Neisseria meningitidis-induced death of cerebrovascular endothelium: mechanisms triggering transcriptional activation of inducible nitric oxide synthase
Abstract
The intense host response to meningococcus reflects marked functional and morphological alterations in blood–brain barriers. We showed previously that mouse-derived cerebrovascular endothelium responded to meningococcal lysates with a robust nitric oxide (NO) response, resulting in the loss of cell viability. To understand how the NO synthase-2 gene in endothelium is activated by meningococcus, we investigated upstream roles for specific protein kinases. Using known kinase inhibitors, and measuring both mRNA expression and nitrite release, we found MAPK/ERK kinase (MEK)2, p38 kinase and phosphoinositide 3-kinase (but not MEK1 or phospholipase C) to be implicated in the NO synthase-2 response. Recruitment of these kinases by meningococcus did not depend on the prior release of the proinflammatory cytokines tumour necrosis factor α or interleukin-1β from endothelium. These endothelial cells were found to express toll-like receptors (TLR) 2, 4 and 9 and antibodies directed against TLR 2 and 4 (but not TLR 9) blocked the NO synthase-2 response to meningococcus. Both meningococcus-induced translocation of nuclear factor-kB (NF-kB) and endothelial cell death were blocked by a known inhibitor of p38 kinase. Calpain inhibitor-1 blocked the NO synthase-2 response to meningococcus, which is further evidence of a role for NF-kB.
Abbreviations used
-
- BBB
-
- blood–brain barrier
-
- CPI
-
- calpain inhibitor
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- MEK
-
- MAPK/ERK kinase
-
- NF-kB
-
- nuclear factor-kB
-
- NO
-
- nitric oxide
-
- NOS-2
-
- inducible NO synthase
-
- p38
-
- k, p38 MAPK
-
- p65
-
- subunit of NF-kB
-
- PI
-
- 3-k, phosphoinositide 3-kinase
-
- RPA
-
- ribonuclease protection assay
-
- TLR
-
- toll-like receptor
-
- TNF
-
- tumour necrosis factor
Neisseria meningitidis is a commensal bacterium of the human nasopharynx which occasionally initiates disease ( Taha et al. 2002 ). The mortality among children and adults with meningitis is 5–15% and one-tenth of those who survive suffer from long-lasting neurological sequelae ( Cripps et al. 2002 ). There is presently a worldwide increase in the incidence of meningococcal infections, particularly due to serogroup B, for which there is no vaccine due to poor immunogenicity of the capsular polysaccharide ( Vogel and Frosch 1999 ). The recent success of the new conjugated capsular polysaccharide vaccines against serogroup C infections emphasizes the need for effective protein-based formulations against group B.
Following mucosal colonization and systemic invasion, the meningococcus evades host defences. The site and mechanisms by which the bacterium gains entry into the brain are now becoming clear and specific proteins expressed on choroid plexus and cerebral capillary endothelial cells appear to facilitate transit into the subarachnoid space (Nassif et al. 2002). The intense host response (oedema, increased intracranial pressure and altered cerebral flow) reflects functional alterations in the blood–brain barriers (BBB), including changes in the properties of cerebral microvascular endothelium (Nau and Bruck 2002). These changes are initiated when bacteria first transit through the vascular wall, may facilitate bacterial entry and would, therefore, be important in the ensuing pathogenesis.
A common downstream response to virulence factors is the transcriptional activation of inducible nitric oxide (NO) synthase (NOS-2), resulting in the persistent production of high levels of NO (Nathan 1997). Patients, and also experimental animals with meningitis, have elevated levels of cerebrospinal fluid nitrite and the changes in cerebral blood flow seen early in pathogenesis appear to be mediated by NO (Boje 1996; Faraci and Heistad 1998; Jaworowicz et al. 1998). This NOS-2 response in the host, while antimicrobial (Fang 1997; Leib et al. 1998), may also contribute to the ensuing pathophysiology (Tunkel and Scheld 1993; Shenep and Tuomanen 1998). Levels of NO correlate with alterations in BBB properties (Buster et al. 1995; Mayhan 2000) and the severity of meningitis (Deng et al. 2001), and also changes in BBB permeability (Buster et al. 1995), is reversed by NOS inhibition. Recently, there have been reports of increased expression of both NOS-2 and NOS-3 in rodents experimentally infected with Streptococcus pneumoniae, correlating with rat blood-labyrinth (Kastenbauer et al. 2001) and mouse BBB disruption (Winkler et al. 2001). By comparison, animals that were NOS-2 deficient or receiving peroxynitrite scavengers showed significantly less disruption.
