White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene
Acknowledgements This work was supported in part by grants DK15070, DK20595, and RR04999 from the National Institutes of Health.
North American usage: mental retardation.
Abstract
Aim Mutations in the SLC16A2 gene have been implicated in Allan–Herndon–Dudley syndrome (AHDS), an X-linked learning disability* syndrome associated with thyroid function test (TFT) abnormalities. Delayed myelination is a non-specific finding in individuals with learning disability whose genetic basis is often uncertain. The aim of this study was to describe neuroimaging findings and neurological features in males with SLC16A2 gene mutations.
Method We reviewed brain magnetic resonance imaging (MRI) findings and neurological features in a cohort of five males aged between 1 year 6 months and 6 years (median 4y) from four families harbouring SLC16A2 gene mutations.
Results The participants presented aged between 4 and 9 months with initial hypotonia and subsequent spastic paraparesis with dystonic posturing and superimposed paroxysmal dyskinesias. Dystonic cerebral palsy was the most common initial clinical diagnosis, and AHDS was suspected only retrospectively, considering the characteristically abnormal thyroid function tests, with high serum tri-iodothyronine (T3), as the most consistent finding. Brain MRI showed absent or markedly delayed myelination in all five participants, prompting the suspicion of Pelizaeus–Merzbacher disease in one patient.
Interpretation Our findings indicate a consistent association between defective neuronal T3 uptake and delayed myelination. SLC16A2 involvement should be considered in males with learning disability, an associated motor or movement disorder, and evidence of delayed myelination on brain MRI. Although dysmorphic features suggestive of AHDS are not always present, T3 measurement is a reliable screening test.
List Of Abbreviations
-
- ADC
-
- Apparent diffusion coefficient
-
- AHDS
-
- Allan–Herndon–Dudley syndrome
-
- MCT8
-
- Monocarboxylate transporter 8
-
- MRS
-
- Magnetic resonance spectroscopy
-
- OFC
-
- Occipitofrontal circumference
-
- rT3
-
- Reverse tri-iodothyronine
-
- T3
-
- Tri-iodothyronine
-
- TFT
-
- Thyroid function test
Allan–Herndon–Dudley syndrome (AHDS) was among the first X-linked learning disability syndrome to be described in a large North American pedigree,1 and has subsequently been reported in additional multigenerational families.2 Causative mutations in the solute carrier family 16, member 2 (SLC16A2) gene on Xq13.8 encoding the monocarboxylate transporter 8 (MCT8) have recently been identified in males with similar clinical features3 and typical AHDS.4 MCT8 has now been characterized as an active and specific thyroid hormone transporter with differential tissue expression,5 suggesting a possible molecular basis for the complex clinical manifestations associated with SLC16A2 gene mutations. In addition to a characteristic pattern of disturbed thyroid function, typical features in affected males include hypotonia and muscle weakness at birth, preceding evolution of a more complex neurological picture featuring profound learning disability, dysarthria, spastic paraplegia, and distal involuntary movements. Although SLC16A2 gene mutations have recently been identified in around 1/10 of previously genetically unresolved cases with initial radiological features suggestive of a Pelizaeus–Merzbacher-like disease type of leukodystrophy and subsequent progression of myelination,6 neuroimaging features associated with AHDS, particularly those indicating abnormal white matter development,7–9 have been documented in only a few cases. AHDS is, therefore, likely to be underrecognized as an important differential diagnosis in males presenting with learning disability, spastic paraplegia with or without additional dystonia, and abnormal myelination on brain magnetic resonance imaging (MRI).
We report clinical features, laboratory abnormalities, and MRI findings in five males with confirmed mutations in the SLC16A2 gene and features of AHDS.
