Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo
Abstract
The tangles of Alzheimer's disease (AD) are comprised of the tau protein displaying numerous alterations, including phosphorylation at serine 422 (S422) and truncation at aspartic acid 421 (D421). Truncation at the latter site appears to result from activation of caspases, a class of proteases that cleave specifically at aspartic acid residues. It has been proposed that phosphorylation at or near caspase cleavage sites could regulate the ability of the protease to cleave at those sites. Here, we use tau pseudophosphorylated at S422 (S422E) to examine the effects of tau phosphorylation on its cleavage by caspase 3. We find that S422E tau is more resistant to proteolysis by caspase 3 than non-pseudophosphorylated tau. Additionally, we use antibodies directed against the phosphorylation site and against the truncation epitope to assess the presence of these epitopes in neurofibrillary tangles in the aged human brain. We show that phosphorylation precedes truncation during tangle maturation. Moreover, the distribution of the two epitopes suggests that a significant length of time (perhaps as much as two decades) elapses between S422 phosphorylation and cleavage at D421. We further conclude that tau phosphorylation at S422 may be a protective mechanism that inhibits cleavage in vivo.
Abbreviations used
-
- AD
-
- Alzheimer's disease
-
- ERC
-
- entorhinal cortex
-
- Fus
-
- fusiform gyrus
-
- ITG
-
- inferior temporal gyrus
-
- MTG
-
- middle temporal gyrus
-
- NFT
-
- neurofibrillary tangle
-
- PMI.
-
- post-mortem interval
-
- STG
-
- superior temporal gyrus
Alzheimer's disease (AD) is characterized by the intracellular accumulation of the microtubule binding protein tau in the form of paired helical and straight filaments, which results in the deposition of neurofibrillary tangles (NFT) in the cerebral cortex. The tau in these filaments displays numerous abnormal alterations, some of which appear to be directly involved in filament formation. One such change involves proteolysis of tau by the caspase class of proteases, which are enzymes essential to the process of apoptosis. Multiple lines of evidence suggest that cleavage of tau at aspartic acid 421 (DMVD421↓S422) by caspase 3 (EC 3.4.22) is intimately involved in NFT formation: in vitro, cleavage at D421 is associated with disruption of the microtubule network (Canu et al. 1998) and results in enhanced fibril formation (Gamblin et al. 2003b); in tissue culture, cortical neurons undergoing β-amyloid-induced apoptosis display a higher level of caspase-cleaved tau than do untreated cultures (Fasulo et al. 2000; Gamblin et al. 2003b); and D421-cleaved tau is found in the neurofibrillar manifestations of AD pathology, including NFT, dystrophic neurites, and neuropil threads in both demented and non-demented aged individuals (Gamblin et al. 2003b; Guillozet-Bongaarts et al. 2005).
Immediately carboxy terminal to D421 is a serine (S422) at the so-called P1′ position that is phosphorylated in AD (Ikegami et al. 1996; Kimura et al. 1996; Augustinack et al. 2002). Intriguingly, phosphorylation at or near the caspase cleavage site in other proteins inhibits proteolysis. For example, presenilin-1 and presenilin-2 contain a serine residue at the P1′ site, and IFκB can be phosphorylated at the P4′ site; in these proteins, phosphorylation at these sites has been shown to inhibit cleavage (Barkett et al. 1997; Walter et al. 1999; Fluhrer et al. 2004). However, it is not known if phosphorylation of tau at S422 regulates caspase cleavage of tau in a similar manner. Accordingly, in the present study, we investigated alterations of caspase cleavage kinetics in vitro caused by pseudophosphorylation of tau at S422, and we demonstrate that the S422E tau pseudophosphorylation mutant is relatively resistant to caspase proteolysis.
We were also compelled to investigate this question in post-mortem human tissue. The possible effects of phosphorylation on cleavage are, of course, harder to discern in the human brain, but it is possible to determine if the S422 phosphorylated epitope precedes that created by cleavage at D421. This staging of events is possible because NFTs undergo an orderly process of maturation as they accumulate progressively in various regions of the temporal lobe in aged individuals and those with AD (Garcia-Sierra et al. 2003; Guillozet-Bongaarts et al. 2005). Here, we perform a quantitative histochemical examination of human tissue for the presence of both the phospho-S422 and D421-cleavage epitopes in order to determine the temporal sequence of these events in tangle maturation. Our results show that phosphorylation at S422 precedes cleavage at D421 and suggests that phosphorylation of tau at S422 is a mechanism by which caspase proteolysis of tau is regulated.
