Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats
Abstract
Recently, we demonstrated that intrahippocampal infusion of the cyclo-oxygenase (COX)-2-specific inhibitor celecoxib impaired spatial memory retention in the Morris water maze. In the present work, we investigated the effects of nicotine, infused in the rat dorsal hippocampus several minutes after infusion of celecoxib, on memory retention in the Morris water maze. Rats were trained for 3 days; each day included two blocks, and each block contained four trials. Test trials were conducted 48 h after surgery. As expected, bilateral intrahippocampal infusion of celecoxib (19 µg/side; 0.1 m) increased escape latency and travel distance in rats, indicating significant impairment of spatial memory retention. We also examined the effects of bilateral infusion of nicotine (0.5, 1.0 and 2.0 µg/side) on memory retention. Infusion of 1 µg nicotine significantly decreased escape latency and travel distance but not swimming speed, compared with controls, suggesting memory retention enhancement by nicotine at this concentration. In separate experiments, bilateral infusion of nicotine, infused 5 min after 0.1 m (19 µg/side) celecoxib infusion, was associated with escape latency, travel distance and swimming speed profiles very similar to those in control animals. Brain tissue sections from several of these animals were subjected to immunohistochemical staining analysis with anti-COX-2 antibodies. Quantification analysis by optical density measurements showed that the celecoxib infusion reduced the immunoreactivity of COX-2-containing neurons in the CA1 area of the hippocampus compared with controls, although this reduction was not significant. However, infusion of a combination of celecoxib and nicotine significantly increased this immunoreactivity compared with levels in control and celecoxib-infused groups. These results suggest that nicotine prevented or reversed the adverse effects of celecoxib on spatial memory retention and protected or restored the immunostaining pattern of COX-2 neurons in the rat dorsal hippocampus.
Abbreviations used
-
- ACh
-
- acetylcholine
-
- ChAT
-
- choline acetyltransferase
-
- COX
-
- cyclo-oxygenase
-
- DMSO
-
- dimethyl sulfoxide
-
- PG
-
- prostaglandin
-
- PBS
-
- phosphate-buffered saline
-
- TX-100
-
- Triton X-100
-
- VAChT
-
- vesicular ACh transporter
Cholinergic hippocampal neurons are considered to be critical for memory formation. In particular, nicotinic acetylcholine (ACh) receptors localized on these neurons have been widely shown to be important for memory and cognition Decker et al. 1995; Stolerman et al. 1995; Kim and Levin 1996; Puma et al. 1999; Hiramatsu et al. 2002; Marti Barros et al. 2004). Some studies indicated that nicotine-binding sites in the human brain were affected by aging or by Alzheimer's disease, and large reductions in the number of nicotinic binding sites have been reported in the brains of patients with Alzheimer's disease (Nordberg and Winblad 1986; Quirion et al. 1986; Whitehouse et al. 1986; Nordberg et al. 1992; Nordberg 2001). Nicotine has been shown to improve cognitive function, including memory and attention, in a variety of experimental animal and human studies (Decker et al. 1995; Whitehouse and Kalaria 1995; Levin et al. 1996; Brioni et al. 1997; Levin and Simon 1998; Bancroft and Levin 2000; Marti Barros et al. 2004).
Experimental studies with rodents have shown a protective effect of nicotine on memory deficits induced in different brain lesions (Decker et al. 1992; Grigoryan et al. 1994) or induced by nicotinic antagonists (Zarrindast et al. 1996) or β-amyloid protein (Itoh et al. 1996; Decker et al. 1997). Nicotine also improved memory in naive rats and mice (Haroutunian et al. 1985; Sansone et al. 1991; Zarrindast et al. 1996). Nicotine facilitated spatial working memory, as measured by improved choice accuracy in the radial arm maze, when administered systemically to rats (Levin and Rose 1990; Levin 1992; Kim and Levin 1996). It is clear that this improvement is caused by central nicotinic effects. This is based on the observation that systemic administration of the peripheral nicotinic antagonist hexamethonium did not impair radial arm maze choice accuracy (Levin et al. 1987; Kim and Levin 1996), whereas systemic administration of mecamylamine as a central nicotinic receptor antagonist significantly impaired the maze choice accuracy (Levin et al. 1987; Kim and Levin 1996). There is also evidence that low concentrations of nicotine enhance hippocampal synaptic transmission, which seems to contribute to the improvement of memory (Hiramatsu et al. 1994, Hiramatsu et al. 2002; Whitehouse and Kalaria 1995). Moreover, it has been reported that nicotine induces release of glutamate and ACh (Toth et al. 1993; Sullivan et al. 1997; Hiramatsu et al. 2002), both of which have been implicated in learning and memory enhancement.