Method
Participants
Five males from four unrelated families (one set of twins) were referred for assessment of developmental delay and an evolving motor disorder presenting from the first year of life. Genetic, clinical, and thyroid function details from all five participants are summarized in Table I. Genetic details for participant 3 have been reported previously.10 Parental informed consent for publication of data was obtained for all participants.
| Participant | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age at presentation (mo) | 9 | 4 | 4 | 6 | 6 |
| Main presenting feature | Dystonic posturing, paroxysmal dyskinesia | Developmental delay, truncal hypotonia | Developmental delay, truncal hypotonia | Developmental delay, truncal hypotonia | Developmental delay, truncal hypotonia |
| Current age (y) | 4 | 4 | 6 | 2 | 2 |
| Current OFC (centile) | 9th | 0.4th | 0.4th | 9th | 9th |
| Motor function | Non-ambulant | Non-ambulant | Non-ambulant | Non-ambulant | Non-ambulant |
| Speech | Absent | Absent | Absent | Absent | Absent |
| Dystonia | Yes | Yes | Yes | Yes | Yes |
| Paroxysmal dyskinesias | Yes | Yes | Yes | No | No |
| Initial diagnosis | Pelizaeus–Merzbacher disease | Dystonic CP | Dystonic CP | Dystonic CP | Dystonic CP |
| TSH (mU/L; normal 0.27–4.2) | 5.66 | 4.4 | 4.55 | 4.0a | 3.7a |
| fT4 (pmol/L; normal 12–22) | 10.9 | 9.6 | 7.1 | 9.2 | 9.2 |
| fT3 (pmol/L; normal 3.1–6.8) | 11.8 | 10.8 | 10.2 | – | – |
| TT3 (ng/dL; normal 90–180) | – | – | – | 323 | 337 |
| Mutation | Del ex2–6 | c. 1306delT (p. C436fs) | c. 683-5delTCT (p. delF229) | c. 962C>T (p. P321L) | c. 962C>T (p. P321L) |
- Total tri-iodothyronine (TT3) measurements were obtained in participants 4 and 5. aMeasurements for participants 4 and 5 came from a different laboratory with a slightly different normal range: N, 0.4–3.6. CP, cerebral palsy; fT3, free tri-iodothyronine; fT4, free thyroxine; OFC, occipitofrontal circumference; TSH, thyroid-stimulating hormone; –, Values not available.
Participants 1, 2, and 3
Participants 1 and 2, aged 4 years, and participant 3, aged 6 years, presented within the first year of life with developmental delay, hypotonia, and poor feeding; all three were diagnosed with an evolving four-limb motor disorder of unknown aetiology. In addition, there were concerns about episodes of unexplained distress, often triggered by nappy change and associated with paroxysmal dyskinesias featuring trunk extensor spasms and choreoathetoid movements of the upper limbs. All participants were born to non-consanguineous parents of Caucasian (participants 1 and 3) or Pakistani (participant 2) origin. Family, as well as antenatal and perinatal, history was unremarkable in all three with the exception of an antepartum haemorrhage at 11 weeks’ gestation and some concerns regarding intrauterine growth retardation at 30 weeks’ gestation in participant 3. Birthweights were between the 0.4th and 25th centile, whereas occipitofrontal circumferences (OFCs) at birth were between the 0.4th and 9th centiles. In the case of participant 2, weight fell from the 25th centile at birth to the 0.4th centile at the time of study, but growth parameters for the other two participants remained on the same centile on subsequent measurements.
Examination findings were very similar in all three participants. They all appeared placid and sociable with no distinctive facial features apart from a myopathic facial expression in the case of participant 1 (Fig. 1) and no nystagmus. There was truncal hypotonia with limb hypertonia and hyperreflexia, muscle wasting, and dystonic posturing mainly affecting the distal upper limbs. Intermittent episodes of extensor posturing and dyskinesias were also noted in all three participants. Investigations including congenital infection screen, creatine kinase, ammonia, plasma amino acids, acylcarnitines, transferrin glycoforms, white cell enzymes, urine organic acids, blood and cerebrospinal fluid (CSF) glucose and lactate, CSF amino acids and neurotransmitters, as well as karyotype and subtelomeric deletion screen were all normal.