Materials and methods
Production and cleavage of recombinant tau
The expression and purification of full-length tau (hTau40, 2N4R) (Goedert et al. 1989) has been previously described (Carmel et al. 1996; Abraha et al. 2000; Gamblin et al. 2003a). Novel mutations containing pseudophosphorylated tau (S422E) or non-phosphorylatable tau (S422A) were generated using hTau40 and a QuickChange Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The forward and reverse mutant primers used in the reaction were as follows: S422E forward GACATGGTAGACGAGCCCCAGCTCGCC and reverse CTGTACCATCTGCTCGGGGTCGAGCGG; S422A forward GACATGGTAGACGCGCCCCAGCTCGCC and reverse CTGTACCATCTGCGCGGGGTCGAGCGG. Mutations were confirmed by DNA sequencing, and tau concentrations were determined using the method of Lowry et al. (1951).
To assess the ability of caspase 3 to cleave the recombinant tau proteins, active caspase 3 (25 μg/mL; Promega, Madison, WI, USA) was added to hTau40, S422A tau, and S422E tau (100 μg/mL) in buffer containing 0.1 m sodium chloride, 0.02 m HEPES pH 7.4 and 1.25 mm dithiothreitol, and incubated at 37°C (Cryns et al. 1996). Samples were withdrawn at various times and analyzed by sodium dodecyl sulfate – polyacrylamide gel electrophoresis and western blotting using Tau-5 (20 ng/mL), Tau-C3 (20 ng/mL), and Tau46.1 (20 ng/mL), monoclonal antibodies that recognize the proline rich region, S210–R230 (Carmel et al. 1996), the D421 truncation (Gamblin et al. 2003b; Guillozet-Bongaarts et al. 2005), and the extreme C-terminus, L428–L441 (Carmel et al. 1996), respectively. Horseradish peroxidase-conjugated secondary antibody and enhanced chemoluminescence were used to visualize bands. Western blots using the Tau-5 antibody to show total tau were digitized; optical densities of the resulting bands were calculated, and the percentage of tau that was cleaved was measured using gel-analysis software (NIH Image, http://rsb.info.nih.gov/nih-image). Statistical software (Prism 3.0; SAS Institute, Cary, NC, USA) was used to fit the data to a single-site hyperbolic binding curve to determine the half-time for substrate cleavage or, in the case of S422E, to a linear regression curve.
Immunohistochemistry
Tissue from 12 non-AD subjects, ranging in pathological severity from Braak stage 0 to IV, was obtained from the Northwestern Alzheimer Disease Center Neuropathology Core. For the purposes of this study, ‘non-AD’ refers to tissue which contained AD-type pathology, but was insufficient to be classified as Braak stage V or VI. Relevant information regarding age, post-mortem interval (PMI), and Braak stage can be found in Table 1. Coronal blocks approximately 2–3-cm thick from each brain were fixed in 4% paraformaldehyde for 30 h, followed by cryoprotection in ascending concentrations of sucrose (10 to 40%) in 0.1 m phosphate buffer, pH 7.4. The tissue block containing the posterior amygdala and/or anterior hippocampus was frozen on dry ice and 40-μm sections were cut and collected in phosphate buffer with 0.02% sodium azide.