Previously, we reported that live and lysed N. meningitidis (both being present in the human disease) activated the NOS-2 gene in mouse cerebrovascular endothelium, irrespective of whether lipopolysaccharide (LPS) was expressed, and that this production of NO resulted in cell death (Constantin et al. 2002). Here we reveal roles for specific toll-like receptors (TLR), members of the MAPK signalling pathway, phosphoinositide 3-kinase (PI 3-k) and also NF-kB in the endothelial cell response to meningococcus.
Materials and methods
Reagents
TriReagent, calpain inhibitor (CPI)-1 and the polyclonal antibody against β-actin (A-2066) were obtained from Sigma (St Louis, MO, USA). The HybSpeed™ RPA (High-Speed Ribonuclease Protection Assay) kit was purchased from AMS Biotechnology Ltd (Abingdon, UK). Polyclonal antibodies against TLR 2 (sc-10739), TLR 4 (sc-10741) and subunit of NF-kB (p65; sc-372) were obtained from Santa Cruz (Autogen Bioclear UK Ltd, Wiltshire, UK). The TLR 9 polyclonal antibody (IMG-431) was from Biocarta (Oxford, UK). The mouse interleukin (IL)-1 receptor antagonist (480-RM) and mouse soluble tumour necrosis factor (TNF)-receptor 1 (425-R1) were purchased from R & D Systems (Minneapolis, MN, USA). Anti-mouse (NXA 931) and anti-rabbit (NA 934) antibodies were from Amersham, Pharmacia Biotech (Little Chalfont, UK). Pierce Supersignal Enhancer West Pico Chemiluminescent mixture was purchased from Pelbio Sciences (Cheshire, UK). To prepare probes for the ribonuclease protection assay (RPA) (α)32P was purchased from NEN Life Science Products (Zaventem, Belgium). The kinase inhibitors were from Calbiochem (San Diego, CA, USA).
Cell lines
MB114En cells derived from mouse cerebral vessels (Moore et al. 1988) were grown as described previously and viability assessed by trypan blue exclusion (Constantin et al. 2002). Primary cultures of cerebral cortical astrocytes were prepared from neonatal C57BL/6 mice and maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum, glutamine (2 mm) and penicillin/streptomycin (20 U/mL). Mouse macrophage-like RAW 264.7 cells were grown in medium of the same composition.
Preparation of meningococcus
Strain MC58, a serogroup B:15:P1.16 isolate of N. meningitidis, was prepared as described earlier (Constantin et al. 2002). Over the course of these experiments a number of different lysates were prepared and their activity varied.
RT-PCR and molecular cloning
Total RNA was isolated from cells using TriReagent according to the manufacturer's protocol. The primers and PCR conditions used for TLR 2 were according to Medvedev et al. (2000). These primers were designed to amplify a 346-bp fragment of the 5′ end of the mouse TLR 2 cDNA. The primers and PCR conditions used for TLR 4 were according to Lehnardt et al. (2002), with the difference that DNase treatment of RNA samples prior to assay was found to be essential. These primers amplify a 507-bp fragment of the 5′ end of the mouse TLR 4 cDNA.
The NOS-2 plasmid construct used as template for the RPA probe was generated by RT-PCR using oligonucleotides iNOS1 (5′-GCTCTGGAATTCACAGCTCA) and iNOS2 (5′-TGTTGTAGCGCTGTGTGTCA). These primers were designed to amplify a 398-bp fragment of the 5′ end of the mouse NOS-2 cDNA. The resulting PCR products were cloned into the PCR II TA cloning vector (Invitrogen, Carlsbad, CA, USA). The β-actin RPA probe was generated from a plasmid template supplied with the RPA kit. Probes for TNFα (303 bp) and IL-1β (142 bp) mRNAs were generously provided by Iain Campbell (Scripps Research Institute, La Jolla, USA).
