Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases
Abstract
The human excitatory amino acid transporter (EAAT)2 is the major glutamate carrier in the mammalian CNS. Defective expression of the transporter results in neuroexcitotoxicity that may contribute to neuronal disorders such as amyotrophic lateral sclerosis (ALS). The serum and glucocorticoid inducible kinase (SGK) 1 is expressed in the brain and is known to interact with the ubiquitin ligase Nedd4-2 to modulate membrane transporters and ion channels. The present study aimed to investigate whether SGK isoforms and the related kinase, protein kinase B (PKB), regulate EAAT2. Expression studies in Xenopus oocytes demonstrated that glutamate-induced inward current (IGLU) was stimulated by co-expression of SGK1, SGK2, SGK3 or PKB. IGLU is virtually abolished by Nedd4-2, an effect abrogated by additional co-expression of either kinase. The kinases diminish the effect through Nedd4-2 phosphorylation without altering Nedd4-2 protein abundance. SGKs increase the transporter maximal velocity without significantly affecting substrate affinity. Similar to glutamate-induced currents, [3H] glutamate uptake and cell surface abundance of the transporter were increased by the SGK isoforms and down-regulated by the ubiquitin ligase Nedd4-2. In conclusion, all three SGK isoforms and PKB increase EAAT2 activity and plasma membrane expression and thus, may participate in the regulation of neuroexcitability.
Abbreviations used
-
- ALS
-
- amyotrophic lateral sclerosis
-
- EAAT
-
- excitatory amino acid transporters
-
- ENaC
-
- epithelial sodium channel
-
- HA
-
- hemagglutinin
-
- NAT
-
- neutral amino acid transporter
-
- PBS
-
- phosphate-buffered saline
-
- PKB
-
- protein kinase B
-
- RLU
-
- relative light unit
-
- SGK
-
- serum and glucocorticoid inducible kinase
-
- wt
-
- wild-type
The human excitatory amino acid transporter (EAAT)2 is primarily expressed in astrocytes (Rothstein et al. 1994; Lehre et al. 1995; Milton et al. 1997) and plays a major role in glutamate re-uptake from the synaptic cleft after neuronal transmission (Rothstein et al. 1994; Lehre et al. 1995; Dehnes et al. 1998; Lehre and Danbolt 1998; Kugler and Schmitt 1999). As glutamate may exert neurotoxic effects (Bergles and Jahr 1997; Diamond and Jahr 1997; Bednarski et al. 1998; Mennerick et al. 1999), defective function or regulation of astroglial EAAT2 expression may foster neuroexcitotoxicity. Lack of EAAT2 has indeed been shown to promote extracellular glutamate accumulation, excitotoxicity and, ultimately, cell death (Rothstein et al. 1996; Tanaka et al. 1997; Vorwerk et al. 2000). Consistent with this idea, a reduction in EAAT2 function has been reported in amyotrophic lateral sclerosis (ALS) (Rothstein et al. 1995).
Despite the fundamental role of EAAT2 in CNS glutamate shuttling, only a few studies have addressed the regulation of trafficking and function of this carrier, which has been shown to be regulated by protein kinase C (Casado et al. 1993; Kalandadze et al. 2002; Kalandadze et al. 2004). Regulation of the closely related glutamate transporter subtype, EAAT1, has recently been shown to involve members of the serum and glucocorticoid inducible kinase (SGK) protein kinase family (Boehmer et al. 2003a). The effect of those kinases on EAAT1 is mediated, in part, by direct phosphorylation of the SGK phosphorylation site on EAAT1, and by interference with the down-regulating effect of the ubiquitin ligase Nedd4-2 (Debonneville et al. 2001; Snyder et al. 2002; Pearce 2003; Verrey et al. 2003). SGK1, SGK3 and the related protein kinase B (PKB) have further been shown to up-regulate EAAT3 (Schniepp et al. 2004).
SGK1 was originally cloned as a glucocorticoid-sensitive gene (Webster et al. 1993a,b; Firestone et al. 2003) from a mammary tumour cell line, and the human orthologue was subsequently identified as a cell volume-sensitive kinase up-regulated by hypertonicity (Waldegger et al. 1997). SGK1 was proven to be a potent stimulator of a variety of ion channels and transporters (Lang et al. 2003).
In the present study, co-expression experiments in Xenopus laevis oocytes have been used to examine the influence of SGK1, its isoforms SGK2 and SGK3, and the related protein kinase PKB on the glial glutamate transporter EAAT2.
Materials and methods
Site-directed mutagenesis of EAAT2
The hemagglutinin (HA)-tagged EAAT2 was generated by two-stage PCR site-directed mutagenesis using the primers EAAT2-HA, s: 5′-GTCCAGCCTGGATTA-CGACGTACCAGATTACGCTGCCTTCCTGGACC-3′ and EAAT2-HA, as: 5′-GGTCCAGGAAGGCAGCGTAATCTGGTACGTCGTAATCCAGG-CTGGAC-3′. EAAT2-HA was sequenced to verify the presence of the desired mutation.