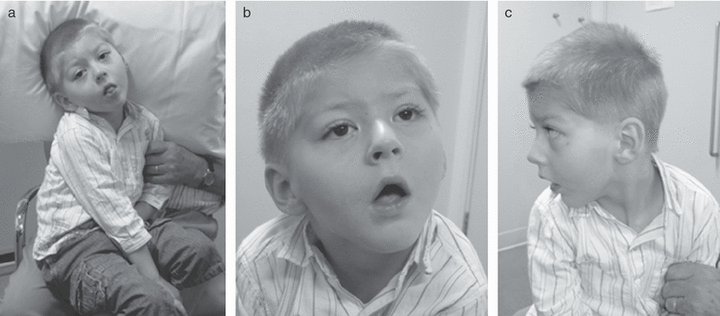
(a) Participant 1 at 4 years of age. Note myopathic facial appearance with (b) inverted v-shaped mouth, low anterior hairline, narrow forehead, (c) receding chin, and large, low-set ears.
In view of markedly delayed myelination on initial brain MRI in participant 1, Pelizaeus–Merzbacher disease or a Pelizaeus–Merzbacher-like disease was suspected, but screening for the proteolipid protein 1 (PLP1) gene was negative. MRI findings in participants 2 and 3 were initially thought to be non-specifically abnormal, and in both cases a diagnosis of cerebral palsy (CP) of unknown cause was made. The characteristically abnormal thyroid function tests (TFTs) prompted screening of the SLC16A2 gene on all three participants (Table I). All participants remain non-ambulant and do not have any speech. There is persistent truncal hypotonia with variable peripheral tone and, apart from the baseline dystonic movements, they all suffer from intermittent dyskinetic episodes, often associated with positional change.
Participants 4 and 5
Identical monochorionic twin males aged 1 year 6 months presented at age 6 months with hypotonia, poor head control, and earlier concerns about reduced spontaneous movements and poor visual behaviour. They were born to healthy, non-consanguineous parents of Ashkenazi Jewish descent and have a healthy older sister. Antenatally, the mother had gestational diabetes and there were concerns about a two-vessel cord in twin I (participant 4), but amniocentesis revealed normal male karyotype in both cases. Perinatal history was unremarkable; birthweight was on the 9th centile for twin I (participant 4) and on the 2nd centile for twin II (participant 5) and OFC was on the 9th centile in both cases. Biochemical and metabolic investigations showed elevated serum levels of lactate and sex hormone-binding globulin but were otherwise unremarkable.
At 13 months both twins exhibited severe developmental delay. Examination revealed that OFC remained on the 9th centile and weight was below the 2nd centile in both twins; additionally they were noted to have dolichocephaly with a broad forehead and wide nasal bridge. There was axial hypotonia with poor head control, variable peripheral tone with hyperreflexia and a positive extensor plantar response, and dystonic hand posturing. Because of prominent dystonia, a trial of l-dopa was initiated without apparent benefit, and the brain MRI appearances led to the suspicion of a disorder of brain myelination. Characteristically abnormal TFTs were noted in both twins and prompted screening for the SLC16A2 gene (Table I); in addition to the TFT results shown in Table I, both had low levels of reverse tri-iodothyronine (rT3; 9.1 and 11.7ng/dL; normal range 15–34ng/dL).
Magnetic resonance imaging
Brain MRI was performed in all five participants; in addition to a baseline scan, participants 1 and 2 had two and one follow-up scans respectively. Brain imaging included routine T1-weighted images, T2-weighted images, fluid-attenuated inversion recovery (FLAIR) sequences, and diffusion-weighted sequences. Apparent diffusion coefficient (ADC) maps were calculated from the diffusion-weighted sequences. Single-voxel proton magnetic resonance spectroscopy (1H-MRS) of the basal ganglia and frontal white matter was performed in participants 4 and 5 using a positron resolved spectroscopy sequence with a voxel size of approximately 15 × 20 × 16mm and using short time to echo (TE; 35ms) and long time to echo (144ms) sequences and a time to repeat (TR) of 1500ms.