Cyclo-oxygenase (COX) catalyses the first committed step in the biosynthesis of a large family of arachidonic acid metabolites comprising prostaglandins (PGs), prostacyclin, and thromboxanes, which are collectively known as prostanoids (Needleman et al. 1986; Teather et al. 2002; Turini and Dubois 2002). COX exists in constitutive and inducible isoforms (Smith et al. 1991; Herschman 1996; Dubois et al. 1998; Chen et al. 2000, 2001; Gorgoni et al. 2001). Three isoforms, COX-1, COX-2 and COX-3, have been identified (Kujubu et al. 1991; Xie et al. 1991; Shaftel et al. 2003). COX-1 is the constitutive form and performs a housekeeping function in regulating normal activities (Smith et al. 1991; Herschman 1996). COX-2 is the inducible isoform, and is rapidly expressed in several cell types in response to growth factors, cytokines and pro-inflammatory molecules (Dubois et al. 1998; Chen et al. 2000, 2001; Gorgoni et al. 2001). COX-2 expression in the brain has been associated with pro-inflammatory activities and is thought to be instrumental in the neurodegenerative processes of several acute and chronic diseases (Minghetti 2004). All the isoforms of COX enzymes are major targets of non-steroid anti-inflammatory drugs (Schwieler et al. 2004).
There are some clinical data that show the effects of COX-2 inhibitors on progression of dementia and Alzheimer's disease. The results from these studies indicate that COX-2 inhibitors do not prevent or slow down cognitive decline and so are not useful therapeutic approaches in patients with established mild to moderate Alzheimer's disease (Aisen et al. 2003; Reines et al. 2004; De Craen et al. 2005; Thal et al. 2005).
In the CNS, COX-2 is expressed under normal conditions and contributes to important brain functions, such as synaptic activity and memory consolidation (Minghetti 2004). Many studies indicate the involvement of COX-2 in the information process and physiological mechanism of memory. For instance, intrahippocampal infusion of celecoxib, a COX-2-specific inhibitor, impaired spatial memory acquisition (Rall et al. 2003) and memory retention (Sharifzadeh et al. 2005a) in rats. In addition, intraperitoneal administration of NS-398, a COX-2 inhibitor, attenuated memory consolidation (Teather et al. 2002). The involvement of COX-2 in passive avoidance memory in chicks has also been reported (Holscher 1995).
It has been reported that nicotine increases COX-2 expression and subsequent PG release in the central and peripheral nervous systems (Adams et al. 1996; Saareks et al. 1998; Baik et al. 1999; Rama Sastry et al. 1999; Du and Role 2001; Chang et al. 2003; Shin et al. 2004). In the present work, we investigated the effects of nicotine, infused into the CA1 region of the hippocampus within minutes of infusion of the COX-2 inhibitor celecoxib, on spatial memory retention in male rats, using the Morris water maze. We also examined the effects of these infusions on the COX-2 immunostaining pattern and conducted quantitative optical density measurements of COX-2-containing neurons in the CA1. Memory retention in our experiments was evaluated 48 h after drug infusion.
Materials and methods
Subjects
Male albino Wistar rats (200–250 g), obtained from the Pasteur Institute of Iran, were used. Rats were housed in groups of five per cage in large cages (41 cm long, 28 cm wide, 15 cm high) before surgery and individually in small cages (26 cm long, 21 cm wide, 14 cm high) after surgery, and were maintained at room temperature, 25 ± 2°C, under a standard 12-h light–dark cycle. Animals were provided with food and water ad libitum. These animal experiments were carried out in accordance with recommendations from the Declaration of Helsinki and the internationally accepted principles for the use of experimental animals.
Drugs
All drugs used in this work were purchased from Sigma (St Louis, MO, USA). Celecoxib and (–)nicotine were dissolved in dimethylsulfoxide (DMSO) (100%) and saline (0.9%) respectively. ketamine and xylazine were used for surgical anesthesia.
Surgery
Rats were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (25 mg/kg, i.p.) and placed in a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). Guide cannulas were implanted bilaterally into the CA1 region and were attached to the skull surface using dental cement and jeweler's screws. Stereotaxic coordinates of the rat brain, based on the atlas of Paxinos and Watson (1997), were: anterior–posterior, 3.8 mm from bregma; mediolateral, ± 2.2 mm from midline; dorsoventral, − 2.7 mm from the skull surface.
Infusion procedure
Intrahippocampal infusions were administered through guide cannulas (21 G) using injection needles (27 G) connected by polyethylene tubing to 5-µL Hamilton microsyringes. Figure 1 shows a representative photomicrograph of an infusion site in the dorsal hippocampus with an arrowhead pointing to the infusion cannula track. The injection needle was inserted 0.5 mm beyond the tip of the guide cannula. The duration of the drug infusion was 1 min, and the needles were left in place for an additional 60 s to allow diffusion of solution away from the needle tip.
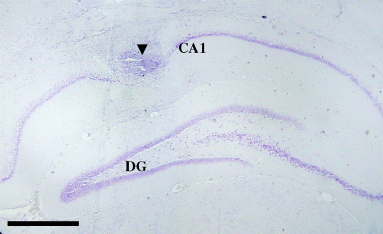
Representative photomicrograph of an infusion site into the rat hippocampal CA1 area. The figure shows the site of infusion in the dorsal hippocampus with the arrowhead pointing to the infusion cannula tract. CA1, Ammon's horn region 1; DG, dentate gyrus. Scale bar = 0.5 mm.
Behavioral training and testing
All animals were subjected to bilateral infusions of saline or DMSO as control groups, celecoxib (19 µg/side, 0.1 m) and nicotine (0.5, 1 or 2 µg/side). For assessing the interactive effects of nicotine and celecoxib on memory retention, we infused nicotine (1 µg/side) 5 min after celecoxib (19 µg/side, 0.1 m) into the CA1 region of the hippocampus immediately after the training trials. The volume of injection in all groups was 0.5 µL per side. The same procedure was performed for the other vehicles.