Electrophysiology
cRNA encoding human wild-type EAAT2 (Shigeri et al. 2001), mouse wild-type neutral amino acid transporter (NAT3) (Gu et al. 2003), human HA-tagged EAAT2, human wild-type Nedd4-2 (Debonneville et al. 2001), human C962SNedd4-2, human S382D,S468DNedd4-2, human SGK1 (Waldegger et al. 1997), human SGK2 (Kobayashi and Cohen 1999), human SGK3 (Kobayashi and Cohen 1999), constitutively active human S422DSGK1 (Kobayashi and Cohen 1999), S356DSGK2 and S419DSGK3, inactive human K127NSGK1 (Kobayashi and Cohen 1999) and constitutively active human T308D,S473DPKB (Alessi et al. 1996) were synthesized as described by Wagner et al. (2000).
Dissection of Xenopus laevis ovaries, and collection and handling of the oocytes, have been described in detail elsewhere (Wagner et al. 2000). Oocytes were injected with 7.5 ng of the respective kinase cRNA, and/or 5 ng Nedd4-2 cRNA or H2O, on the first day after preparation of the oocytes and subsequently, with 10 ng EAAT2 or NAT3 cRNA. All experiments were performed at room temperature (21°C) 4 days after injection. Two-electrode voltage-clamp recordings were performed at a holding potential of −60 mV. The data were filtered at 10 Hz, and recorded with MacLab A/D-D/A converter and software for data acquisition and analysis (ADInstruments, Castle Hill, Australia). The control solution (superfusate/ND96) contained 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2 and 5 mm HEPES, pH 7.4. Glutamate was added to the solutions at the indicated concentrations. In the case of NAT3-injected oocytes, substrate-induced currents were evoked by the addition of 1 mm alanine. The final solutions were titrated to pH 7.4 using NaOH. The flow rate of the superfusion was 20 mL/min and a complete exchange of the bath solution was reached within about 10 s.
Tracer flux measurements
The transport assay was performed 4 days after cRNA injection with 5–10 single oocytes in 0.50 mL ND96-Na (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2 and 5 mm HEPES, pH 7.4) containing 1 µCi [3H]l-glutamate and 10 µm cold l-glutamate, or ND96-Ch where NaCl was substituted by ChCl. After incubation for 10 min with l-glutamate (linear range of uptake), uptake was terminated by washing the oocytes four times with 3 mL ice-cold ND96-Ch containing 1 mm unlabelled l-glutamate. Oocytes were individually transferred into scintillation vials and dissolved by adding 200 µL 10% sodium dodecyl sulfate (SDS) before the radioactivity was determined. Uptake values obtained with ND96-Ch were always subtracted from the values obtained with ND96-Na.
Western blotting
To analyse the expression of SGK1 and Nedd4-2, oocytes were homogenized in lysis buffer containing 50 mm Tris (pH 7.5), 0.5 mm EDTA (pH 8.0), 0.5 mm EGTA, 100 mm NaCl, 1% Triton X-100, 100 µm sodium orthovanadate and protein inhibitor cocktail (Roche, Mannheim, Germany) at the recommended concentration. Proteins were separated on an 8% polyacrylamide gel and transferred to nitrocellulose membranes. After blocking with 5% non-fat dry milk in phosphate-buffered saline (PBS)/0.15% Tween 20 for 1 h at room temperature (21°C), blots were incubated overnight at 4°C with a rabbit Nedd4-2 antibody (diluted 1 : 1000 in PBS/0.15% Tween 20/5% non-fat dry milk) or a rabbit anti-SGK1 antibody (Upstate, Waltham, MA, USA; diluted 1 : 1000 in PBS/0.15% Tween 20/5% non-fat dry milk). Secondary peroxidase-conjugated sheep anti-rabbit IgG (diluted 1 : 1000 in PBS/0.15% Tween 20/5% non-fat dry milk) was used for chemiluminescent detection with an enhanced chemiluminescent (ECL) kit (Amersham, Freiburg, Germany).
Detection of cell surface expression by chemiluminescence
Defolliculated oocytes were first injected with SGK/PKB cRNA (7.5 ng/oocyte) and/or Nedd4-2 cRNA (5 ng/oocyte) and 1 day later, with EAAT2-HA which contains an HA epitope inserted into an extracellular site. Oocytes were incubated with 1 µg/mL primary rat monoclonal anti-HA antibody (clone 3F10, Boehringer, Ingelheim, Germany) and 2 µg/mL secondary, peroxidase-conjugated affinity-purified F(ab′)2 goat anti-rat IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA). Individual oocytes were placed in 20 µL SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA), and chemiluminescence was quantified in a luminometer by integrating the signal over a period of 1 s. Results are given in relative light units (RLU). RLU obtained with H2O-injected oocytes (control oocytes) were subtracted from the values obtained with oocytes injected with EAAT2-HA alone or together with the respective SGK isoforms and/or Nedd4-2. In the relevant range of protein expression, the chemiluminescence amplitude correlates linearly with the protein abundance at the cell membrane (Zerangue et al. 1999).