| Case no. | Age | Gender | PMI | Braak stage | Severity level |
|---|---|---|---|---|---|
| 1 | 68 | F | 6 | 0 | 0 |
| 2 | 81 | F | 9.5 | I | 0 |
| 3 | 87 | F | 8 | I | 0 |
| 4 | 81 | F | 7 | II | 2 |
| 5 | 90 | M | 2.5 | III | 3 |
| 6 | 95 | F | 3.25 | III | 3 |
| 7 | 90 | F | 15 | I | 4 |
| 8 | 92 | F | 3.5 | III | 4 |
| 9 | 91 | M | 12 | III | 4 |
| 10 | 77 | M | 17 | II | 5 |
| 11 | 102 | F | 5.5 | IV | 5 |
| 12 | 90 | M | 3 | IV | 5 |
To determine the relationship between phosphorylation at S422 and cleavage at D421 in tissue, sections were single- or double-labeled with the monoclonal antibody Tau-C3 (400 ng/mL) (Gamblin et al. 2003b; Guillozet-Bongaarts et al. 2005), and the polyclonal antibody pS422 (1 : 1000, Biosource, Camarillo, CA, USA), which recognizes tau phosphorylated at S422 (Reed et al. 1998; Kang et al. 2005; Pedraza et al. 2005; Rahman et al. 2005). For bright-field microscopy, species-specific secondary antibodies conjugated to biotin or alkaline phosphatase (1 : 1000, Jackson ImmunResearch, West Grove, PA, USA) were employed, followed by enhancement with the Vector Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). For single-label bright-field studies, the reaction product was visualized with diaminobenzidine (DAB); for double-label bright-field studies, the reaction product was visualized using the Nova Red kit and the AP substrate kit III (Vector Laboratories). For confocal microscopy, secondary antibodies conjugated to Texas Red, FITC, or CY-5 were used as previously described (Garcia-Sierra et al. 2003; Guillozet-Bongaarts et al. 2005). In some cases, tissue was also labeled with Thiazine Red to identify compact tangles. All antibodies were titered on tissue from cases with severe AD to find an appropriate subsaturating concentration, thus allowing efficacious comparisons between antibodies of varying affinities.
Tissue analysis
With the aid of StereoInvestigator software (Microbrightfield), temporal lobes from double-labeled sections were traced, and the position of each tangle or pre-tangle that labeled with either or both of the antibodies was plotted. All five major gyri of the temporal lobe were mapped: parahippocampal gyrus/entorhinal cortex (ERC), fusiform/occipito-temporal gyrus (Fus), inferior temporal gyrus (ITG), middle temporal gyrus (MTG), and superior temporal gyrus (STG). Only gyri that contained more than 15 NFT were included for statistical analysis; of the 12 cases studied, three cases contained too few NFT in any gyrus to be used in statistical analysis (Braak stage 0-I). The remaining nine cases displayed a widely varied distribution of NFT.
In order to assess the association of the different epitopes with tangles of different maturational stages, we took advantage of the ordered progression of pathology through the temporal lobe that occurs during normal aging (Braak and Braak 1991). To do so, the gyri in each case were assigned a severity level ranging from 1 to 5, with 1 representing the area with the least number of NFT, and each increasingly affected area receiving a higher rating (Fig. 1). Therefore, the maximum severity rating was suggestive of the severity of pathology. All cases that received a severity rating of ‘3’ contained staining in the entorhinal cortex, fusiform gyrus, and inferior temporal gyrus. Those with NFT in the middle temporal gyrus either retained a maximum severity rating of ‘3’ if the number of NFT in the MTG did not exceed 10, or received a maximum rating of ‘4’ if the number in the MTG exceeded 10. This allowed us to separate the population of tangles based on their average age as determined by their anatomical locations. The percentage of tangles within each severity level that were labeled by either of the two antibodies alone was compared using a Student's t–test; possible interactions between the epitope and the severity level were assessed with a factorial anova. Linear regression was performed to assess the relationship of Tau-C3 and pS422 labeling. anova analysis revealed no significant difference in PMI between the different Braak stages or severity levels, and no difference in age for those subjects used in statistical analysis. All statistical analyses were performed using Statview Software version 5.0.1 (SAS Institute).
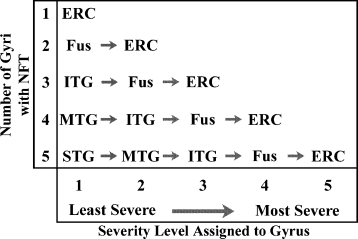
In order to compare tangles at the same relative stage of maturation, the gyri were ordered in severity from the least severe to the most severe. In an individual with mild NFT accumulation reaching only the fuisform gyrus, the tangles within the fusiform should be comparable in maturation to the tangles found in the STG of an individual with more extensive tangle formation. The arrows show the increase in the severity of pathology within a single case, from least severe to most severe gyrus.
