Histopathological diagnosis of Japanese spotted fever using formalin-fixed, paraffin-embedded skin biopsy specimens
Usefulness of immunohistochemistry and real-time PCR analysis
Abstract
Clin Microbiol Infect 2012; 18: 260–267
Japanese spotted fever (JSF) is caused by Rickettsia japonica, and lethal cases are reported yearly in southwest Japan. We thus established the method of diagnosing JSF by immunohistochemistry (IHC) and real-time PCR (RT-PCR) using formalin-fixed, paraffin-embedded skin biopsy specimens. Two monoclonal antibodies were used for IHC, and the 17k genus common antigen gene served as the target of RT-PCR. We collected skin biopsy (n = 61) and autopsy (n = 1) specimens from 50 patients clinically suspected of JSF. Immunohistochemically, the rickettsial antigens were localized as coarse dots in the cytoplasm of endothelial cells and macrophages. Thirty-one seropositive cases plus one autopsy case (group A) and nine seronegative cases but with positive IHC and/or RT-PCR (group B) were judged as JSF. Nine cases were regarded as non-JSF disorders based on negative serology, IHC and RT-PCR (group C). Of 50 biopsies (eschar 34, eruptions 10, and scabs 6) from groups A and B, IHC and RT-PCR positivities were 94% (32/34) and 62% (21/34) for eschar, 80% (8/10) and 30% (3/10) for eruptions, and 33% (2/6) and 50% (3/6) for scabs. For IHC, eschar was most suitable, and scabs were insufficient. Unexpectedly, 18 biopsies happened to be fixed in 100% formalin, and this lowered the detection rate by RT-PCR, but IHC was tolerant. Sequence analysis using five skin biopsy specimens confirmed a 114 bp DNA stretch homologous to that reported for the target gene of R. japonica. In 26 (84%) of the 31 seropositive patients, the diagnosis was made by IHC and/or RT-PCR earlier than serology.
Introduction
There are two rickettsioses endemic in Japan: Japanese spotted fever (JSF) caused by Rickettsia japonica (Rj) and Tsutsugamushi disease caused by Orientia tsutsugamushi (Ot). JSF was first reported by Mahara, et al. [1,2] in 1984 in Tokushima, Shikoku Island. JSF is thus one of the newcomers to the spotted fever group (SFG) rickettsioses [3]. In Japan, JSF cases must be reported to the health authorities once confirmed [4,5]. Because fatal JSF cases have recently been seen in southwest Japan [6,7], establishment of reliable diagnostic assays is needed.
Serological screening of IgM and IgG antibodies and detection of Rj DNA in blood by polymerase chain reaction (PCR) are the main diagnostic tests for JSF [8,9]. Serology requires a minimal twofold increase of antibody titres between the initial and second blood samples. It usually takes a 2-week period; therefore this cannot be used in an emergency situation. Indeed, fatal JSF cases have occurred within 24 h of hospitalization. Empirical treatment was started on clinical suspicion, and serological diagnosis was made retrospectively [6].
We have established diagnostic immunohistochemistry (IHC) using two monoclonal antibodies in formalin-fixed, paraffin-embedded (FFPE) specimens biopsied from eschar and eruptions [10,11]. When tiny samples or superficial scabs were submitted, false-negative results happened. Therefore, we started detecting Rj DNA extracted from FFPE skin biopsies with TaqMan® real-time PCR (RT-PCR). RT-PCR was targeted at the 17k genus common antigen gene to yield short-length products. We utilized FFPE specimens not only to establish histopathological diagnostic tools but also to avoid possible biohazard during handling of the biopsy material.
The aims of the present study are to establish the method for diagnosing JSF with IHC and RT-PCR in FFPE skin biopsies, and to compare these two assays with serology.