Statistical analysis
Data are provided as means ± SEM, n represents the number of oocytes investigated. All experiments were repeated with at least three batches of oocytes; in all repetitions, qualitatively similar data were obtained. All data were tested for significance using anova, and only results with p < 0.05 were considered as statistically significant.
Results
Application of 1 mm glutamate to the bath induced in EAAT2 expressing Xenopus oocytes an inward current (IGLU) approaching 27.0 ± 1.6 nA (n = 81) at a holding potential of −60 mV, while water-injected control oocytes showed no detectable electrogenic glutamate transport (0.1 ± 0.1 nA, n = 4). The constitutively-active S422DSGK1 activated the EAAT2-mediated current by 87.2 ± 10.7% (n = 53), while the inactive K127NSGK1 did not significantly modify IGLU (−3.1 ± 7.9%, n = 32). Both mutant kinases are expressed at levels comparable with the wild-type (wt)SGK1 (Fig. 1). In humans, SGK1–3 are not constitutively active but require activation though the PI3 kinase signalling pathway (Kobayashi and Cohen 1999). In the oocyte expression system, the activation of the kinases is not limiting as the wild-type SGK isoforms are similarly as effective as the consitutively-active kinases (Lang et al. 2000; Friedrich et al. 2003). The SGK1 isoforms SGK2 and SGK3, and the closely related protein kinase B (PKB), similarly stimulated EAAT2 transport. SGK2 co-expression enhanced glutamate-induced current by 78.2 ± 18.9% (n = 30), SGK3 by 138.9 ± 22.0% (n = 28) and T308D,S473DPKB by 49.7 ± 13.3% (n = 26, Fig. 1).
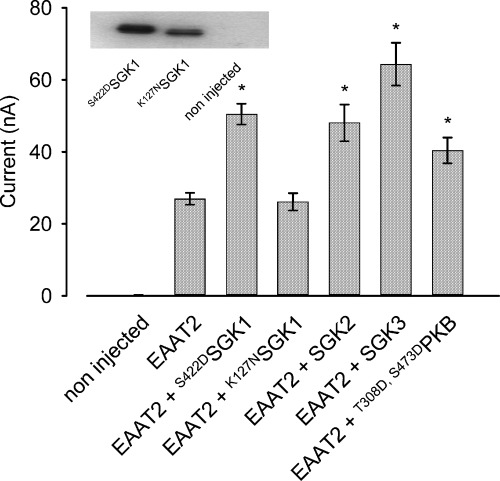
Glutamate (1 mm)-induced inward currents (IGLU) in EAAT2-expressing oocytes are activated by the constitutively active serum and glucocorticoid inducible kinase S422DSGK1 but not the inactive mutant K127NSGK1. When injected at identical mRNA concentrations, SGK2 and SGK3, as well as T308D,S473DPKB, produce similar effects on IGLU as compared with cells co-injected with EAAT2 and SGK1. Arithmetic means ± SEM; *indicates statistically significant difference (p < 0.05, anova) to the current in Xenopus oocytes expressing EAAT2 alone; n = 4 for non-injected cells; n = 30–32 for all other groups. Insert: western blot demonstrating proper expression of the mutant S422DSGK1 and K127NSGK1.
SGK kinases were reported to modulate their targets not only by direct phosphorylation but also by interaction with cytosolic mediators. One of these proteins is the ubiquitin ligase Nedd4-2, which was shown to play a key role in the regulation of the epithelial sodium channel, ENaC. The ubiquitin ligase bears two putative SGK phosphorylation sites on positions 382 and 468. IGLU was significantly decreased by 87.5 ± 1.3% (n = 28) following co-expression of the ubiquitin ligase Nedd4-2. The effect was abrogated by additional co-expression of the constitutively-active S422DSGK1. In Xenopus oocytes co-expressing EAAT2 together with both S422DSGK1 and Nedd4-2, IGLU was not significantly different (− 15.8 ± 12.5%, n = 28) from IGLU in oocytes expressing EAAT2 alone, but was more than sixfold higher than IGLU in oocytes expressing EAAT2 together with Nedd4-2. In contrast, co-expression of the inactive mutant K127NSGK1 did not significantly modify the IGLU in Xenopus oocytes expressing EAAT2 and Nedd4-2. The inhibitory effect of the ubiquitin ligase Nedd4-2 was similarly reversed by the co-expression of the kinase isoforms SGK3 and PKB and, to a lesser extent, SGK2. As shown in Fig. 2, co-injection of oocytes with cRNA encoding each of these kinases with cRNAs for Nedd4-2 and EAAT2 modulated the effect of Nedd4-2.