Genetic studies
Sequencing of exons 1–6 of the SLC16A2 gene was performed in all five participants based on fluorescent sequencing analysis using a Mutation Surveyor (Soft Genetics LLC, PA, USA) or Applied Biosystems 3730XL sequencer (Applied Biostystems Inc., CA, USA). The parents of participants 2, 3, 4, and 5 were also tested. Mutation nomenclature was based on GenBank accession number NM_006517, where +1 is the A of the ATG translation initiation codon.
Results
Magnetic resonance imaging
Brain MRI was performed serially in participant 1 at the ages of 21 months, 30 months, and 4 years (Fig. 2). The first scan showed a marked delay in myelination, which gradually improved over the course of the next two studies to achieve a near-normal pattern by the age of 4 years on T1-weighted sequences. However, although there was also some improvement on T2-weighted sequences, these remained substantially abnormal, suggesting a persistent myelination defect. The FLAIR sequences and ADC maps also showed persistent white matter high signal, suggesting immaturity, consistent with the delay in myelination on T2-weighted sequences. In addition, there was generalized prominence of the extra-axial CSF spaces, in keeping with a global lack of cerebral white matter bulk.
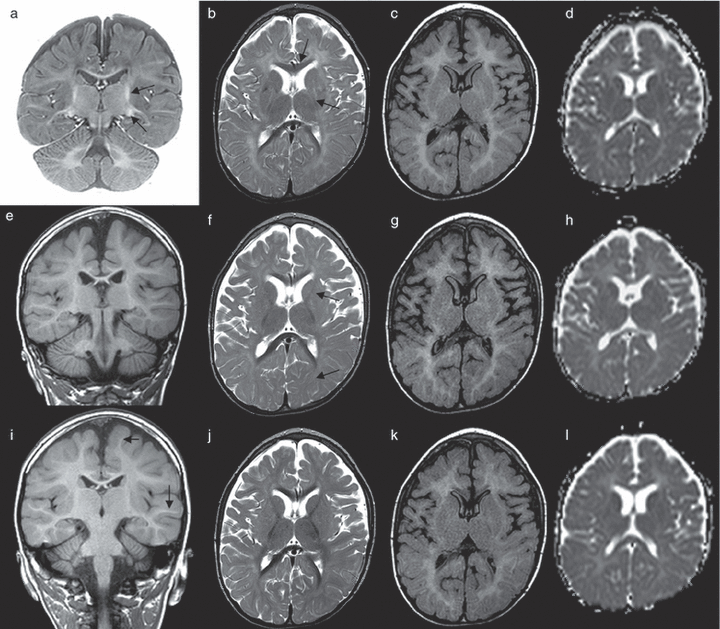
Magnetic resonance images from participant 1. (a) T1-weighted image, (b) T2-weighted image, (c) fluid-attenuated inversion recovery (FLAIR) image, and (d) apparent diffusion coefficient (ADC) map obtained at 21 months. (e–h) Corresponding images obtained at age 30 months. (i–l) Corresponding images obtained at age 4 years. On the first series of images (a–d) there is marked delay in myelination; myelin is apparent only in the posterior limb of internal capsule (PLIC) (arrow), the lateral geniculate body (arrow), the corpus callosum, the optic radiations, and patchily in the parietal and superior temporal lobes (a). On the T2-weighted image (b), myelin is seen in the PLIC and genu of the corpus callosum (arrows) but is not apparent within the anterior limb of internal capsule (ALIC) and the majority of the hemispheric white matter. Normally, the brain shows nearly complete myelination at this age, with the minor exception of some subcortical U-fibres and peritrigonal terminal zones. The FLAIR image (c) and ADC map (d) also reveal diffusely high signal within the hemispheric white matter, again reflecting immaturity (particularly noticeable on the FLAIR image because of the normally observed slight delay on this sequence). Note that myelination has gradually progressed on the second set of scans obtained at 30 weeks (e–f). On T2-weighted images, note the gradual progress of myelination in the ALIC (f, arrows) and fontal/occipital white matter (f, arrow indicating isointensity of grey–white matter in the occipital lobe marking the crossover between grey and white matter, which is normally seen at approximately 8–10 months of age). At 4 years (i–l), although myelination appears nearly complete on the T1-weighted image (i, arrows indicate myelination reaching subcortical U-fibres), there is still some delay in myelination on the other sequences, probably due to the relatively smaller amount of myelin or myelin immaturity. In addition, note the generalized prominence of the extra-axial cerebrospinal fluid spaces indicating less white matter bulk.