Results
It has been postulated that phosphorylation/dephosphorylation of some substrates may serve as a regulatory mechanism by which proteolytic cleavage is controlled (Tozser et al. 2003). Tau contains a putative regulatory site in the P1′ position (S422) of its known caspase 3 cleavage site (Gamblin et al. 2003b), but there have been no previous reports suggesting that this site, which is phosphorylated in NFT (Kimura et al. 1996), acts as a regulator of caspase cleavage. We therefore sought to determine if phosphorylation of S422 inhibited caspase cleavage at D421 and, if so, whether evidence of that inhibition could be found in the brain.
Effect of pseudophosphorylation on tau cleavage by caspase 3
As a result of the extensive number of phosphorylation sites on tau, and the phosphorylation of multiple sites by individual kinases, it is nearly impossible to control the phosphorylation of a specific residue on the tau molecule when using exogenous kinases. For this reason, a specific residue was pseudophosphorylated by site-directed mutagenesis, mutating the serine at position 422 to a glutamic acid in order to mimic phosphoryalation (S422E), or to an alanine as a control mutation (S422A). Pseudophosphorylation of pathologically relevant sites has been used extensively to examine the effects of phosphorylation at both single and multiple specific residues of the tau molecule (Abraha et al. 2000; Fath et al. 2002; Necula and Kuret 2004, 2005).
Recombinant hTau40, S422E tau, and S422A tau were incubated with active caspase 3 for varying lengths of time, and the percentage of cleaved tau was determined. Cleavage of non-pseudophosphorylated hTau40 was apparent within 5 min, and tau was completely proteolyzed by 1 h of incubation (Fig. 2a, top). In contrast, the pseudophosphorylated tau mutant, S422E, was much less susceptible to caspase 3 cleavage (Fig. 2a, bottom), with only about 33% of the S422E tau cleaved after 2 h of incubation with caspase 3. To determine the specificity of this effect, we also examined the cleavage kinetics of a tau protein in which S422 was mutated to alanine (S422A, Fig. 2b). Unlike S422E tau, S422A tau had cleavage kinetics similar to hTau40 (cleavage half-time = 13.7 min and 14.3 min, respectively). Caspase cleavage at D421 was confirmed both by a loss of reactivity to the C-terminal specific antibody, Tau46.1 (Fig. 2c), and by an increase in reactivity to the D421-cleavage-specific antibody, Tau-C3 (Fig. 2d). These cleavage half-times are as rapid as, if not more so than, those reported for other caspase 3 substrates, such as poly(ADP-ribose) polymerase (PARP) (Casciola-Rosen et al. 1996), suggesting that tau is an excellent substrate for caspase 3.
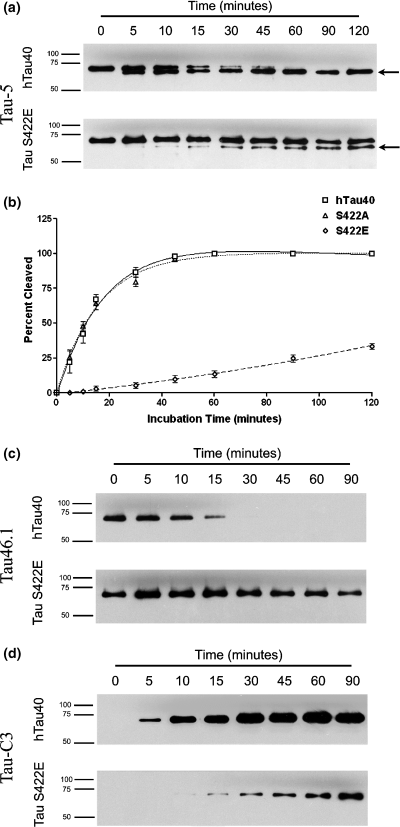
(a) Cleavage of recombinant hTau40 and pseudophosphorylated tau. The cleavage product migrates at a slightly lower molecular weight (arrow). Tau was detected with the Tau-5 antibody which recognizes both full-length and truncated tau. (b) Recombinant tau and the control mutant (S422A) show similar cleavage profiles, but the pseudophosphorylated mutant (S422E) demonstrates a much lower rate of cleavage at all time points. (c, d) Cleavage of both hTau40 and S422E tau is accompanied by a loss of the C-terminus as shown by reactivity to the antibody Tau 46.1 (c) and an emergence of tau cleaved at D421 as shown by reactivity to the Tau-C3 antibody (d).