Ribonuclease protection assay
The RPA was performed using the HybSpeed kit. The RNA probes were synthesized by in vitro transcription from linearized plasmid templates with incorporation of 32P-UTP. Probes were DNase treated to remove residual template and gel purified. Sample RNA (10 µg) was mixed with 10 000 cpm β-actin probe as the internal control and 30 000 cpm of the other probes and ethanol precipitated using sodium acetate. The resulting pellet was redissolved in 10 µL pre-heated hybridization buffer by incubation at 95°C. Hybridization was carried out for 10 min at 68°C, followed by RNase digestion at 37°C for 30 min. The RNase was inactivated by addition of inactivation/precipitation mix and protected RNA duplexes were ethanol precipitated. Samples were re-dissolved in gel loading buffer and fragments were separated by 5% denaturing polyacrylamide gel electrophoresis. Gels were exposed to X-ray film and signal was quantified using densitometry.
Nitrite assay
Following the addition of meningococcal lysate, cell supernatant fluids were removed from the culture at various time points and nitrite assayed by the Griess reaction. Briefly, 150 µL of supernatant fluid were diazotized with sulfanilamide (final concentration 1 mm) and HCl (final concentration 0.1 m). N-(1-Naphthyl) ethylenediamine was then added to a final concentration of 1 mm and the absorbance of the sample was read at 550 nm. The amount of nitrite (µm) was calculated from a standard curve for sodium nitrite. This varied between batches of cells (see figure legends).
Isolation of nuclei and cytosolic fractions
This was performed as previously described by Shafer and Murphy (1997).
Western blotting
Cell lysates were thawed and centrifuged at 15 000 g for 20 min at 4°C. Samples (100 µg protein) were separated in 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membrane. Immunoblotting was performed as described previously (Constantin et al. 2002).
Statistical analysis
The data were analysed using two-way (time and condition) anova for repeated measurements and Student's t-test. Where the anova resulted in a significant F ratio (p < 0.05), the location of significance was determined using either least significant difference or paired t-test. Statistical significance for all analyses was accepted as p < 0.05.
Results
Specific kinases are implicated in the activation of the NOS-2 gene
To understand how meningococcus caused an increase in NOS-2 mRNA expression, MB114En cells were pre-incubated with a number of known kinase inhibitors and their effects on NO production screened by nitrite assay. These cells express a low level of NOS-2 mRNA endogenously, as we have reported previously (Borgerding and Murphy 1995; Shafer and Murphy 1997). Following exposure to meningococcal whole cell lysates, the accumulation of nitrite increased three- to sixfold (Fig. 1a). While the known inhibitors of MAPK/ERK kinase (MEK)1 (PD98059) and phospholipase C γ (U73122) were without effect, SB203580 (p38 kinase), U0126 (MEK1/2) and LY294002 (PI 3-k) significantly reduced nitrite accumulation in response to MC58. This reduction was not due to the loss of cell viability as basal nitrite release from unstimulated cells was unaffected by the three compounds (data not shown). Subsequent RPA analysis (Fig. 1b) revealed reductions in NOS-2 mRNA levels in MC58 exposed cells pre-treated with SB203580, U0126 and LY294002, while basal expression of NOS-2 mRNA was unaffected.
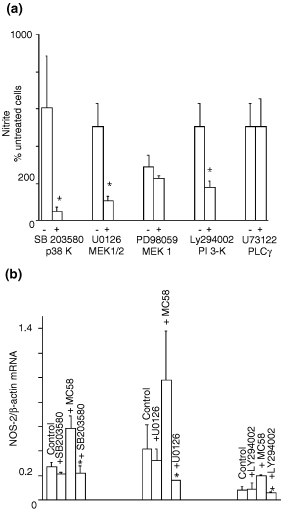
Specific kinase inhibitors block meningococcus-induced inducible nitric oxide synthase (NOS-2) expression in endothelium. MB114En cells were pre-incubated with (+) or without (–) SB203580 (2 µ m ), U0126 (10 µ m ), Ly294002 (10 µ m ), PD98059 (10 µ m ) and U73122 (1 µ m ) for 30 min and then exposed to sonicated strain MC58 (10 µg/mL). (a) Nitrite accumulation in cell conditioned medium after 48 h (untreated cells, 10 µ m = 100%), as determined by the Griess reaction. Values represent the mean ± SEM of at least three separate experiments (* p < 0.05). (b) Ratio of NOS-2/β-actin mRNAs after 8 h, as determined by ribonuclease protection assay. Values represent the mean ± SEM of at least three separate experiments (* p < 0.05 vs. MC58 treated). p38 k, p38 MAPK; MEK, MAPK/ERK kinase; PI 3-k, phosphoinositide 3-kinase; PLC, phospholipase C.