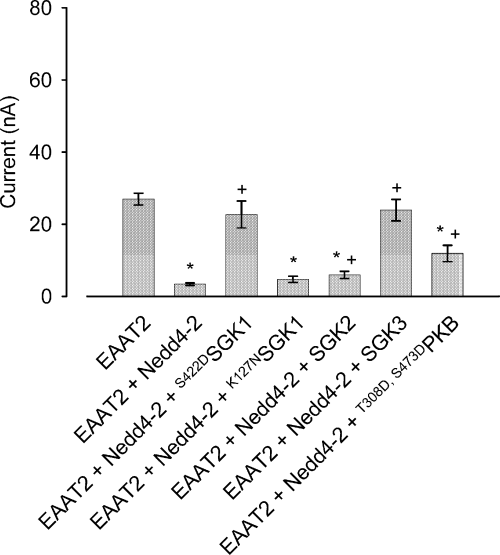
Co-expression of the ubiquitin ligase Nedd4-2 inhibits glutamate-induced current IGLU significantly. Down-regulation of EAAT2 by Nedd4-2 is abrogated by the constitutively active serum and glucocorticoid inducible kinase S422DSGK1 but not the inactive mutant K127NSGK1. The SGK1 kinase isoform SGK3 and the related kinase T308D,S473DPKB are similarly capable of blunting the inhibitory action of Nedd4-2 on IGLU. *Indicates statistically significant difference (p < 0.05, anova) to the current in Xenopus oocytes expressing EAAT2 alone; + indicates statistically significant difference (p < 0.05, anova) to the current in Xenopus oocytes co-expressing EAAT2 and Nedd4-2; n = 20–28.
Destruction of the catalytic domain in the mutant C962SNedd4-2 (Debonneville et al. 2001) abolished the down-regulation of EAAT2 by the ubiquitin ligase. The inward current in control cells (15.0 ± 1.8 nA, n = 7) was not significantly different to that in oocytes expressing EAAT2 together with C962SNedd4-2 (15.8 ± 1.9 nA, n = 9). The current was up-regulated to 20.1 ± 1.2 nA (n = 9) upon additional co-expression of the constitutively-active S422DSGK1 in cells expressing EAAT2 and C962SNedd4-2 (Fig. 3). These observations are consistent with two models of EAAT2 regulation: first, the ubiquitin ligase could down-regulate the transporter and this effect is abrogated by an inactivation of Nedd4-2 by the protein kinase SGK; second, the inhibitory effect of the ubiquitin ligase could be compensated by an additive effect of the SGK protein that up-regulates the transporter by an independent mechanism.
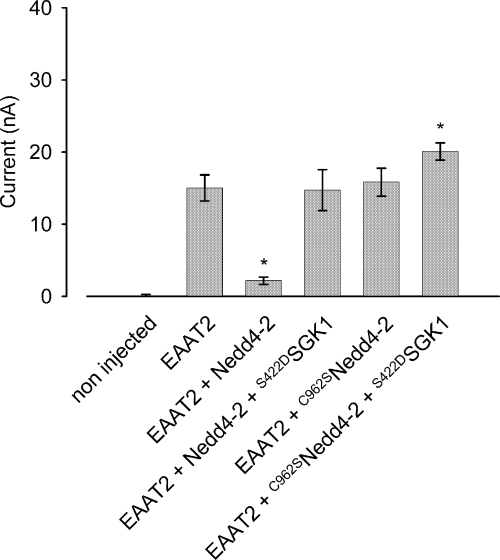
Co-expression of the catalytically inactive C962SNedd4-2 does not affect IGLU in EAAT2-expressing oocytes. Co-expression of S422DSGK1 with C962SNedd4-2 leads to activation of IGLU in EAAT2 expressing-oocytes. *Indicates statistically significant difference (p < 0.05, anova) to the current in Xenopus oocytes expressing EAAT2 alone; n = 7–9.
Co-expression of the SGK protein kinases did not significantly alter the expression of the ubiquitin ligase. According to immunoblotting, Nedd4-2 protein abundance was unaffected by co-expression of the respective kinases (Fig. 4). Conversely, SGK1 expression was not significantly altered by co-expression of the ubiquitin ligase Nedd4-2 (Fig. 5).