Pathological tau structures labeled by pS422 and Tau-C3
Labeling for phosphorylation at S422 revealed the fibrillar manifestations of AD tau pathology, including NFT, dystrophic neurites and neuropil threads (Figs 3a and b). In addition, the pS422 antibody also highlighted numerous pre-tangle neurons throughout the cortex. In pre-tangle cells, labeling was punctate, and was found in the soma as well as in apical and basal dendrites of pyramidal cells (Fig. 3a).
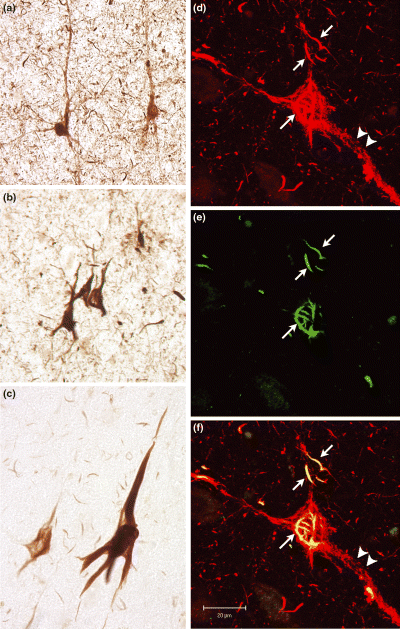
(a–c) Tau phosphorylated at S422 is found in pre-tangle neurons (a) which display punctuate staining throughout the somato-dendritic compartments, as well as in NFT (b). Neuropil threads and dystrophic neurites are also visible with the pS422 antibody. Tau cleaved at D421 is found in NFT and neuropil threads (c), but not in pre-tangle neurons as shown by labeling with the Tau-C3 antibody. (d–f) Confocal microscopy showing that pS422 (d, red) labels both punctuate and fibrillar tau deposits, while Tau-C3 (e, green) labels primarily the fibrillar portions of the deposit. Co-localization of the two epitopes appears yellow in the merged image (f). Fibrillar deposits are identified by the arrows, while arrowheads point to punctate deposits. Scale bar = 20 μm.
Labeling with the Tau-C3 antibody included mature fibrillar tangles, dystrophic neurites, and neuropil threads (Fig. 3c). Notably, Tau-C3 failed to label pre-tangle neurons, suggesting that truncated tau is not present in aggregated form in pre-tangles. The consistency with which Tau-C3 highlighted mature NFT was evident in a high degree of co-localization with Thiazin Red (data not shown). Although Thiazin Red-positive tangles could be found in the absence of Tau-C3 labeling, this occurred predominantly in the entorhinal cortices of subjects with more severe pathology, and probably represents the loss of the Tau-C3 epitope to additional cleavage events late in the disease process (Garcia-Sierra et al. 2001, 2003).
Next, we examined co-localization of the pS422 and the Tau-C3 epitopes. The extent to which these epitopes co-localized varied among subjects, on average comprising approximately 15% of labeled cells, but accounting for as much as 63% in one individual. When pS422 labeling was found co-localized with Tau-C3 labeling, the labeled structures were almost always fibrillar in nature. Utilizing confocal microscopy, occasional cells could be found in which both punctate and fibrillar labeling by pS422 was evident. However, in these instances, pS422 positive fibrils co-localized with Tau-C3. Because a single molecule of tau cannot be positive for both epitopes simultaneously, this indicates that truncated and full-length tau can coexist within the polymer. Meanwhile, areas with punctuate pS422 labeling never exhibited evidence of truncation at D421 (Figs 3d–f).
Distribution of tau epitopes during aging
The pS422 epitope was found in a pattern consistent with that of tangle development described by Braak and Braak (1991). In cases with minimal pathology, pS422 tau was found exclusively in the entorhinal and transentorhinal cortices, where tangles initially form. In cases containing more severe pathology, pS422 was found in the lateral temporal cortices (i.e, the ITG, MTG, and STG), which become involved later in the disease. Consistent with the distribution of tangles in aging, pS422-positive cells were found in the lateral temporal regions only when they were also present in medial temporal lobe structures (i.e. entorhinal cortex and fusiform gyrus).