Six to seven days after recovery from surgery the animals were trained for 3 days in the Morris water maze. A circular, black painted pool (136 cm diameter, 60 cm high) filled to a depth of 25 cm with water (22 ± 1°C) was used. The pool was divided into four quadrants with four starting locations called north (N), east (E), south (S) and west (W) at an equal distance from the rim. An invisible platform (10 cm diameter) made of Plexiglas was submerged 1 cm below the water line and placed in the center of the northern quadrant. Each training day included two blocks, and each block consisted of four trials. Each trial was initiated by placing the animals randomly in one of the four quadrants. Animals were allowed to find the hidden platform during a period of 60 s. The rats rested for 5 min between two consecutive blocks, and the intertrial interval time was 30 s in each block. A video camera was mounted directly above the water maze pool. This camera was also linked to a computer and used to record the rats' swim paths. The escape latency (time to reach the hidden platform), distance traveled (length of swim path) and swimming speed for each rat were all recorded automatically by a video tracking system. Immediately after the last trial, on the third day of training, we bilaterally infused rats with either saline, DMSO, celecoxib or nicotine. The interval between drug infusions and testing trials was 48 h. Rats were tested in the Morris water maze in order to assess their spatial memory retention. The testing included one block of 4 trials.
Histology
Following each behavioral testing trial, animals were killed by decapitation, and their brains removed. For histological examination of cannulas and needle placement in the CA1 region, the brains were cut into sections 30–40 µm thick on a cryostat, mounted on glass slides, and stained with cresyl violet. The sections were then examined under a light microscope to find the bilateral sites of cannulas and infusion tracts and sites to ascertain whether the sites were in the dorsal hippocampus (Fig. 1). Data collected from animals whose cannulas and sites of infusions were not in the dorsal hippocampus were discarded.
Immunohistochemistry
Brain tissues from four animals in each experiment were obtained and processed according to standard protocols (Roghani et al. 1998; Woolf et al. 2001). Animals were deeply anesthetized and then transcardially perfused with 100 mL phosphate-buffered saline (PBS) followed by 300 mL 4% paraformaldehyde in 0.1 M phosphate buffer containing 0.15% picric acid at 4°C. The brains were post-fixed in the paraformaldehyde/picric acid solution overnight followed by incubation in PBS containing 30% sucrose. After embedding in Optimal Cutting Temperature (OCT), the brains were sectioned at 40-μm intervals through the rostrocaudal extent of the medial septal area and hippocampus in the frontal plane (Paxinos and Watson 1997). The tissue sections through the vertical diagonal band nucleus and CA1 of hippocampus were saved. These tissue sections were immunostained for COX-2. Antibodies directed against COX-2 were used to immunostain the COX-2 neurons in the hippocampus. Free-floating tissue sections were washed five times (for 5 min each) in PBS to remove the OCT and picric acid. They were then permeabilized in PBS containing 0.4% Triton X-100 (TX-100) and 1% normal goat serum for 45–60 min. The sections were then washed with PBS (three times each for 5 min) followed by incubation in PBS containing 0.3% hydrogen peroxide solution for 30 min. After washing the tissue in PBS (three times each for 5 min), the sections were blocked for 60 min in PBS containing 0.3% TX-100 and 3% normal goat serum. The sections were then incubated with a polyclonal antiserum to COX-2 (diluted 1 : 100 in PBS containing 1% normal goat serum and 0.3% TX-100) for 48 h on a shaking platform at 4°C. An affinity-purified polyclonal anti-rabbit COX-2 antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA) was used in our study. The sections were washed extensively with PBS with 1% normal goat serum (eight times each for 5 min) followed by incubation with biotinylated anti-goat IgG secondary antibody (diluted 1 : 200 in PBS and 1% normal goat serum; Vector Laboratories, Burlingame, CA, USA), for 60 min at room temperature. Following three washes in a PBS solution with 1% normal goat serum, the sections were incubated in avidin–biotin complex detection solution (ABC Elite Kit; Vector Laboratories) diluted 1 : 150 in PBS for 45 min at room temperature. The sections were washed (three times each for 5 min) in PBS containing 1% normal goat serum and then incubated with a PBS solution containing 0.02% diaminobenzidine-4HCl (Sigma), 0.3% nickel sulfate and 0.03% hydrogen peroxide for 5–15 min to achieve the desired staining intensity. Staining was stopped by washing the sections three times with PBS. The sections were mounted on gelatin-coated Super Frost Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA) and air-dried overnight. After a brief wash in distilled water, they were dried again as before, incubated in xylene for 5 min, and coverslipped with a Cytoseal® mounting medium containing xylene (Baxter Diagnostics, Inc., McGrow Park, IL, USA). These tissue sections were analyzed with a BX51 Olympus microscope (Olympus, Tokyo, Japan). The extent of the infusions was mapped on templates from the atlas of Paxinos and Watson (1997). On the basis of anatomical landmarks, the region of CA1, located 4.5 mm posterior and 3.0 mm lateral to bregma, was selected with the microscope set at × 10, and the photomicrographs were taken at × 10 and × 40 magnification.