Materials and Methods
Clinical specimens
In the period 2004–2010, we collected specimens from 50 patients clinically suspected of having JSF. The clinics and hospitals supplying samples included Mahara Clinic (Anan, Tokushima, n = 17), Yamada Red Cross Hospital (Ise, Mie, n = 13), Myojin Clinic (Kozagawa, Wakayama, n = 9), Uwajima Municipal Hospital (Uwajima, Ehime, n = 7), Hyogo Prefectural Awaji Hospital (Sumoto, Hyogo, n = 1), Shinano Hospital (Tomi, Nagano, n = 1), Yasu Hospital (Yasu, Shiga, n = 1) and Notogawa Hospital (Higashiomi, Shiga, n = 1).
Biopsy samples were taken from 49 cases, and autopsy material from one. The skin samples (n = 61) included eschar (n = 42), eruptions (n = 12) and scabs (n = 7). All but the autopsied tissues, fixed in formalin, were sent to our department within 24–72 h. Normal skin sampled at autopsy served as a negative control. For serological assays, sera were sent to Ohara Research Laboratory, Fukushima, or Prefectural Institutes of Public Health. In total, 89 sera (acute 46 and convalescent 43) were analysed serologically, as described earlier [12]. The cut-off value was set at <×40 for both IgM and IgG. The Rj Aoki strain was used as the antigen. Rickettsia was isolated in limited cases in Ohara Research Laboratory, as reported previously [12].
Cultivation of rickettsial strains and preparation of cell blocks
Rickettsiae (Aoki and Katayama strains of Rj and Kato, Karp and Gilliam strains of Ot) were sent from Ohara Research Laboratory. All strains were passed in cultured L929 cells (fibroblast-like cells of a C3H/An mouse) at a biosafety level 3 containment laboratory in the Department of Microbiology, Wakayama Medical University, Wakayama.
The cells were grown at 32°C in 25 cm2 plastic cell culture flasks containing Dulbecco’s modified Eagle’s minimal essential medium (Nissui, Tokyo, Japan) supplemented with 5% fetal calf serum (Hyclone, Logan, UT, USA). The cells harvested 5 to 7 days after inoculation were fixed in 10% formalin overnight. Cell blocks of uninfected and infected L929 cells were prepared by a gelation method using sodium alginate membranes [13]. Cell blocks were also prepared from R. conorii (Malish strain)-infected monkey Vero cells (Fuller Laboratories, Fullerton, CA, USA) fixed in 10% formalin.
Monoclonal antibodies and IHC
Mouse IgM monoclonal antibodies, clones S3 and X1, directed to the Rj Aoki strain were a gift from Dr Yosaburo Oikawa, Department of Parasitology, Kanazawa Medical University, Kanazawa. Both clones react with the epitope common to SFG rickettsiae, but do not cross-react with Ot [14,15].
Sections, 4 μm thick, were prepared from cell blocks and tissue specimens. After inactivating endogenous peroxidase with 0.3% H2O2 in methanol for 20 min, sections were heat-retrieved in 10 mM citrate buffer, pH 7.0 for 10 min with a pressure cooker. Incubation with the monoclonals (dilution: 1:100) at room temperature overnight and then amino acid polymers (Simple Stain MAX-PO, Nichirei, Tokyo, Japan) for 30 min at room temperature followed [10,11]. Antigen localization was visualized in 50 mM Tris–HCl buffer, pH 7.6 containing 1 mM 3,3′-diaminobenzidine and 0.006% H2O2. Nuclei were counterstained with haematoxylin.
DNA preparation
Five 4-μm-thick FFPE sections were collected in Eppendorf’s tubes. After deparaffinization, DNA was extracted using a QIAamp DNA FFPE Tissue kit (#56404; Qiagen, Hilden, Germany). At sample processing, microtome blades were renewed to prevent sample-to-sample contamination.
Real-time PCR
The 17k genus common antigen gene of SFG rickettsia origin was amplified by RT-PCR, according to the previous reports [9,16,17]. Primer pairs for Rj consisted of 5′-ATG AAT AAA CAA GGT ACA GGA ACA-3′ (forward: 24mer) and 5′-AAG TAA TGC ACC TAC ACC TAC TC-3′ (reverse: 23mer), generating products of 114 bp length (GenBank D16515). Both primers were 100% homologous to R. conorii (GenBank M28480) and R. rickettsii (M28479), while three bases (forward) and one base (reverse) were mismatched with R. typhi (M28481) and R. prowazekii (M28482). Signals were detected with a TaqMan® hybridization probe FAM-GGT GGC GCA TTA CTT GGT TCT CAA TTC GGT AAG GG-TAMRA for Rj (Applied Biosystems, Foster City, CA, USA). The number of bases mismatched with the TaqMan® probe (35mer) was one base for R. conorii, two bases for R. rickettsii, three bases for R. prowazekii and four bases for R. typhi.