Meningococcus does not induce IL-1β or TNFα release from MB114En cells
Activation of p38, MEK1/2 and PI 3-ks could be secondary to meningococcus-induced release of proinflammatory cytokines from endothelium. To determine whether meningococcus up-regulated endothelial IL-1β and TNFα mRNAs we performed RPA on cells 8 h later but there was no trace of transcripts in either untreated or treated endothelium (Fig. 2a). We performed RT–PCR and RPA for IL-1β at earlier time points (1 and 4 h) but mRNA could not be detected in these cells (data not shown). Finally, we investigated the effects of adding mouse IL-1 receptor antagonist and mouse soluble TNF-receptor 1 on meningococcus-induced nitrite release from MB114En cells. According to the supplier, the concentrations that we selected were sufficient to neutralize 50 pg/mL IL-1 and 100 pg/mL TNFα in cell reporter assays and, in our hands, inhibited the NOS-2 response to these cytokines in RAW cells. However, generation of nitrite from MB114En cells in response to meningococcus was unaffected by the presence of mouse IL-1 receptor antagonist and mouse soluble TNF-receptor 1 (Fig. 2b).
Endothelium-derived tumour necrosis factor (TNF)α and interleukin (IL)-1β are not involved in the mechanism of transcriptional activation of inducible nitric oxide synthase by meningococcus. (a) The expression of TNFα and IL-1β mRNA was determined by ribonuclease protection assay in MB114En cells in the presence or absence of strain MC58 (10 µg/mL) for 8 h. The β-actin transcript served as a verification of loading and RAW cells as a positive control. C1, C2, MC58-1 and MC58-2 represent untreated and treated cells in two experiments. The free probes were run in the first three lanes of the gel and the positions of the RNA duplexes in the remaining lanes are indicated by arrows. (b) MB114En cells were incubated with either mouse soluble TNF-receptor 1 (TNF-R1; 1.5 µg/mL) or mouse IL-1 receptor antagonist (IL-1ra; 60 ng/mL) in the presence of MC58 (10 µg/mL) and nitrite accumulation determined by the Griess reaction. Values (untreated cells, 10 µ m = 100%) represent the mean ± SEM of three separate experiments.
MB114En cells express toll-like receptors
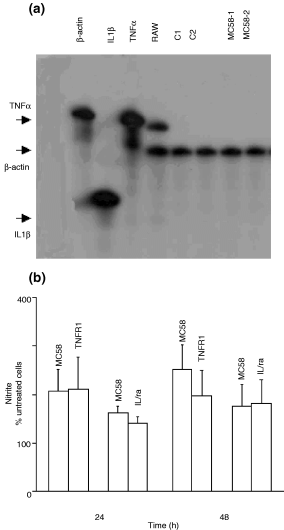
Pathogens engage TLRs on host cells and TLRs 2, 4 and 9 mediate many of the responses to Gram-negative and -positive bacteria. Using RT–PCR (Figs 3a and c), we determined that MB114En cells express both TLRs 2 and 4, irrespective of their exposure to meningococcus. We were able to detect TLR 4 expression in MB114En cells via immunoblotting and expression was unchanged following exposure to meningococcus (Fig. 3b).