Quantification of COX-2 immunostaining
Following immunohistochemical staining, COX-2 immunoreactivity was evaluated by light microscopy and the captured images were digitized using Olysia software (Olympus) and analyzed with Scion Image (Scion Corporation, Frederick, MD, USA). Briefly, the CA1 region was selected in the image and the average optical density or pixel intensity was calculated in each brain section from control, celecoxib and celecoxib–nicotine groups. The optical density of background was obtained from areas adjacent to the COX-2 immunoreactive areas in the CA1 region in each brain section, as described previously (Lee et al. 2004). After subtraction of the background density from the optical density of measured CA1 areas, the normalized values were averaged and values in control, celecoxib and celecoxib–nicotine groups were compared statistically.
Statistical analysis
We used a one-way anova in most cases and a two-way anova when specified. A Newman–Keulz multiple comparison test was performed to assess differences in behavioral scores. A p-value of 0.05 or less was considered statistically significant.
Results
Effects of training on escape latency, distance traveled and swimming speed in the Morris Water Maze
After 3 days of training in the Morris water maze, all animals (those selected to receive bilateral infusion of saline, DMSO, celecoxib or nicotine), learned how to find the hidden platform as indicated by a decrease in escape latencies and distance traveled (Table 1). There was a significant difference (p < 0.001) between the third and first day of training in terms of escape latency and distance traveled for finding the hidden platform. The swimming speed was not significantly altered by training trials in any of the groups (Table 1).
| Training (day) | Escape latency (s) | Travel distance (cm) | Speed (cm/ s) |
|---|---|---|---|
| 1 | 32.7 ± 1.973 | 701.2 ± 96.49 | 24.25 ± 0.590 |
| 2 | 14.53 ± 1.292*** | 355.4 ± 33.25*** | 24.88 ± 0.989 |
| 3 | 9.225 ± 0.840*** | 244.3 ± 20.72*** | 26.00 ± 0.823 |
- In our experiments, all groups of animals (sham operated as well as those infused with saline, DMSO, celecoxib (0.1 m/side) or nicotine (0.5, 1 or 2 µg/side) learned how to find the hidden platform. A significant difference (***p < 0.001, One-way Anova) between the first and third days of training was observed in escape latency and distance traveled. There was no significant difference in swimming speed between the first, second and third days of training. Values are mean ± SEM. six to seven animals per group were used in these experiments.
Comparison of celecoxib and DMSO on time and distance of finding hidden platform during the test trials
Bilateral infusion of DMSO (0.5 µL/side) into the CA1 of the hippocampus after training did not cause any significant changes in escape latency, distance traveled or swimming speed compared with values in saline-infused and sham-operated rats (Fig. 2). These results are similar to those obtained in our previous studies (Sharifzadeh et al. 2005a,b). Infusion of celecoxib (19 µg/side, 0.1 m) into the CA1 region caused significant differences in escape latency (p < 0.001) and distance traveled (p < 0.001) compared with values in the control group (DMSO-treated animals) (Figs 2a and b). However, the swimming speed was not significantly affected by the celecoxib infusion (Fig. 2c).
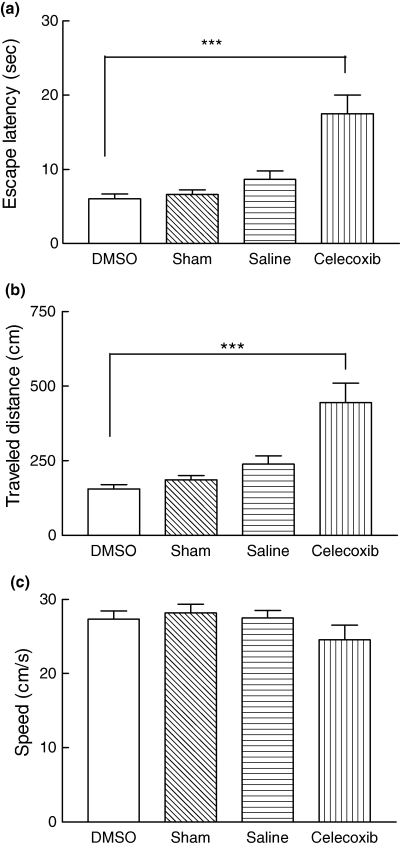
Comparative effects of DMSO, saline, sham operation and celecoxib on the memory retention test. In all experiments, the testing trials were performed 48 h after any infusion. There was no significant difference in escape latency (a), distance traveled (b) or swimming speed (c) during testing between sham-operated animals and animals infused with saline or DMSO. The celecoxib-infused rats showed spatial memory deficits during testing trials. Infusion of celecoxib (0.1 m/side, dissolved in DMSO) caused spatial memory deficits. The escape latency (a) and distance traveled (b) were significantly increased by infusion of celecoxib (0.1 m) compared with values in animals that received DMSO (control group) (***p < 0.001, Newman-Keuls multiple comparison post test). Celecoxib did not cause any significant alteration in swimming speed compared with that in the control group. Values are mean ± SEM for at least seven animals.
Effects of nicotine infusion on escape latency, traveled distance and swimming speed
Bilateral infusion of nicotine (0.5, 1 and 2 µg/side) after training led to an improvement in escape latencies and distance traveled (Figs 3a and b). Infusion of all three concentrations of nicotine caused a reduction in escape latency and travel distance compared with values in the saline control group. However, a significant difference (p < 0.05) was observed only with 1 µg nicotine. The swimming speed was similar in all groups, indicating no motor disturbances in the animals (Fig. 3c). This finding shows that intrahippocampal administration of nicotine (1 µg) after training enhances spatial memory retention in the Morris water maze.