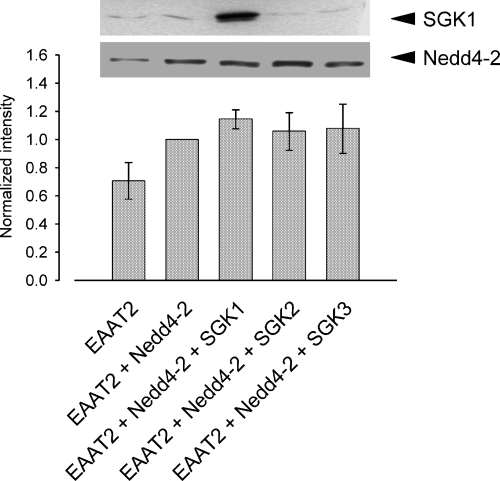
Nedd4-2 expression remains unaffected upon injection of SGK protein kinases. The histogram depicts the quantification of the intensities from three different western blots. All data were normalized to cells expressing EAAT2 + Nedd4-2. Arithmetic means ± SEM.
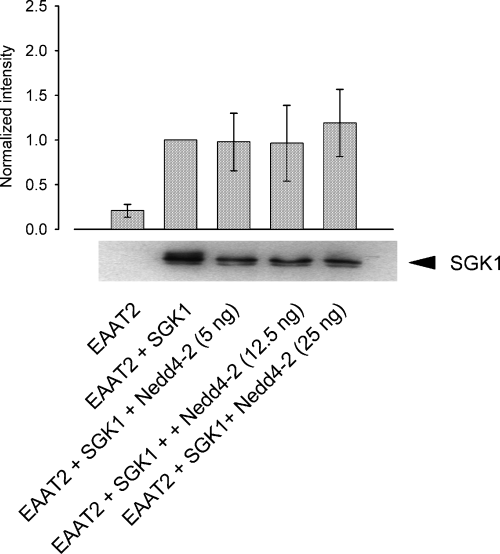
SGK1 expression remains unaltered upon Nedd4-2 expression. Immunoblots with a specific SGK1 antibody show that expression of the kinase is not modulated by the ubiquitin ligase Nedd4-2. The histogram depicts the quantification of the intensities from three different western blots. All data were normalized to cells expressing EAAT2 + SGK1. Arithmetic means ± SEM.
Kinetic analysis revealed that all three SGK isoforms and protein kinase B increased the maximal current without significantly modifying the substrate concentration required for half maximal carrier activation (Fig. 6 and Table 1). Thus, the kinases enhanced the maximal transport rate without affecting substrate affinity of EAAT2.
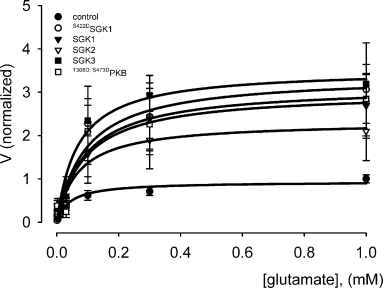
Normalized glutamate-induced currents as a function of extracellular glutamate concentration in oocytes co-expressing EAAT2 and the respective wild-type kinases SGK1–3, constitutively active S422DSGK1 or active T308D,S473DPKB. Arithmetic means ± SEM of the current relative to control (EAAT2 co-injected with water, 1 mm glutamate); n = 5–6.
| Protein kinase | Imax (normalized) | K0.5 (Glu) | n |
|---|---|---|---|
| Control | 1.02 ± 0.10 | 69.66 ± 13.26 | 6 |
| SGK1 | 3.17 ± 0.89, p < 0.05 | 73.26 ± 15.28, n.s. | 6 |
| SGK2 | 2.33 ± 0.83, p < 0.05 | 67.10 ± 15.09, n.s. | 6 |
| SGK3 | 3.50 ± 0.63, p < 0.05 | 69.66 ± 18.54, n.s. | 5 |
| S422DSGK1 | 3.39 ± 0.90, p < 0.05 | 71.16 ± 16.76, n.s. | 6 |
| T308D,S473DPKB | 3.28 ± 0.67, p < 0.05 | 71.86 ± 5.64, n.s. | 5 |
- Oocytes were injected with 10 ng EAAT2 and 7.5 ng of the respective protein kinase cRNA and incubated for 4 days prior to the experiment. Oocytes were superfused with ND 96, pH 7.4, and glutamate was applied at concentrations ranging from 0.001 to 1 mm. K0.5 and Imax were calculated from the modified Hill equation.
The up-regulation of EAAT activity did not significantly affect the voltage dependence of the transporter. As is obvious from Fig. 7, co-expression of none of the three SGK isoforms resulted in alterations of current-voltage behaviour or a shift of the reversal potential of the carrier.