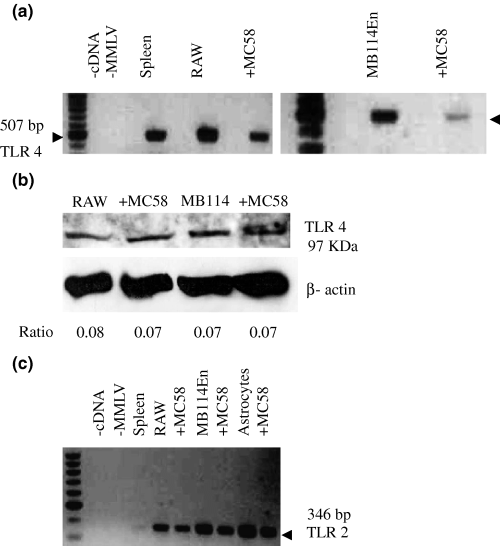
MB114En cells express toll-like receptors (TLR) 2 and 4. (a) Two gels showing results of RT–PCR with primers specific for TLR 4 and amplification of a 507-bp product from MB114En cells. RAW cells and whole spleen were included as positive controls. RAW and MB114En cells were exposed to strain MC58 for 8 h. The first of each pair of lanes is the control for genomic DNA (negative). (b) Expression of TLR 4 protein was verified in MB114En cells by immunoblotting using a specific polyclonal antibody. RAW cells served as the positive control. Loading is shown by β-actin and the TLR 4 : β-actin ratio is indicated below each lane. RAW and MB114En cells were exposed to MC58 for 8 h. (c) RT–PCR with primers specific for TLR 2 amplified a 346-bp product from MB114En cells and astrocytes. Spleen and RAW cells served as positive controls. Negative controls included minus cDNA and minus Moloney murine leukaemia virus (MMLV) for genomic DNA. RAW, MB114En cells and astrocytes were exposed to MC58 for 8 h.
The TLR 9 receptor is responsive to bacterial DNA and so could mediate the MB114En cell response to MC58 lysate. On immunoblots we could detect TLR 9 in MB114En cells and the level of expression was unaltered by exposure to MC58 (Fig. 4a). However, prior treatment of meningococcus lysates with DNase did not diminish the NOS-2 mRNA response, as revealed by RPA (Fig. 4b). Increasing the DNase concentration further (10-fold) did not affect lysate activity (data not shown).
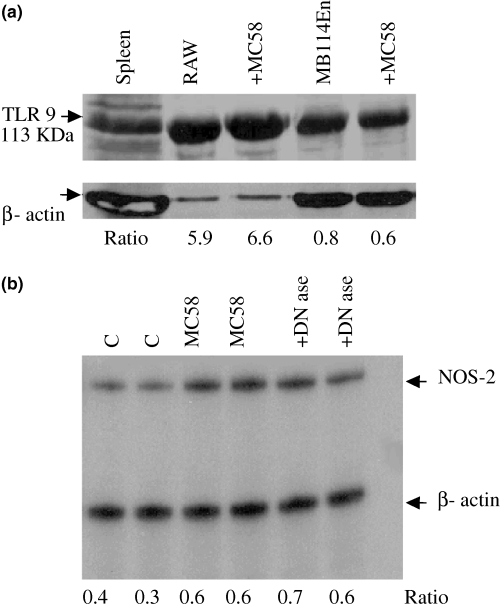
MB114En cells express toll-like receptor (TLR) 9. (a) Expression of TLR 9 was determined by immunoblotting using a specific polyclonal antibody. RAW cells served as the positive control and loading is shown by β-actin. The TLR 9 : β-actin ratio is indicated below each lane. RAW and MB114En cells were exposed to strain MC58 for 8 h. (b) Meningococcal lysates were treated with and without DNase (0.04 U/µL) for 1 h at 37°C, prior to incubation with MB114En cells. After 8 h, RNA was prepared and inducible nitric oxide synthase (NOS-2)/β-actin transcripts detected by ribonuclease protection assay (two separate experiments with the same lysate; ratios indicated below the lanes).