Assays were carried out in 20 μL final volume containing 1.5–3.0 μL of sample DNA, 2× reaction mixture (10 μL, Premix Ex Taq™; TaKaRa Bio, Otsu, Shiga, Japan), 10 pmol primers, and 10 pmol TaqMan® probe. RT-PCR was performed using the DNA Engine Opticom® System (Bio-Rad, Berkeley, CA, USA), with initial holding temperature of 95°C for 30 s, followed by 50 cycles with two-step PCR at 95°C for 5 s and at 60°C for 30 s with fluorescence monitoring on 6-carboxy fluorescein aminohexyl amidite (FAM) channel.
β2-microglobulin (β2m) served as an internal control for effective DNA extraction [18]. Primers designed with Primer3 software (SourceForge, Mountain View, CA, USA) consisted of 5′-TGC TGT CTC CAT GTT TGA TGT ATC T-3′ (forward) and 5′-TCT CTG CTT CCC CAC CTC TAA GT-3′ (reverse) for human/monkey β2m (GenBank NM_004048), and 5′-CAG TGT GAG CCA GGA TAT AG-3′ (forward) and 5′-GAA GCC GAA CAT ACT GAA CTG CTA C-3′ (reverse) for mouse β2m (GenBank NM_009735). The product sizes were 86 bp for human/monkey and 152 bp for mouse.
Sequencing analysis
For sequencing, the SYBR Green method (Qiagen) using the same primer pairs was employed. RT-PCR was performed using Rotor-Gene Q (Qiagen) according to the QIAGEN SYBR-Green PCR Handbook (2009), with initial holding temperature of 95°C for 15 min, followed by 45 cycles with four-step PCR at 95°C for 20 s, at 55°C for 30 s, at 72°C for 30 s and at 57°C for 15 s. The melting curve was checked in the respective reactions. DNA from FFPE sections of L929 cells infected with Rj (Katayama strain), Vero cells infected with R. conorii and skin biopsies from five cases (eschar: A4, A10, A14 and B1, and scab: B8) were examined. When the plateau was not obtained in the amplification curve in the first run, the second PCR was performed by adding, as a template, 1.5 μL of 1:1000 diluted PCRed aliquot to reaction mixture. After electrophoresis in 1% Agarose gel, the amplified products were extracted with the QIAquick gel extraction kit (Qiagen). Direct sequencing analysis with the dye terminator method [19] was performed in FASMAC Co. (Atsugi, Kanagawa, Japan). The comparison was done using the BLAST program (National Center for Biotechnology Information, Bethesda, MD, USA).
Results
Clinical features of JSF
Table 1 summarizes 31 seropositive cases and one autopsy case (group A) and nine seronegative cases but with positivity of the SFG rickettsial antigen and/or genome (group B). All group B cases exhibited clinical features of JSF, response to antibiotic therapy, and positivities of IHC and/or RT-PCR. Case distribution in groups A and B (n = 41) is shown on Japan’s map (Fig. 1). The male to female ratio was 23:18. The mean age was 62.0 years (range, 28–87). Infection occurred during April–December with a peak in September (n = 10). Serologically, Rj antibodies of IgM and/or IgG types got elevated in group A, except for case A32 who died acutely. Rj was isolated from two cases (A1 and A6) [12].