Truncated tau, as demonstrated by Tau-C3 staining, was also found in a pattern consistent with tangle formation, but appeared to lag behind the appearance of the pS422 epitope. For example, in cases where only minimal labeling was observed with pS422, labeling with Tau-C3 was virtually absent. In cases with mild to moderate labeling with pS422, labeling with Tau-C3 was limited to paralimbic cortex, especially layer II of the entorhinal cortex. Truncated tau was found in the lateral temporal cortex only when the pS422 epitope was prominent as well (Figs 4a and b). In these cases, the layer II islands of the ERC displayed the truncation epitope almost exclusively, suggesting that the earlier pS422 epitope had been replaced with the Tau-C3 epitope. To verify this pattern, we quantified the percentage of cells that labeled for pS422 only, Tau-C3 only, or with both antibodies. The percentage of tangles labeled only with pS422 was greatest in more lateral temporal neocortex and lowest in the medial temporal/paralimbic cortex. Conversely, the percentage of tangles labeled with only Tau-C3 was greatest in the paralimbic cortex and lowest in the more lateral neocortex (Fig. 4a). The percentage of cells labeling for phosphorylated tau (pS422) was inversely proportional to the percentage of tangles labeled for cleaved tau (Tau-C3) (Fig. 4b; r2 = 0.626, p < 0.0001), consistent with the loss of the pS422 epitope occuring as a result of cleavage at D421. Additionally, a significant interaction between the degree of severity for each temporal gyrus (i.e. the stage of tangle maturation) and the reactivity to either phosphorylated or cleaved tau was found (Fig. 4c). This further indicates that a shift in the predominant epitope from phosphor-S422 tau to D421-truncated tau accompanies the progression of tangle maturation.
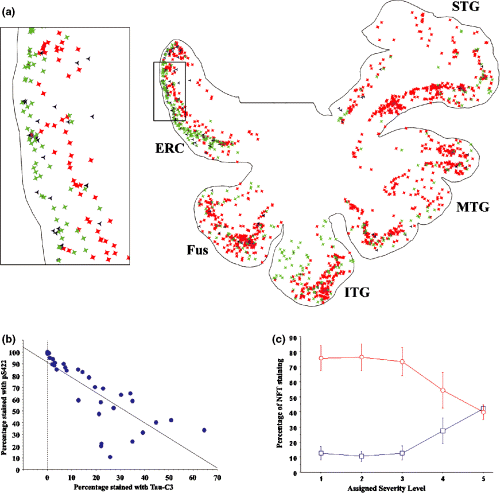
(a) Tracing of the temporal lobe from a non-demented individual, showing the location of pS422-only labeled NFT (red), double-labeled NFT (black), or Tau-C3-only labeled NFT (green). Inset shows the entorhinal cortex outlined in (a), demonstrating a higher proportion of Tau-C3 (green) labeling in the layer II islands, while the deeper layer V cells are predominantly labeled for pS422. (b) Correlation plot showing a decrease in the percentage of cells labeled only by pS422 as the percentage of NFT labeled only with Tau-C3 increases. Each point reflects the percentage of label for each gyrus in each individual. (c) Interaction plot comparing the percentage of NFT staining with either pS422 (red circles) or Tau-C3 (blue squares) based on the severity of neurofibrillar pathology in the different gyri. There is a significant interaction between the severity level of the gyrus (reflecting maturation stage of the tangles) and the label.
Discussion
The effect of phosphorylation on cleavage
Initiation of the apoptotic cascade and the resulting activation of caspases central to the apoptotic process can result from a toxic insult to the cell (Cryns and Yuan 1998), including exposure to Aβ (Gamblin et al. 2003b). In neurons, Aβ-induced apoptosis is dependent on the presence of tau (Rapoport et al. 2002), which is itself a caspase substrate. Cleavage of tau by caspase results in a molecule that is more toxic than full-length tau (Chung et al. 2001) and that assembles into filaments more readily (Berry et al. 2003; Gamblin et al. 2003b; Rissman et al. 2004), providing a mechanism by which tangle formation may be enhanced or possibly stabilized (Binder et al. 2005).
It has been postulated that phosphorylation/dephosphorylation of some caspase substrates may serve as a mechanism by which cleavage is regulated (Tozser et al. 2003). Phosphorylation at the P1′ through P4′ sites has been shown to inhibit caspase cleavage of the AD-associated proteins presenillin-1 (Fluhrer et al. 2004), presenillin-2 (Walter et al. 1999), and the amyloid precursor protein (Gervais et al. 1999), as well as of numerous proteins not associated with AD (for review, see Tozser et al. 2003). We therefore attempted to determine if tau phosphorylation could regulate caspase cleavage of tau at D421.