These TLR antibodies are IgGs which recognize epitopes in the extracellular domains of the mouse receptors. Therefore, we reasoned that these antibodies might block receptor activation by MC58. Incubation of MB114En cells with TLR 2 and 4 antibodies for 5 h prior to exposure to MC58 prevented activation of the NOS-2 gene, as revealed by nitrite assay (Fig. 5). The TLR 9 antibody was without effect, supporting our findings from the DNase experiment in Fig. 4(b) and suggesting that TLR 9 plays no role in initiating the NOS-2 response in MB114En cells.
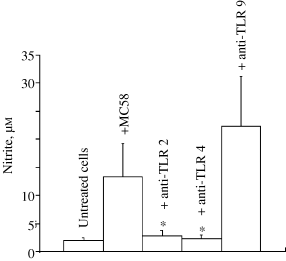
Roles for toll-like receptors (TLR) 2 and 4 (but not TLR 9) in the endothelial cell response to meningococcus. MB114En cells were pre-incubated for 5 h in the presence or absence of TLRs 2, 4 and 9 antibodies (5 µg/mL) and exposed to strain MC58 (10 µg/mL) for 48 h. The antibodies contained sodium azide (0.09%) and this was added as vehicle to untreated and MC58-treated cells (final concentration 0.002%). Nitrite accumulation (µ m ) was determined by the Griess reaction and values represent the mean ± SEM of three cell cultures (* p < 0.05).
Meningococcus activates the NOS-2 gene via the p38 kinase/NF-kB pathway
Activation of either TLR 2 or 4 may be expected to initiate the translocation of NF-kB from the cytoplasm to the nucleus. To determine whether this occurred in MB114En cells, we isolated nuclei and cytoplasmic fractions from these cells at different times following exposure to MC58 and performed immunoblotting with an antibody against p65. No p65 was present in the nuclear fraction until 30 min after exposure to MC58 and translocation was increased by MC58 treatment (Fig. 6a, upper panel). The response was still present at 120 min but not thereafter (data not shown). In addition, cells were exposed to the known p38 MAPK (p38 k) inhibitor SB203580 (or the vehicle dimethylsulphoxide) prior to 30 min treatment with MC58. The addition of dimethylsulphoxide itself resulted in p65 accumulation. However, the effect of MC58 on p65 translocation to the nuclear fraction was still apparent (twofold over control) and this effect was abolished by SB203580 (Fig. 6a, lower panel).
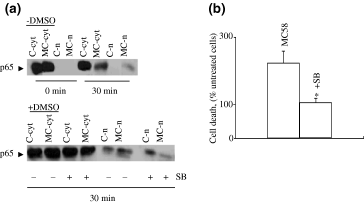
SB203580 blocks meningococcus-induced translocation of NF-kB in MB114En cells and also abrogates cell death. (a) Nuclei (n) and cytoplasmic fractions (cyt) were prepared from MB114En cells treated with or without strain MC58 (MC, 10 µg/mL, zero or 30 min) or SB203580 (SB, 2 µ m , 30 min prior), separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblotted for the p65 subunit of NF-kB. The lower panel illustrates the effects of dimethylsulphoxide (DMSO) (final concentration 0.02%), which was included as the vehicle control for SB. The blots shown are representative of two separate experiments. (b) MB114En cells were treated with or without MC58 (10 µg/mL) or SB203580 (SB, 2 µ m , 30 min prior) and cell death determined by trypan blue exclusion 48 h later. Values (% untreated cells) represent the mean ± SEM of three cell cultures (* p < 0.05).
Previously, we reported that a large proportion of MB114En cells eventually die in response to MC58, via an NO-mediated mechanism (Constantin et al. 2002). To determine whether cells could be rescued by interrupting the signalling pathway upstream of NF-kB activation, MB114En cells were exposed to SB203580 prior to MC58. This known p38 k inhibitor had a dramatic protective effect and abolished MC58-mediated cell death (Fig. 6b).
As we had already shown that CPI-1 blocked NF-kB activation in MB114En cells (Borgerding and Murphy 1995), we predicted that CPI-1 would also reduce the NOS-2 response to MC58. Indeed, incubation of MB114En cells with CPI-1 1 h prior to MC58 abolished the increase in NOS-2 mRNA (Fig. 7a) and also the subsequent accumulation of nitrite (Fig. 7b).