| Patient no | District | Date of onset | Age (year) | Sex | History of tick bite | Rash | Fever >38°C | Rickettsia isolation | IHC | Real-time PCR | Serological assay of R. japonica antibodies | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Convalescent | ||||||||||||||||
| Skin biopsyTaq-Man® | Sybr-Green sequencing | IgM | IgG | IgM | IgG | ||||||||||||
| A1 | Tokushima | 19 July 2005 | 87 | M | Yes | Yes | Yes | Blood & eschar | + (Es) | + (Es) 41.3 Cy | <40 | 40 | 640 (2w), 160 | 640 (2w), 5120 | Recovered | ||
| A2 | Mie | 19 September 2007 | 60 | F | Yes | Yes | Yes | + (Es) + (Er) | + (Es) 39.3 Cy + (Er) 40.5 Cy | <40 | <40 | 320 | 1280 | Recovered | |||
| A3 | Mie | 26 October 2007 | 55 | M | Yes | Yes | Yes | + (Sc) | − (Sc) | 80 | 80 | 320 | 1280 | Recovered | |||
| A4 | Ehime | 27 October 2009 | 71 | F | Yes | Yes | Yes | + (Es) | + (Es) 34.5 Cy | + | <40 | <40 | 640 | 640 | Recovered | ||
| A5 | Tokushima | 26 May 2008 | 79 | F | Yes | Yes | Yes | + (Es) − (Er) | + (Es) 37.4 Cy − (Er) | <40 | <40 | 640 | 640 | Recovered | |||
| A6 | Tokushima | 25 July 2004 | 76 | F | Yes | Yes | Yes | Blood | + (Es) + (Er) | − (Es) − (Er) | <40 (2w) | <40 (2w) | 640 | 160 | Recovered | ||
| A7 | Tokushima | 24 August 2004 | 51 | M | Yes | Yes | Yes | + (Es) | − (Es) | 80 | 320 | Recovered | |||||
| A8 | Mie | 6 October 2006 | 63 | F | Yes | Yes | Yes | + (Es) | + (Es) 40.7 Cy | <40 | <40 | 320 | 640 | Recovered | |||
| A9 | Wakayama | 31 May 2010 | 74 | F | Yes | Yes | Yes | + (Es) | + (Es) 37.3 Cy | <40 | <40 | 320 | 640 | Recovered | |||
| A10 | Ehime | 7 October 2009 | 59 | F | Yes | Yes | Yes | + (Es) | + (Es) 33.8 Cy | + | <40 | <40 | 320 | 160 | Recovered | ||
| A11 | Ehime | 16 June 2010 | 60 | F | Yes | Yes | Yes | + (Es) | + (Es) 35.2 Cy | <40 | <40 | 320 | 160 | Recovered | |||
| A12 | Ehime | 3 July 2009 | 28 | M | Yes | Yes | Yes | + (Es) | + (Es) 34.6 Cy | <40 | <40 | 180 | 160 | Recovered | |||
| A13 | Mie | 21 September 2007 | 62 | M | Yes | Yes | Yes | + (Es) | + (Es) 48.0 Cy | 20 | 80 | Recovered | |||||
| A14 | Wakayama | 13 August 2009 | 49 | F | Yes | Yes | Yes | + (Es) | + (Es) 34.7 Cy | + | <40 | <40 | 160 | 320 | Recovered | ||
| A15 | Tokushima | 23 April 2009 | 65 | F | Yes | Yes | Yes | + (Es) | + (Es) 37.1 Cy | <40 | <40 | 160 | 320 | Recovered | |||
| A16 | Ehime | 25 September 2009 | 60 | F | Yes | Yes | Yes | + (Es) | + (Es) 33.2 Cy | <40 | <40 | 160 | 320 | Recovered | |||
| A17 | Ehime | 27 April 2009 | 71 | M | Yes | Yes | Yes | + (Es) | + (Es) 39.5 Cy | 40 | <40 | 80 | 80 | Recovered | |||
| A18 | Ehime | 29 July 2010 | 60 | M | Yes | Yes | Yes | + (Es) | + (Es) 35.6 Cy | <40 | <40 | 160 | 320 | Recovered | |||