In vitro, pseudophosphorylation of S422 (S422E) was capable of inhibiting cleavage. These results were not due to manipulation of the protein, but specifically to the pseudophosphorylation at S422, because mutating the serine to an alanine resulted in cleavage kinetics indistinguishable from that of wild-type tau. It is to be noted that pseudophosphorylation has been shown to be a reliable model for the effects of tau phosphorylation (Abraha et al. 2000; Fath et al. 2002; Necula and Kuret 2004, 2005). Therefore, there is ample in vitro evidence to suggest that the phosphorylation state of S422 could regulate caspase cleavage of tau at D421. Although numerous kinases, such as JNK, ERK, and p38, have been shown to phosphorylate this site in vitro and indirectly in vivo (Reynolds et al. 1997a,b; Illenberger et al. 1998; Kins et al. 2001, 2003) the exact kinase(s) and phosphatase(s) that are involved in vivo, and the extent to which they actually phosphorylate and de-phopshorylate this site is unknown.
Phosphorylation precedes cleavage in tangle evolution
To discern whether phosphorylation might be acting as a regulator of cleavage in vivo as well, we made use of the consistent pattern with which NFT appear in aging and AD. Tangles appear in the course of normal aging, emerging first in the entorhinal and transentorhinal cortices, spreading to the hippocampus, amygdala, and other paralimbic cortices, and eventually developing in the temporal neocortex (Braak and Braak 1991). Therefore, NFT found in areas that are initially affected, such as the entorhinal cortex and hippocampus are, on average, older than NFT in lateral temporal regions, independent of the clinical status of the subject.
The formation of many tau epitopes follows a spatial distribution pattern that is consistent with the appearance of tangles in aging and in Alzheimer's disease. Thus, the location of labeled NFT provides a method for staging the appearance and disappearance of various epitopes. In AD, tangles within the medial temporal cortex tend to display epitopes that are indicative of older, more mature NFT, such as Tau-66 (in which the proline-rich region comes into close proximity with the microtubule binding repeat) and MN423 (in which tau is cleaved at glutamic acid 391) (Garcia-Sierra et al. 2003). Conversely, NFT in the frontal and parietal cortices are relatively younger and display epitopes indicative of less-evolved NFT, such as phosphorylation at the AT8 site (Braak et al. 1994; Braak and Braak 1995) and the presence of an intact C-terminus (Garcia-Sierra et al. 2003).
In this report, all individuals examined displayed some pre-tangle cells labeled with pS422, and the majority of cases contained tangles labeled with Tau-C3 as well. However, in individuals with the mildest accumulation of tau deposits, the pS422 epitope predominated. In individuals with pathology extending into the lateral temporal regions (i.e. more advanced pathology), these regions were characterized by a predominance of pS422-reactive tau, while, in the entorhinal cortex, tangles contained predominantly cleaved, Tau-C3 reactive tau. This distribution is consistent with a maturation process in which phosphorylation at S422 precedes cleavage at D421. We did not extend the quantitative aspects of this study into more severe cases (i.e. Braak stages V–VI) as it would have proved redundant. We had previously shown that the Tau-C3 epitope is removed upon additional cleavage events resulting in the formation of the MN423 epitope (cleavage at E391; Guillozet-Bongaarts et al. 2005). Hence, we limited our investigation to individuals with less severe pathology in whom the majority of tangles within the temporal lobe had not matured past the Tau-C3 stage.
We have previously examined the D421 truncation with respect to other alterations in the tau molecule, and found that this truncation appears to be a relatively early event in NFT evolution (Guillozet-Bongaarts et al. 2005), consistent with other reports (Rissman et al. 2004). The data presented here suggests that S422 becomes phosphorylated prior to cleavage, making it an even earlier event than tau cleavage at D421. It must be mentioned that our results contradict a previous report that phosphorylation at S422 is a later alteration that persists throughout the life of a tangle into the ghost tangle stage (Augustinack et al. 2002). Furthermore, that report is contradicted by an earlier report stating that phosphorylation at S422 occurs in both pre-tangles and tangles, but not in the later-staged ghost tangles (Kimura et al. 1996); our findings are consistent with this. Additionally, we have found a high degree of overlap between labeling with the pS422 antibody and the AT8 antibody, especially with regards to pre-tangle cells (data not shown). AT8 positive cells have been identified in human brains prior to the formation of NFT [i.e. pre-tangles (Braak et al. 1994; Braak and Braak 1995)] as well as in non-tangle bearing cells in the brains of other mammals (Braak et al. 1994; Hartig et al. 2000; Bi et al. 2001; Masliah et al. 2001). The co-localization of pS422 and AT8 in pre-tangle cells strongly suggests that these phosphorylations are earlier, rather than later, events.