Two sets of MRI examinations were performed in participant 2 at the ages of 2 years 9 months and 3 years 7 months (data not shown). At the initial scan, the T1-weighted inversion recovery sequence suggested near-complete myelination (with the exception of the subcortical U-fibres, which showed further myelination on the follow-up scan), whereas T2-weighted sequences showed hyperintensity in large areas of the central and peripheral white matter of the frontal, parietal, and temporal lobes, indicating a significant myelination delay. Myelination was, however, observed on T2-weighted sequences in the internal capsules and corpus callosum, and patchily in the deep central region and subcortical white matter of the occipital, frontal, and perirolandic regions. Generalized prominence of extra-axial CSF spaces and ventricles was also noted, reflecting a lack of white matter bulk. The ADC maps revealed increased signal globally in the hemispheric white matter excepting the internal capsule and corpus callosum, consistent with diffuse white matter immaturity or abnormality. There was very little progression in myelination on T2-weighted sequences between the two studies.
The single MRI brain was performed in participant 3 at age 9 months showed generalized prominence of extra-axial CSF spaces, in keeping with lack of white matter bulk, as well as high signal of the periventricular white matter on T2-weighted images, suggestive of delayed myelination (data not shown).
Virtually identical findings of delayed myelination were found on brain MRI brain performed on participants 4 (Fig. 3) and 5 at 9 months of age. T2-weighted images indicated myelination in the posterior limbs of the internal capsules, splenium, optic radiations, and corona radiata, corresponding to the pattern seen normally at approximately 3 to 4 months of age. Single-voxel proton MRS in participants 4 (Fig. 3) and 5 showed a relatively larger peak for choline than for N-acetyl aspartate with no evidence of lactate. Although absolute quantification of the metabolites is not available, metabolite ratios are provided in Figure 3. It was felt that this spectral pattern was probably in keeping with delayed maturation or myelination.
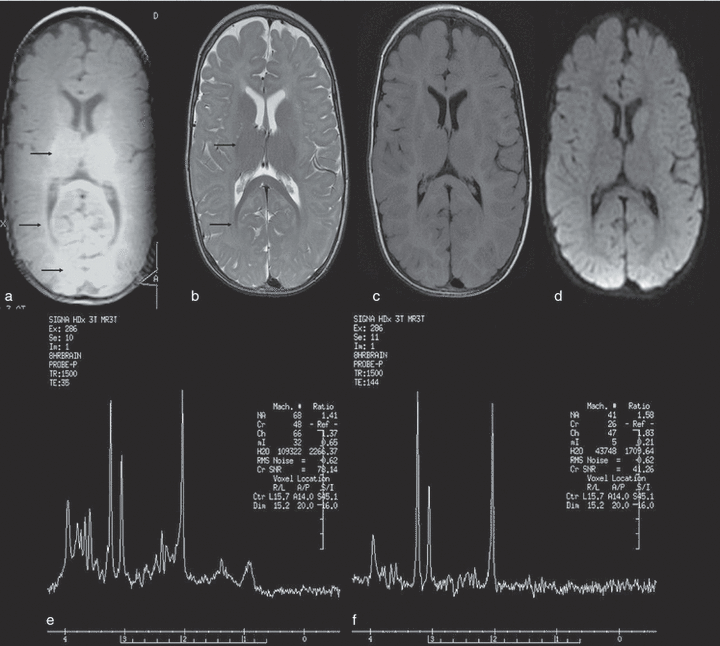
(a) T1-weighted, (b) T2-weighted, (c) fluid-attenuated inversion recovery (FLAIR) image, (d) diffusion-weighted image, and (e and f) single-voxel 1H-magnetic resonance spectroscopy (MRS) images from participant 4 obtained at 9 months of age. There is evidence of myelination in the splenium, posterior limb of internal capsule, and optic radiations (a and b, arrows) but a relative lack of myelin in the genu and anterior limb of internal capsule, corresponding to a pattern that would be seen normally at age 3 to 4 months. Single-voxel MRS images of the frontal white matter at (e) time to echo (TE) of 35ms and (f) TE 144ms show respectively, a relatively low N-acetyl aspartate peak (seen at 2.01ppm) and a relatively higher choline peak (seen at 3.21ppm) (the N-acetyl aspartate to creatinine ratios [NAA:Cr] are 1.41 and 1.58, and choline to creatinine (Cho:Cr) ratios are 1.37 and 1.83 at TE of 35ms and 144ms respectively). There is no evidence of lactate.