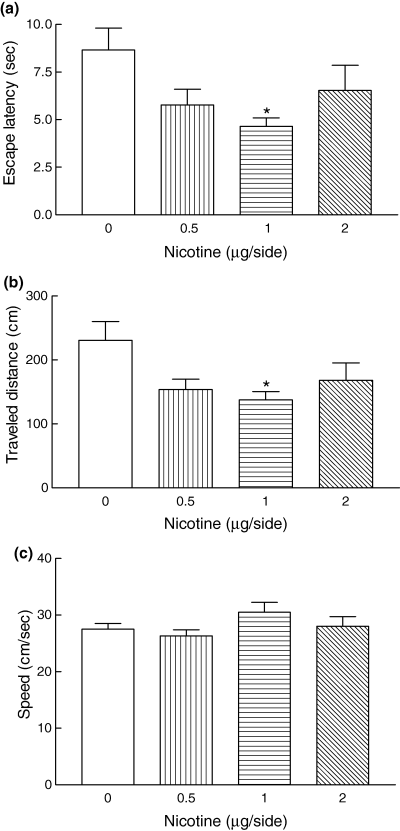
Nicotine-infused rats showed spatial memory improvement during the testing trials. The escape latency (a) and distance traveled (b) were significantly decreased by nicotine (1 µg) infusion compared with values in the control group (saline-treated animals) (*p < 0.05, One-way, anova). The swimming speed in the nicotine-induced animals did not show any significant changes (c). Values are mean ± SEM for seven animals.
Protective effects of nicotine against celecoxib-induced spatial memory deficits
Bilateral post-training intrahippocampal infusion of nicotine (1 µg) 5 min after celecoxib (19 µg/side, 0.1 m) infusion prevented the onset of spatial memory deficit normally observed after celecoxib infusion in the Morris water maze (Fig. 4). The celecoxib-induced increase in escape latency and distance traveled was reversed by nicotine infusion to a level similar to that in the control group (Figs 4a and b). The swimming speed was very similar in control, celecoxib-infused and nicotine plus celecoxib-infused groups (Fig. 4c).
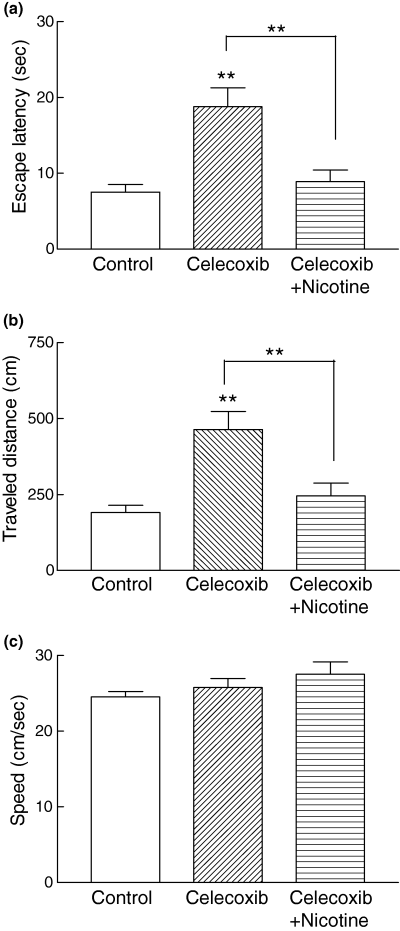
The celecoxib-induced spatial memory deficit was reversed by nicotine administration (1 µg). In all experiments, the testing trials were performed 48 h after any infusion. The increased escape latency (a) and distance traveled (b) induced by celecoxib (0.1 m) was reduced significantly by nicotine (**p < 0.01, Newman-Keuls multiple comparison post test). No significant difference was observed between the control group and animals that received celecoxib with nicotine. The swimming speed was similar in all groups, indicating that there were no motor disturbances (c).
Compared with the control group (Fig. 5a), a decrease and increase in travel paths were observed following the infusion of nicotine (1 µg/side) and celecoxib (19 µg/side, 0.1 m) respectively (Figs 5b and c). Figure 5(d) illustrates and confirms that intrahippocampal administration of nicotine (1 µg/side) after training substantially decreased the travel path in celecoxib-infused animals.
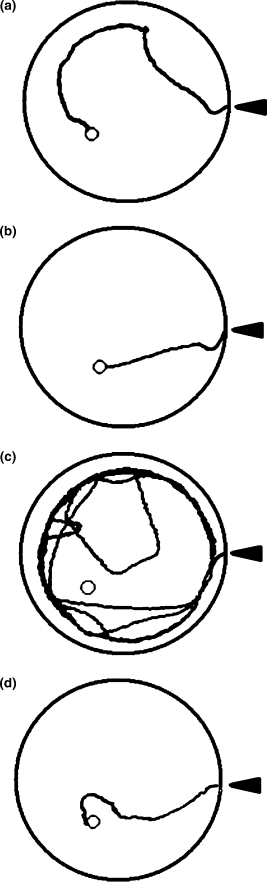
(a–d) Representative traces of travel path in the control (a) nicotine (b; 1 µg), celecoxib (c; 0.1 m), and celecoxib plus nicotine (d) groups. Nicotine decreased (c) and celecoxib increased (c) travel paths on the test day compared with the distance travelled in the control group (a). Intrahippocampal infusion of nicotine (0.1 µg) following celecoxib (0.1 m) infusion caused a marked decrease in travel path (d), compared with celecoxib (c) alone. The circles designate the position of the hidden platform.