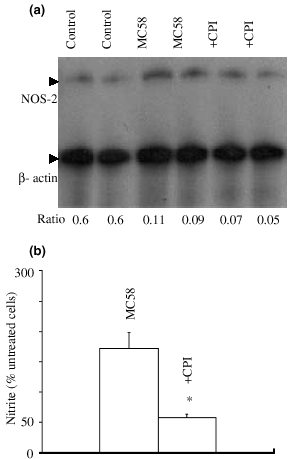
Inhibition of NF-kB activation abolishes the inducible nitric oxide synthase (NOS-2) response to meningococcus. (a) MB114En cells were pre-incubated for 1 h in the presence or absence of calpain inhibitor (CPI)-1 (20 µ m ), exposed to strain MC58 (10 µg/mL) for 8 h and the expression of NOS-2 mRNA determined by ribonuclease protection assay (two separate experiments). The β-actin transcript served as a verification of loading and the NOS-2 : β-actin ratio is indicated below each lane. (b) MB114En cells were incubated as above and nitrite accumulation determined by the Griess reaction 48 h later. Values (untreated cells, 3 µ m = 100%) represent the mean ± SEM of three separate experiments (* p < 0.05).
Discussion
Our major finding is that meningococcus transcriptionally activates the NOS-2 gene in cerebrovascular-derived endothelium via a mechanism involving TLRs 2 and 4, the recruitment of specific kinases and the activation of NF-kB.
While we cannot rule out non-specific effects of the kinase inhibitors used on other signalling molecules or pathways, numerous studies have revealed roles for specific kinases in NOS-2 expression in various cell types. For example, Bhat et al. (1998, 2002) have revealed the involvement of both p38 k and ERK in rat astrocytes and microglia, while Hua et al. (2002) showed that inhibition of p38 k could prevent IL-1β-mediated NOS-2 induction. Interferon regulatory factor-1 and MAPK participate in the induction of NOS-2 in retinal pigmented epithelial cells (Faure et al. 1999). It is clear, however, that other signalling pathways impinge upon the MAPK cascade to regulate NOS-2 expression. For example, Pahan et al. (1999, 2000) showed that blockade of PI 3-k could potentiate endotoxin- and IL-1β-stimulated NOS-2 induction.
The recruitment by meningococcus of signalling kinases, such as p38 k, MEK1 and PI 3-k, could result from activation of a variety of receptors on the endothelial cell surface, including those for IL-1 and TNF. Indeed, the severity of meningococcal disease directly correlates with the production of these proinflammatory cytokines (Kolb-Maurer et al. 2001). However, we found no evidence to suggest that NOS-2 expression in MB114En cells resulted from the autocrine release of IL-1β and TNFα. As Taha (2000) has already shown, endothelial cells in vitro cannot be induced to express TNFα when exposed to meningococcus unless macrophages are also present.
Our data suggest that MB114En cells express TLRs 2, 4 and 9. These receptors are known to trigger the host response to Gram-negative and -positive bacteria and TLR activation recruits numerous protein kinase cascades (Takeda et al. 2003), including those described here. Taken together, the persistence of NOS-2 inducing activity in DNase-treated meningococcal lysate and the lack of effect by the TLR 9 antibody argue against a role for this receptor.
We found that antibodies directed against extracellular epitopes of TLRs 2 and 4 could block the NOS-2 response to meningococcus. Results from studies in cells from TLR knockout mice (Takeuchi et al. 1999) and in HEK293 cells expressing TLRs (Chow et al. 1999; Schwander et al. 1999) suggest that TLR 4 mediates signalling induced mainly by LPS whereas TLR 2 is involved in responses to lipopeptides. While TLR 4 has been identified as a component of the LPS receptor signalling complex that controls the immune response in vivo (Schwander et al. 1999), we and others have found that an LPS-deficient mutant of N. meningitidis is still able to evoke host cell responses (Steeghs et al. 1999; Constantin et al. 2002) and that these responses are mediated by TLR 2 rather than TLR 4 (Ingalls et al. 2001; Pridmore et al. 2001). Likewise, neisserial porins have been shown to stimulate B cells via TLR 2 (Massari et al. 2002). In a murine model of experimental pneumococcal meningitis, Koedel et al. (2003) also found that TLR 2 has a significant role. While TLR 2 has been shown to heterodimerize with other TLRs (Takeda et al. 2003), we are not aware of any reported association with TLR 4.