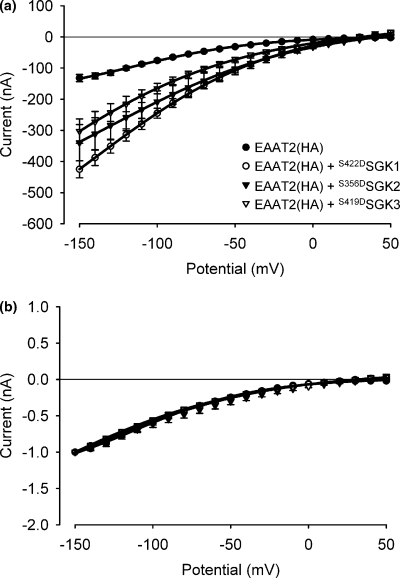
The current-voltage behaviour or the reversal potential of the carrier is not affected by co-expression of any of the constitutively active SGK protein kinases (S422DSGK1, S356DSGK2, S419DSGK3). (a) Absolute net current evoked by the application of 1 mm glutamate. (b) Normalized net current evoked by the application of 1 mm glutamate. Arithmetic means ± SEM; n = 5–6.
Similar to the glutamate-induced current, EAAT2-mediated glutamate uptake increased upon co-expression of the three SGK isoforms (Fig. 8). The activation of glutamate-induced currents and [3H]l-glutamate uptake were similar, indicating that the stimulation of transport was not paralleled by alterations of stochiometry. The tracer uptake studies were performed with an EAAT2 construct containing an HA tag for determination of chemiluminescence (see below). The currents mediated by this construct were virtually identical to the currents in oocytes injected with wild-type EAAT2, indicating that the tag did not affect the carrier function.

Comparison of the effects of the constitutively active SGK protein kinases (S422DSGK1, S356DSGK2, S419DSGK3) on glutamate-induced currents (a) and labelled glutamate uptake (b) in EAAT2-expressing oocytes. The activation of glutamate-induced currents by the three constitutively active SGK isoforms is virtually identical to the effects on [3H]l-glutamate uptake by EAAT2(HA). *Indicates statistically significant difference (p < 0.05, anova) to Xenopus oocytes expressing EAAT2 alone; n = 15–18 for electrophysiological recordings; n = 21–23 for uptake experiments.
As the EAAT2 transporter does not bear an SGK consensus site it is unlikely that SGK directly phosphorylates the transporter. However, SGKs might be effective through phosphorylation of Nedd4-2. This hypothesis is supported by the fact that the mutant S382D,S468DNedd4-2 mimicking the phosphorylation state by SGKs is unable to modulate EAAT2 activity, as both glutamate-induced current and [3H]l-glutamate uptake remained unaffected (Fig. 9). The catalytically-inactive mutant C962SNedd4-2 does not influence [3H]l-glutamate uptake, either.
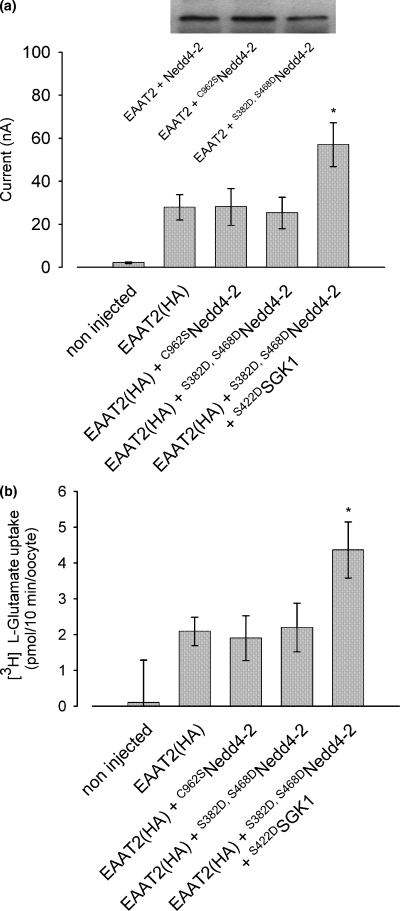
The Nedd4-2 mutant mimicking its phosphorylation state by SGKs does not affect EAAT2 transport activity. The mutant S382D,S468DNedd4-2 is unable to modulate EAAT2 transport activity as both, glutamate-induced current (a) and [3H]l-glutamate uptake (b) remain unaffected. The mutant C962SNedd4-2 bearing a destructed catalytic domain influences neither glutamate-induced current (a) nor [3H]l-glutamate uptake (b). Insert: both ubiquitin ligase mutants are properly expressed compared with the wtNedd4-2, as demonstrated in the western blot included in the left panel. *Indicates statistically significant difference (p < 0.05, anova) to glutamate-induced current or glutamate uptake in Xenopus oocytes expressing EAAT2 alone; n = 6–9 for electrophysiological recordings; n = 10–12 for uptake experiments.
To explore whether the increase in glutamate transport by EAAT2 following co-expression of the different SGK kinase isoforms is due to enhanced EAAT2 abundance at the plasma membrane, we quantified cell surface expression of the carrier by a chemiluminescence assay. As demonstrated in Fig. 10, carrier abundance in the oocyte membrane was similarly up-regulated by SGK1, SGK2, SGK3 and PKB.