Effects of intrahippocampal infusion of nicotine and celecoxib on the density of COX-2-containing neurons in the dorsal hippocampus
Rat brain sections from the control (DMSO) group and animals infused with celecoxib, nicotine or celecoxib combined with nicotine (1 µg/side) were immunostained with anti-COX-2 antibodies. Figures 6a and b show the pattern of immunostaining in a representative rat that received a bilateral infusion of DMSO into the CA1 region of the hippocampus, with photomicrographs taken at × 10 (Fig. 6a) and × 40 (Fig. 6b) magnification. Bilateral infusion of nicotine into the CA1 region of the hippocampus did not appear to significantly alter the number and density of immunostained COX-2-containing neurons, as shown in representative sections at × 10 (Fig. 6c) and × 40 (Fig. 6d) compared with control sections (Figs 6a and b). Quantification of immunostaining data by taking optical density measurements from six animals confirmed our qualitative assessment (Fig. 7). Bilateral infusion of celecoxib (19 µg/side, 0.1 m) appeared to somewhat reduce the COX-2-containing neuron immunostaining density in the dorsal hippocampus as displayed at × 10 (Fig. 6e) and × 40 (Fig. 6f), although there was no significant reduction compared with the control group (Fig. 7). The density and immunostaining intensity of the CA1 hippocampal COX-2-containing neurons present in a representative animal that received nicotine (1 µg/side) 5 min after infusion of celecoxib (19 µg/side, 0.1 m/side) were visibly and qualitatively increased, as shown at × 10 (Fig. 6g) and × 40 (Fig. 6h) in comparison to levels in celecoxib-infused animals (Figs 6d and f). Subsequent quantification of COX-2 immunostaining in the CA1 regions of tissue sections confirmed that there was a significant increase in COX-2 immunoreactivity in rats treated with a combination of celecoxib and nicotine compared with levels in the celecoxib group (p < 0.01) and control group (p < 0.05) (Fig. 7).
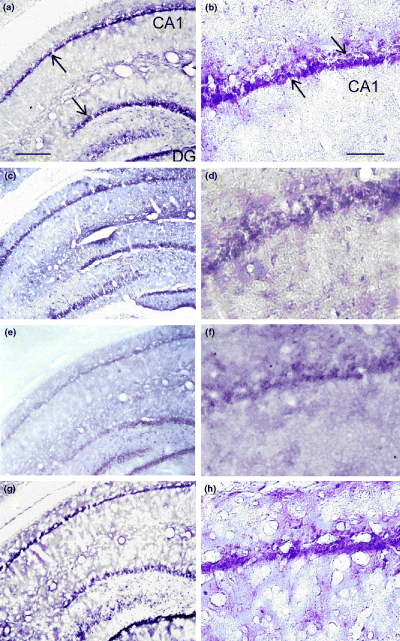
Interactive effects of intrahippocampal infusion of nicotine and celecoxib on the immunostaining density of COX-2-containing neurons in the dorsal hippocampus. (a, b) Pattern of staining in a representative rat dorsal hippocampus in the control group, with photomicrographs taken at × 10 (a) and × 40 (b) magnifications. The arrows indicate COX-2-containing neurons. (c, d) Patterns of staining in animals infused with nicotine (1 µ g) at × 10 (c) and × 40 (d). (e, f) Patterns of staining in a rat infused with 0.1 m celecoxib at × 10 (e) and × 40 (f). (g, h) Immunostaining patterns in a representative rat infused with a combination of celecoxib (0. m) and nicotine (1 µg), at × 10 (g) and × 40 (h). Nicotine infused 5 min after celecoxib appeared to prevent the reduction in the density and staining intensity of the CA1 hippocampal COX-2-containing neurons. Scale bar for (a), (c), (e) and (g) 200 µm; scale bar for (b), (d), (f) and (h) 25 µm.
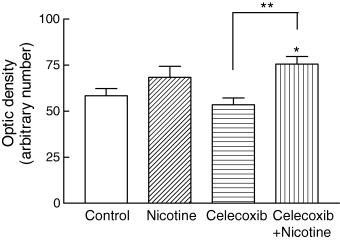
Quantification of COX-2 immunoreactivity in the hippocampal CA1 in control animals and those infused with nicotine (1 µg), celecoxib (19 µg, 0.1 m) and a combination of celecoxib plus nicotine. All groups were tested for spatial memory retention 48 h after the drug infusions. Optical density measurements for COX-2 immunoreactivity in the CA1 regions for all brain sections in each group of animals were measured and the respective background staining subtracted. Infusion of nicotine and celecoxib caused an increase and a decrease respectively in COX-2 immunoreactivity, but these changes were not significant. However, there was a significant increase (**p < 0.01, Newman-Keuls multiple comparison post test) in the immunoreactivity of COX-2-containing neurons in the CA1 region in celecoxib–nicotine-treated animals compared with the celecoxib-infused group. A significant difference (*p < 0.05, One-way anova) was also observed between the celecoxib–nicotine-infused animals and the control group. Values are mean ± SEM for tissue sections from at least six animals in each group.