The engagement of TLRs 2 and 4 by pathogens can lead to the activation of NF-kB (Takeda et al. 2003) and this transcription factor is well known as a key regulator of NOS-2 expression (Bhat et al. 2002). We found that exposure of MB114En cells to meningococcus resulted in the translocation of p65 from cytoplasm to nucleus and this response was sensitive to SB203580. In addition, we observed that SB203580 and CPI-1, known inhibitors of p38 k and NF-kB activation, blocked the endothelial cell NO response. Thus, similar to the reported response to pneumococcus (Koedel et al. 2003), meningococcus appears to activate NOS-2 in host cells via NF-kB.
In the current study we have not addressed the identity of the component(s) of N. meningitidis responsible for transcriptional activation of the NOS-2 gene in cerebrovascular endothelium. Clearly, this is important and could offer the potential for novel therapeutic approaches to limit the neurological sequelae of meningitis. We have ruled out LPS (Constantin et al. 2002) and, while bacterial DNA has been implicated in evoking the host response (Deng et al. 2001), we found no evidence in the current study for its role in the endothelial response to MC58. There are other potential candidates. Helicobacter pylori causes NOS-2 expression in epithelium through the release of urease (Gobert et al. 2002) and peptidoglycan-associated lipoprotein is implicated in NOS-2 expression in macrophages (Hellman et al. 2002). Alternatively, the NOS-2 response may require release of an unidentified host cell product. For example, heat shock proteins are reported to be ligands for both TLRs 2 and 4 and extracellular matrix components (fibronectin, hyaluronic acid and heparan sulphate) can activate TLR 4 (Takeda et al. 2003).
Recently, the intracellular Nod1 protein has been shown to detect Gram-negative peptidoglycan in permeabilized epithelium, resulting in NF-kB translocation (Girardin et al. 2003). This is an unlikely explanation for our current observations in intact endothelial cells. However, it will be interesting to discover whether meningococcus, well known to transcytose across the BBB in vivo (Nassif et al. 2002), could also provoke an NOS-2 response en route.
In summary (Fig. 8), an as yet unidentified (and not LPS) component(s) within a meningococcal lysate causes up-regulation of NOS-2 in cerebrovascular endothelium through TLRs 2 and 4 (but not TLR 9) receptor activation and the subsequent (CPI-1-sensitive) translocation of NF-kB. The modulatory effects of known inhibitors implicate specific kinases in this cell response and defining their roles further will require more selective strategies, such as the use of dominant-negative or siRNA constructs. The NOS-2 response is not secondary to the release of inflammatory cytokines (IL-1β and TNFα). Although this host response is defensive and potentially bactericidal the NO produced can compromise cell viability through a bystander effect. In vivo, such a response could contribute to alterations in the integrity of the BBB, so characteristic of bacterial meningitis. In terms of future therapeutic development, while NOS inhibitors are effective in reversing the BBB changes in experimentally infected animals, loss of the bactericidal component of the host response may make this an incomplete clinical strategy.
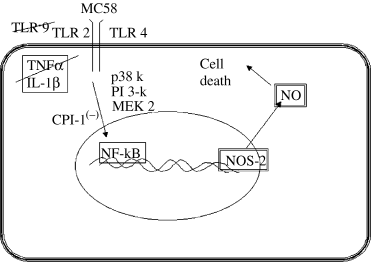
A summary of the proposed signal transduction mechanisms involved in inducible nitric oxide (NO) synthase (NOS-2) up-regulation by meningococcus. CPI, calpain inhibitor; IL, interleukin; MEK, MAPK/ERK kinase; NF-kB ; p38 k, p38 MAPK; pI 3-k, phosphoinositide 3-kinase; TLR, toll-like receptor; TNF, tumour necrosis factor.
Acknowledgements
We would like to thank Steven A. Moore (University of Iowa, lowa City, IA, USA) for MB114En cells and Iain Campbell (Scripps Research Institute) for RPA probes.