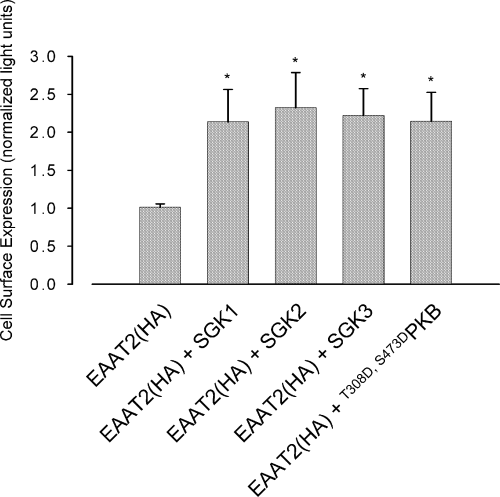
EAAT2 cell surface abundance is similarly up-regulated by the protein kinases SGK1–3 and T308D,S473DPKB. Relative light units (RLU) in Xenopus oocytes expressing EAAT2 with or without additional co-expression of wild-type SGK1, SGK2, SGK3 or constitutively active T308D,S473DPKB. *Indicates statistically significant difference (p < 0.05, anova) to the current in Xenopus oocytes expressing EAAT2 alone; n = 35–37.
To test whether the observed increase in cell surface expression is caused by non-specific trafficking effects of the kinases and Nedd4-2, several other membrane transporters were tested which appeared to be insensitive to the SGK isoforms and/or Nedd4-2. These include the human cationic amino acid transporters CAT, the rat sulfate transporter NaSi or the mouse neutral amino acid transporter NAT3. As illustrated in Fig. 11, the co-expression of neither any of the SGK isoforms nor PKB led to a significant increase in the arginine-induced current in oocytes expressing NAT3 (SLC38A4). Thus, the kinases do not significantly modify the function of this amino acid transporter.
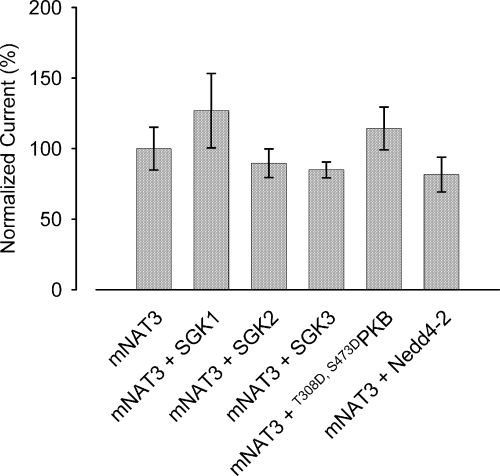
The SGK isoforms and Nedd4-2 do not alter alanine (1 mm)-induced currents in NAT3-expressing oocytes. The NAT3 (SLC38A4) carrier was expressed in Xenopus oocytes with and without wild-type SGK1, SGK2, SGK3 or constitutively active T308D,S473DPKB. Data were tested for significant differences by anova; n = 20–21.
Discussion
The present data reveal a novel mechanism for regulation of EAAT2. The down-regulation of the substrate-induced current in EAAT2-expressing oocytes by Nedd4-2 indicates that this ubiquitin ligase is a regulator of EAAT2. The EAAT2 transporter does not bear a PY motif, a target structure for recognition of the targets by Nedd4-2. Thus, EAAT2 may not be directly ubiquinated by Nedd4-2. The fact thatC962SNedd4-2 does not affect EAAT2 currents indicates that the catalytic activity of the protein is required and thus, ubiquitination is involved in the regulation of EAAT2 by Nedd4-2. Similar observations have been made for regulation of the voltage-gated cardiac Na+ channel (Abriel et al. 2000) and the epithelial Na+ channel, ENaC (Debonneville et al. 2001; Snyder et al. 2002).
The effect of Nedd4-2 is abrogated by SGK1, which thus enhanced carrier protein abundance and transport rate. In a previous study utilizing brefeldin A, a drug interfering with the exocytotic pathway, we were able to demonstrate that SGK1 delays the clearance of other transport proteins from the cell membrane (Yun et al. 2002b). However, in the case of EAAT2, it is not known whether delayed clearance of the transporter from the membrane causes its increased cell surface abundance. Further studies are required to clarify this issue.
The EAAT2 transporter does not bear an SGK1 consensus motif. Instead, SGK1 is, at least in part, effective through regulation of Nedd4-2. We and others have shown that SGK binds to and phosphorylates Nedd4-2, hence decreasing the affinity of the ubiquitin ligase to its target proteins (Debonneville et al. 2001; Embark et al. 2004; Palmada et al. 2004). Therefore, SGK might be effective through phosphorylation of Nedd4-2, an assumption supported by the observation that the Nedd4-2 mutant mimicking the phosphorylated form is not effective. The effect of Nedd4-2 is not completely abolished by SGK1, which may indicate that SGK1 cannot completely suppress Nedd4-2 function. On the other hand, SGK1 may not be effective exclusively through phosphorylation of Nedd4-2.
