serine phosphorylation on both TH and AADC in animal models (Zhu et al. 1992, 1993; Hadjiconstantinou et al. 1993; Cho et al. 1997; Salvatore et al. 2000; Hakansson et al. 2004). These data suggest that physiological stimuli simultaneously activate both TH and AADC to increase neuronal dopamine synthesis. AADC activity in a-Syn cells was affected for both L-DOPA and l-tryptophan, the substrate precursors for dopamine and serotonin, as measured using HPLC. Interestingly, an effect on AADC activity has been described for rats treated in vivo with serotonin receptor antagonists, in which serotonergic neurons were found to increase their serotonin levels (Tohyama et al. 2001). However, AADC phosphorylation was not examined in those studies. While our data using a phosphoserine antibody are very suggestive of an effect of a-Syn on AADC phosphorylation, additional experiments, such as proteomics analyses, are required to verify serine phosphorylation in our cells.
We previously demonstrated that dephosphorylation of TH Ser40 in a-Syn MN9D and PC12 cells occurred in parallel with an activation of PP2A (Peng et al. 2005). As PP2A regulates dephosphorylation of many cellular substrates, including the protein kinase Akt, we assessed the phosphorylation state of Akt in our cells. Because phosphorylated Akt enhances cell viability, and because we have previously demonstrated that a-Syn overexpression does not reduce viability of either MN9D or PC12 cells (Perez et al. 2002; Peng et al. 2005), we were not surprised that the phosphorylation state of Akt in our a-Syn overexpressing cells was normal. Importantly, others have shown that the a-Syn homolog, beta-Syn, can interact with Akt while a-Syn does not (Hashimoto et al. 2004), and our Akt data appear to corroborate this finding because a-Syn overexpression did not affect Akt phosphorylation. Furthermore, our data lead us to speculate that dephosphorylation of PP2A substrates in a-Syn overexpressing cells occurs only for substrates that interact with a-Syn.
In summary, we find significantly diminished serine phosphorylation and reduced AADC activity in dopaminergic cells that overexpress a-Syn. We previously confirmed that these cells have enhanced PP2A activity (Peng et al. 2005). Therefore, the most parsimonious interpretation of our data is that a-Syn diminishes phosphorylation and activity of TH and AADC by activating PP2A, although studies to tease apart the mechanisms for a-Syn effects on AADC through PP2A are still required. Nonetheless, our findings reveal that in addition to interacting with, and inhibiting TH activity, a-Syn also interacts with, and inhibits the AADC enzyme. Cumulatively, our findings further implicate a-Syn in regulating dopamine homeostasis, a function that may be compromised during PD pathogenesis as Lewy bodies and Lewy neurites form.
Acknowledgements
This work is dedicated to M. J. Fox, R. Byer and J. Cordy, and to the memory of Lester ‘Rusty’ Lanelli. We thank Michael Zigmond for the gift of rat tissues, Juliann Jaumotte for critical reading of the manuscript, Nicole Kotchey for technical assistance, Al Heller and Lisa Won for baseline MN9D cells, Leonidas Stefanis for transfected PC12 cells, and Edward Burton for helpful comments and suggestions. We are grateful for financial support from the Michael J. Fox Foundation and NINDS, NS42094.