Discussion
The neural basis of memory may involve the interaction of a number of different endogenous substances and neurotransmitters. There is considerable evidence implicating ACh in higher brain functions such as attention, learning and memory (Hasselmo and McGaughy 2004; Descarries et al. 2005). Cholinergic hypofunction is currently thought to be one of the principle causes of dementia and cognitive deficit in Alzheimer's disease (Hiramatsu et al. 2002). Recent evidence has suggested a role for the nicotinic ACh receptor and nicotine and its analogs in memory deficits induced by cholinergic dysfunction (Potter et al. 1999; Levin and Rezvani 2000; Hiramatsu et al. 2002). The positive effects of nicotine on memory in intact rats and mice have been reported in several studies (Sansone et al. 1991; Zarrindast et al. 1996; Puma et al. 1999). Some experiments on rodents showed that nicotine counteracted the memory deficit induced by muscarinic or nicotinic antagonists (Zarrindast et al. 1996). Our results indicated that intrahippocampal infusion of nicotine improved or enhanced spatial memory retention when the time interval between infusion of the drug and testing trials was 48 h.
A considerable body of evidence has shown the critical role of hippocampal NMDA glutamate receptors in memory function (Wozniak et al. 1990; Toth et al. 1993; Richter-Levin et al. 1995; Toth 1996; Levin et al. 2003). The direct interactions of nicotinic and glutamatergic systems have also been reported previously (Perez De La Mora et al. 1991; Levin et al. 2003). Nicotine has been shown to stimulate the release of glutamate and a variety of nicotine's pharmacological effects are probably mediated by this mechanism (Perez De La Mora et al. 1991; McGehee et al. 1995; Levin et al. 2003). The increase in glutamate release induced by nicotine is important for cognitive function in the hippocampus (Toth et al. 1993; Toth 1996; Levin et al. 2003). Nicotine-induced glutamate release can influence the neural communication and plasticity important in memory processes (Bezzi and Volterra 2001; Amateau and McCarthy 2002; Rall et al. 2003). Therefore, the enhancement in spatial memory retention by nicotine observed in this study might be related to interactions between nicotine and the glutamatergic systems in the dorsal hippocampus.
Nicotine also appears to increase the release of dopamine, GABA, ACh, norepinephrine and serotonin in brain areas associated with cognitive function (Levin and Simon 1998; Levin and Rezvani 2000; Yin and French 2000; Hefco et al. 2003; Singer et al. 2004). Several effects of nicotine on memory function may be mediated through neuromodulatory potentiation of the release of these neurotransmitters. It has been shown that the dopamine system might be involved in the effects of nicotine on memory, because dopaminergic neurons from the ventral tegmental area have both nicotinic and muscarinic ACh receptors. Systemic or in vitro administration of nicotine excites dopamine neurons in the ventral tegmental area (White 1996; Yin and French 2000; Hefco et al. 2003). Co-administration of D2 dopamine receptor antagonists with nicotine decreases memory performance compared with nicotine administration (Addy and Levin 2002). The importance of both nicotinic and GABA systems in memory function and the interactions between these two transmitter receptor systems have also been reported (Levin et al. 2004). For instance, nicotine significantly reversed the memory deficit induced by GABA agonist (Levin et al. 2004). Thus, the positive effects of nicotine on spatial memory retention observed in our studies may be related to effects of nicotine on a variety of neurotransmitter systems.
In our study, the effect of a 2-µg dose nicotine was not as significant as that produced by 1 µg. This might result from complex effects of nicotine on a variety of neural systems. For example, it has been shown that systemic administration of nicotine at a low dose improved working memory in the radial arm maze (Gatto et al. 1998), but higher doses did not improve working memory performance (Levin et al. 2003) or even impaired radial maze choice accuracy (Mundy and Iwamoto 1988). Like all promnestic drugs, nicotine at moderate doses improved cognitive function and at higher doses either had less beneficial effects or produced impairment. It appears that local infusion of nicotine causes nicotine receptor deactivation at a high local concentration. In addition, other factors such as method of administration, type of behavioral task, and selection of the site of infusion in the brain may have an impact on the effects of nicotine on memory. Thus, in our study it is possible that the intrahippocampal infusion of nicotine at 1 µg/side was at the threshold of the switch from positive to negative effects of nicotine on spatial memory retention in the Morris water maze.
In our study, celecoxib for infusion was dissolved in DMSO (100%) and nicotine was dissolved in saline. We found that DMSO and saline were suitable and effective solvents for celecoxib and nicotine respectively. DMSO-infused animals showed no significant differences in escape latencies, distance traveled or swimming speed compared with naive or saline-infused rats during testing trials. Therefore, it appeared that DMSO infusions did not cause motor disturbances or have significant effects on spatial memory measurements. The effective use of DMSO as a vehicle has been reported previously (Packard et al. 1996; Naghdi et al. 2001; Sharifzadeh et al. 2005a, 2005b).
We observed no significant differences in swimming speed between the first, second and third days of training. In addition, the animals receiving celecoxib or nicotine infusions did not show any significant differences in swimming speed compared with the speed of animals receiving saline or DMSO infusions (control groups). These observations demonstrate that celecoxib or nicotine infusions did not apparently cause any undesirable motor disturbances. Such observations provide support for our conclusions that celecoxib infusion impaired spatial memory retention and nicotine infusion enhanced memory retention in rats.