The SGK isoforms regulate a wide variety of transporters including the renal epithelial Na+ channel, ENaC (Chen et al. 1999; Naray-Fejes-Toth et al. 1999; Böhmer et al. 2000; Lang et al. 2000; Shigaev et al. 2000; Pearce 2003; Verrey et al. 2003), the voltage-gated Na+ channel, SCN5A (Boehmer et al. 2003b), the K+ channels ROMK1 (Yun et al. 2002b), KCNE1/KCNQ1 (Embark et al. 2003) and Kv1.3 (Gamper et al. 2002a,b; Wärntges et al. 2002), the Na+/H+ exchanger NHE3 (Yun et al. 2002a), the dicarboxylate transporter NaDCT (Boehmer et al. 2004), the glutamate transporters EAAT1 (Boehmer et al. 2003a) and EAAT3 (Schniepp et al. 2004) and the Na+/K+-ATPase (Henke et al. 2002; Setiawan et al. 2002). Nevertheless, several channels and transporters have been shown to be insensitive to regulation by SGK1 and Nedd4-2 (Palmada et al. 2004). As found here, co-expression of Nedd4-2 and SGK1 did not significantly modify the NAT3 (SLC38A4) amino acid co-transporter expressed in X. laevis oocytes. Thus, the regulation of EAAT2 by the SGK isoforms is not unspecific.
Nedd4-2 and SGK1 may participate in the signalling of neuroexcitability and neuroexcitotoxicity. According to the present results, Nedd4-2 would down-regulate EAAT2 and thus delay the clearance of glutamate from the extracellular space. As a result, the effects of glutamate may be enhanced. SGK1, on the other hand, is expected to accelerate the clearance of glutamate from the extracellular space and thus, foster termination of an excitatory signal. Enhanced expression of EAAT2 has been shown to protect against excitotoxic neuronal death (Guo et al. 2003; Wisman et al. 2003), and deranged function of EAAT2 has been implicated in several neuronal diseases including amyotrophic lateral sclerosis (Rothstein et al. 1992; Rothstein 1995; Altomare et al. 1998; Meyer et al. 1999; Honig et al. 2000; Trotti et al. 2001; Howland et al. 2002; Munch et al. 2002; Legay et al. 2003), epilepsy (Dutuit et al. 2002; Sander et al. 2000; Proper et al. 2002) and Alzheimer's disease (Honig et al. 2000; Rodriguez-Kern et al. 2003). Moreover, lack of EAAT2 function was shown to potentiate retinal ganglion cell death (Vorwerk et al. 2000). In axotomy, or after spinal cord injury, EAAT2 has been shown to be up-regulated, an effect thought to play a protective role (Lopez-Redondo et al. 2000; Vera-Portocarrero et al. 2002), and overexpression of EAAT2 appears to protect against motor neurone degeneration and epilepsy (Sutherland et al. 2001). During ischaemia, on the other hand, a reversed mode function of EAAT1 and EAAT2 has been postulated to enhance extracellular glutamate concentration (Douen et al. 2000). Inappropriate regulation of the carriers by Nedd4-2 and/or SGK1 could similarly participate in the pathophysiology of those diseases.
The ability of SGK1 to stimulate EAAT2 activity is also observed with the isoforms SGK2 and SGK3, and with protein kinase B. Thus, these kinases have the potential to replace SGK1 in this functional capacity, which may be one reason for the apparently mild phenotype of the SGK1-knockout mouse (Wulff et al. 2002). Complete knockout of PDK1, i.e. the kinase upstream of all three SGKs and PKB, is not compatible with survival (Lawlor et al. 2002).
In conclusion, the present observations demonstrate that the serum and glucocorticoid inducible kinase isoforms SGK1, SGK2 and SGK3, and the related kinase PKB, can regulate the glutamate transporter EAAT2. The kinases phosphorylate the ubiquitin ligase Nedd4-2 and thus, stimulate the activity and cell surface expression of EAAT2. The mechanism is likely to participate in the regulation of neuronal excitability.
Acknowledgements
The authors thank Sir Philip Cohen for providing the constructs of active S422D SGK1, inactive K127NSGK1 and constitutively-active T308D,S473DPKB. They gratefully acknowledge the technical assistance of B. Noll. This study was supported by the Deutsche Forschungsgemeinschaft, Nr. La 315/4–4, La 315/5–1, Br 1318/2–4, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Center for Interdisciplinary Clinical Research, 01 KS 9602) and the DAAD.