Genetic studies
Molecular genetic analysis on the five participants revealed four different hemizygous SLC16A2 gene mutations (Table I). Analysis of the mother of participant 3 revealed the mutation at low level, which is likely to represent somatic mosaicism, whereas the mothers of participants 2 and 4 and 5 were found to be heterozygous for the mutations identified in their sons.
Discussion
In addition to clinical and laboratory features in keeping with the phenotype previously associated with SLC16A2 gene mutations and AHDS, the five males reported in this series had consistent evidence of profoundly delayed myelination on brain imaging.
In particular, suggestive clinical features3,4 included initial hypotonia subsequently evolving into a complex motor disorder characterized by upper motor neuron signs with dystonic posturing, mainly affecting the distal upper limbs, and additional spontaneous or provoked paroxysmal dyskinesias, previously reported only in isolated cases.11,12 Other recognized features of AHDS4 also found consistently in our participants included an OFC below average (0.4th–9th centile), profound learning disability with absent speech, and a particularly placid and sociable personality. Thyroid hormone profiles were consistent, with high free or total T3 (fT3 or TT3), normal or mildly decreased free thyroxine (fT4), and normal or slightly elevated thyroid-stimulating hormone (TSH), as previously documented in other males with SLC16A2 gene mutations and some female carriers.3,4 A high T3 level, especially when associated with a reduced serum rT3 concentration, was consistent and is the most useful screening tool in males with suggestive clinical features.13
The dysmorphic features associated with SLC16A2 gene mutations are variable and not necessarily distinct, leading to suspicion of a primary neurological disorder before a syndromic diagnosis of AHDS is considered. In particular, although earlier in life an elongated myopathic facial appearance with ptosis, hypotonia, weakness, muscle wasting, contractures, pectus excavatum, and scoliosis2–4,7 may suggest an underlying myopathy, dystonic or athetoid CP is a common misdiagnosis later in life once the associated motor disorder has manifested. It is of note that in all our participants – as well as in the first cases with identified SLC16A2 gene mutations – genetic testing was guided by the characteristic abnormalities in the TFTs rather than a syndromic diagnosis of AHDS, suggesting that thyroid hormone levels (specifically T3 and rT3) ought to be checked in all males with learning disability and/or an unexplained motor and movement disorder. Our findings also suggest that SLC16A2 involvement may be easily missed, particularly in isolated cases without any family history of AHDS.