Choline acetyltransferase (ChAT) and vesicular ACh transporter (VAChT) represent two known cholinergic markers (Roghani et al. 1994; Bejanin et al. 1994; Erickson et al. 1994; Woolf et al. 2001; Sharifzadeh et al. 2005a, 2005b). In the cholinergic presynaptic terminals, the enzyme ChAT synthesizes ACh, and VAChT protein is responsible for the transport of ACh into synaptic vesicles for regulated exocytotic release (Parsons et al. 1993; Usdin et al. 1995; Woolf et al. 2001). The expression of VAChT and ChAT genes is co-regulated in certain neuronal systems (Berrard et al. 1995; Berse and Blusztajn 1995; Shimojo et al. 1998). COX-2 pathway products are known to affect the cholinergic system in the brain (Bugajski et al. 2002). Therefore, the celecoxib-induced spatial memory retention deficits observed in our study may be in part due to the reduction in expression of cholinergic markers, such as ChAT and VAChT.
The major finding of the present study is that the memory retention deficit induced by celecoxib as a COX-2 inhibitor (Engelhardt et al. 1996; Talley et al. 2000) was prevented or reversed by nicotine. The involvement of COX-2 and its metabolite in spatial memory has been demonstrated previously in rats (Teather et al. 2002; Rall et al. 2003; Sharifzadeh et al. 2005b). These findings suggest an important role for COX-2 in spatial memory. A reduction in COX-2 metabolites, particularly PGE2, and its effects on glutamate release and neural plasticity are the principle suggested mechanisms by which memory deficit is induced by a COX-2 inhibitor (Rall et al. 2003).
In our present work, intrahippocampal infusion of nicotine appeared to show neuroprotective effects against celecoxib-induced spatial memory retention impairment. Nicotine has different functions in a variety of brain areas and cascading effects mediated by nicotine-induced release of a variety of transmitters (Wonnacott 1997; Levin et al. 2003), including glutamate (McGehee et al. 1995; Levin et al. 2003) have been described. There is some recent evidence showing that nicotine-induced stimulation of NMDA glutamate receptors increased glutamate release, which is critical for nicotine-induced memory improvement (Levin et al. 2003). Other recent findings indicate that nicotine increased COX-2 expression, PGE2 release, as well as activation of extracellular signal-regulated protein kinase (Chang et al. 2003; Shin et al. 2004), all of which were prevented by a COX-2 inhibitor (Shin et al. 2004). The stimulatory effect of nicotine on PGE2 formation has also been reported by other investigators (Saareks et al. 1998; Rama Sastry et al. 1999; Du and Role 2001). PGE2 has an important role in influencing neural plasticity and communication. For instance, it has been reported that the addition of exogenous PGE2 overcomes COX-2 inhibition and rescues the induction of long-term potentiation in hippocampal slice cultures (Chen et al. 2002; Rall et al. 2003). In the normal brain, COX-2 is expressed dynamically in neurons and is associated with NMDA receptor-related excitability (Adams et al. 1996; Baik et al. 1999). It has been suggested that PGE2 increases the release of glutamate, which appears to improve memory function (Bezzi and Volterra 2001; Amateau and McCarthy 2002; Rall et al. 2003). Data from these studies, along with our present finding that nicotine prevented spatial memory retention deficit induced by celecoxib, suggest an indirect interaction between nicotine and glutamate systems, mediated by COX-2 activation by nicotine. Therefore, it is reasonable to deduce that COX-2 activation and the subsequent increase in PGE2 formation can stimulate glutamate release. This increased release may underlie the observed protective effects of nicotine against celecoxib-induced memory retention deficits.
In addition to modulatory effects of PGE2 on glutamate neurotransmission, previously published reports indicate an interactive role for the COX-2-mediated PG pathway and cholinergic function in neural plasticity (Bugajski et al. 2002). Nicotine is known to increase expression of VAChT (Prendergast and Buccafusco 1998). It is therefore reasonable to assume that the preventive effect of nicotine on spatial memory retention in animals infused with celecoxib was caused, in part, by an increase in vesicular release of ACh in the CA1 of the hippocampus.
We also found that the optical density of immunostained COX-2-containing neurons in the CA1 region was significantly increased in animals that received an intrahippocampal CA1 infusion of nicotine (1 µg/side) 5 min after infusion of celecoxib (0.1 m/side). This apparently neuroprotective effect of nicotine probably results from an increase in COX-2 protein expression levels caused by nicotine-induced stimulation of COX-2 activity.
In conclusion, our data and those of others, discussed above, provide evidence for an interaction between nicotine and the COX-2 pathway in the brain. Increasing our knowledge of the cellular and molecular mechanism(s) underlying this interaction may require a more in-depth analysis and a better understanding of the roles of nicotine and COX-2 in memory function, which should be achievable in our future studies.
Acknowledgements
We thank Dr Kurdistan Sharifzadeh, Mr Reza Abbasgholizadeh, Mr Julio Payan and Ms Melissa Murphy for help with the preparation of the figures in the manuscript. This study was supported in part by funds from Tehran University of Medical Sciences to MS and a seed grant from Texas Tech University Health Sciences Center to AR.