Phosphorylation and cleavage in vivo
To understand whether or not phosphorylation at S422 may be inhibiting cleavage in vivo, we must examine the approximate time frame for these changes. The appearance of NFT in aging and AD constitutes a continuum of pathological progression. The presence of NFT in the entorhinal cortex is common in individuals over the age of 60, and NFT have been found in even younger individuals (Braak and Braak 1997). The presence of NFT in the lateral temporal cortex correlates with more advanced age (75+ years). Therefore, we can estimate a delay of approximately 15 years between the initial appearance of NFT in the entorhinal cortex and their later appearance in the lateral temporal cortex.
Similarly, our data indicate that the percentage of tangles in which the tau is completely cleaved by caspase begins to increase in the entorhinal cortex and fusiform gyrus only when tau deposition (as indicated by phosphorylation at S422) has progressed into the middle and superior temporal gyri. Hence, we also estimate a time course of at least 15 years from the time that phosphorylated tau appears in the entorhinal cortex to the time that the tau in these neurons becomes fully cleaved to a detectable extent. This seems consistent with work by Morsch et al. (1999) which, using a mathematical model, suggested that neurons may live for decades with NFT before reaching the ghost tangle stage or disappearing altogether. Furthermore, our ability to detect NFT that are labeled by both the pS422 and Tau-C3 antibodies suggests that the epitopes coexist for some time. This further implies that caspases are active in the NFT-bearing neurons, but that the transition from a tangle that contains no truncated tau to one that contains only caspase-cleaved tau is a slow process that occurs over many years. The retardation of caspase cleavage at D421 by phosphorylation at S422 may explain this extended time course of tangle formation.
While the inhibitory effect of phosphorylation of S422 on caspase cleavage of tau may partly explain the delayed appearance of Tau-C3-positive NFT, the eventual appearance of Tau-C3 reactive tangles makes it clear that phosphorylation at S422 does not completely block cleavage in situ. Because phosphorylation is reversible, it is possible that S422 becomes dephosphorylated at some point, thereby allowing truncation to occur more readily. Alternatively, phosphorylation at this site may successfully inhibit cleavage when caspase activity is present at low levels, but may be overwhelmed as caspase activity levels increase.
It is possible that phosphorylation of tau at this site occurs as part of a general increase in phosphorylation of additional substrates as a response to caspase activation. Given the number of caspase substrates that have been identified, and the regulation of many of these by phosphorylation, it seems plausible that a pan-phosphorylation of multiple substrates associated with AD is a mechanism by which irreversible cleavage can be regulated in the early stages of apoptosis.
In summary, we have outlined two lines of evidence, in vitro and in vivo, suggesting that neurons use phosphorylation at S422 as a protective mechanism to inhibit caspase cleavage of tau. If this is the case, then proteolysis of tau by caspase is an exquisitely regulated event controlled by the phosphorylation or dephosphorylation of S422. We believe that failure to phosphorylate S422 or, conversely, dephosphorylation of this site, would expedite the transition to a truncated state in vivo, and thus accelerate fibril formation or stabilize filaments already present. However, it is unclear whether a scenario such as this is what distinguishes normal aging from the more accelerated tangle formation seen in AD. More studies need to be carried out to examine this hypothesis, including in situ studies that explore the effects of phosphorylation on the progression of apoptosis and fibril formation.
Acknowledgements
The authors would like to thank Dr Virginia Lee for the use of the Tau 46.1 antibody, and Dr Eileen Bigio and the Northwestern Alzheimer Disease Center and the NADC Neuropathology Core (P30 AG13854) for providing tissue for this study. We would also like to thank Biosource for providing the pS422 antibody used in this study. This work was supported by NIH grants AG020506, AG021661, AG000257 and AG09466.