All our participants showed markedly reduced myelin on brain MRI on the baseline scan and, although some myelination had occurred in the interim, this remained delayed on longitudinal studies. Although delayed myelination is a non-specific finding in a wide range of metabolic and genetic conditions, AHDS is not usually considered in the differential diagnosis of this MRI appearance.14 Although the great majority of reports on AHDS to date do not include MRI data, delayed myelination has been documented in three case reports without longitudinal follow-up data7–9 and is also indicated by a recent MRS study.15 As in the latter, a spectral pattern of relatively increased choline over N-acetyl aspartate was noted in participants 4 and 5, which is consistent with delayed myelination.16 Although normal myelination has been suggested for five other participants in the literature,11,12,17–19 some of these reports do not contain information on whether appropriate MRI sequences were used, and in others MRIs were obtained at a more advanced age; delayed myelination earlier in life could therefore not be excluded. Other reported MRI abnormalities in single cases of AHDS have been non-specific, including subtle cortical and subcortical atrophy,17 mild cerebellar atrophy,19 and high T2 signal in the putamina.12,18 Taking into account an evolving spastic paraparesis in a male with profoundly reduced myelin on MRI, one of our participants (participant 1) had an initial diagnosis of Pelizaeus–Merzbacher disease, a disorder of myelination caused by mutations in the PLP1 gene on Xq22 encoding proteolipid protein 1, thus indicating that SLC16A2 gene mutation screening should be considered in the differential diagnosis of males presenting with features of Pelizaeus–Merzbacher-like disease or X-linked spastic paraplegia (SPG2),20 a condition allelic to Pelizaeus–Merzbacher disease. Similarly, participants 4 and 5 were also thought to have a disorder of brain myelination on the basis of their neuroimaging findings. Our observations are strongly supported by the recent identification of SLC16A2 gene mutations in cases with clinical and radiological features of Pelizaeus–Merzbacher-like disease;6 in these cases, similarly to our participants and unlike Pelizaeus–Merzbacher disease, myelination was initially severely delayed but eventually progressed, although perhaps not to a normal stage.
SLC16A2 gene mutations, previously associated with AHDS,3,4 were identified in all our participants. Female somatic mosaicism, as in the case of the mother of participant 3, has not, to our knowledge, been previously described. The precise prevalence of SLC16A2 gene mutations as a cause of X-linked learning disability is not known, but the recent rapid identification of mutations in more than 49 families around the world suggests that SLC16A2 gene mutations may be among the more prevalent X-chromosomal changes causing learning disability.
SLC16A2 gene encodes for MCT8, an active thyroid hormone transporter with preferential substrate specificity for T3 in humans, which is early and widely expressed in brain and other tissues.21 In vitro studies have demonstrated that SLC16A2 gene mutations result in a reduced or absent supply of T3 to neurons,10 and that genotype–phenotype correlations may depend on residual T3 transport capacity.22 In addition, recently created SLC16A2 knockout mice models accurately replicated the human thyroid profile and identified impaired T3 uptake into the brain as the primary event leading to this phenotype.23
Although participants harbouring SLC16A2 gene mutations rarely exhibit signs of hyperthyroidism,24 additional symptoms are likely to reflect a relative hyperthyroid state in tissues which are not exclusively dependent on MCT8-mediated thyroid hormone uptake, such as liver17 and muscle.19 The observed muscle wasting, for instance, is thought to be due to a hyperthyroid metabolic myopathy, as supported by the increased serum lactate concentrations in some participants.19 Similarly, excess thyroid hormone in the liver results in an increased serum sex hormone-binding globulin concentration. Treatment with propylthiouracil and thyroxine, although possibly beneficial in reducing the hyperthyroid features, has no effect on the neurological phenotype.24
The finding of markedly delayed myelination in our participants and other individuals6 harbouring SLC16A2 gene mutations suggests AHDS as a possible model for abnormal white matter development and for the role of thyroid hormone transporters in brain development. Thyroid hormones play a crucial role in brain development both in early and late stages. With regard to myelination, in particular, in vitro studies have demonstrated that T3 influences differentiation of oligodendrocytes and elongation of their processes and at later stages affects the distribution of myelin proteins.25 Additionally, thyroid hormones influence oligodendroglial morphology in vivo26 and have been shown to promote remyelination in experimental models of multiple sclerosis.27
Given the crucial role of thyroid hormones in oligodendroglial development and myelination, our finding of delayed myelination on brain MRI in participants harbouring a specific central nervous system thyroid hormone transporter defect is not entirely unexpected. Reports of a normal myelin pattern in a few older participants with AHDS suggest either that myelination, although delayed, will eventually occur or that residual MCT8 activity associated with some SLC16A2 gene mutations22 is sufficient for normal myelination to progress. The latter hypothesis is also supported by the observation that in congenital hypothyroidism, in which neuronal thyroid hormone levels are presumably reduced but not completely abolished, myelination is usually normal.28 More extensive longitudinal MRI studies of individuals with AHDS will be required to explore further the prevalence and natural history of the white matter disorder associated with this condition.