| A19 | Tokushima | 12 September 2004 | 65 | F | Yes | Yes | Yes | + (Es) + (Er) | − (Es) − (Er) | <40 | <40 | 160 | 320 | Recovered | |||
| A20 | Tokushima | 29 September 2007 | 68 | M | Yes | Yes | Yes | + (Es) | − (Es) | <40 | <40 | 160 (4w) | 80 (4w) | Recovered | |||
| A21 | Wakayama | 24 September 2008 | 61 | F | Yes | No | Yes | + (Es) | + (Es) 36.0 Cy | <40 | <40 | 160 | Recovered | ||||
| A22 | Tokushima | 22 June 2005 | 52 | F | Yes | Yes | Yes | + (Es) | + (Es) 41.3 Cy | <40 | 40 | 80 (2w) | 160 (2w) | Recovered | |||
| A23 | Mie | 7 September 2006 | 84 | M | Yes | Yes | Yes | + (Er) + (Sc) | − (Er) + (Sc) 42.7 Cy | <40 | <40 | 80 | 320 | Recovered | |||
| A24 | Tokushima | 22 July 2004 | 77 | M | Yes | Yes | Yes | + (Es) + (Er) | − (Es) − (Er) | <40 | 80 | Recovered | |||||
| A25 | Mie | 19 September 2006 | 70 | M | Yes | Yes | Yes | + (Es) | − (Es) | <40 | <40 | <40 | 160 | Recovered | |||
| A26 | Wakayama | 4 September 2009 | 56 | M | Yes | Yes | Yes | − (Es) | − (Es) | <40 | <40 | 40 | 80 | Recovered | |||
| A27 | Mie | 4 October 2006 | 51 | M | Yes | Yes | Yes | − (Sc) | − (Sc) | <40 | <40 | 320 | 640 | Recovered | |||
| A28 | Wakayama | 1 October 2007 | 71 | M | Yes | Yes | Yes | − (Sc) | − (Sc) | <40 | <40 | 40 | 160 | Recovered | |||
| A29 | Mie | 2 November 2007 | 50 | F | Yes | Yes | Yes | + (Es) + (Er) | + (Es) 38.7 Cy + (Er) 44.7 Cy | + | + | Recovered | |||||
| A30 | Wakayama | 28 July 2010 | 77 | M | Yes | Yes | Yes | + (Es) | + (Es) 30.5 Cy | + | + | Recovered | |||||
| A31 | Wakayama | 28 June 2008 | 56 | M | Yes | Yes | Yes | + (Es) | − (Es) | + | + | Recovered | |||||
| A32 | Hyogo | 19 December 2005 | 77 | M | Yes | Yes | Yes | + (Au) | + (Au) 41.5 Cy | <40 | <40 | Died | |||||
| B1 | Tokushima | 23 October 2006 | 71 | F | Yes | Yes | Yes | + (Es) − (Er) | + (Es) 40.0 Cy − (Er) | + | <40 | <40 | Recovered | ||||
| B2 | Mie | 2 October 2006 | 64 | M | Yes | Yes | Yes | − (Es) + (Er) | − (Es) + (Er) 38.7 Cy | <40 | <40 | <40 | <40 | Recovered | |||
| B3 | Wakayama | 25 May 2009 | 65 | M | Yes | Yes | No | + (Es) | + (Es) 33.6 Cy | <40 | <40 | <40 | <40 | Recovered | |||
| B4 | Mie | 2 November 2006 | 50 | M | Yes | Yes | Yes | + (Es) + (Er) | − (Es) − (Er) | <40 | <40 | <40 | <40 | Recovered | |||
| B5 | Tokushima | 7 June 2008 | 38 | M | Yes | Yes | Yes | + (Es) | − (Es) | <40 | <40 | <40 (2W) | <40 (2W) | Recovered | |||
| B6 | Tokushima | 23 May 2009 | 54 | F | Yes | Yes | Yes | + (Es) | − (Es) | <40 | <40 | <40 | <40 | Recovered | |||
| B7 | Mie | 29 September 2006 | 29 | F | Yes | Yes | Yes | + (Es) | − (Es) | <40 | <40 | <40 | <40 | Recovered | |||
| B8 | Tokushima | 6 May 2007 | 64 | M | Yes | Yes | Yes | − (Sc) | + (Sc) 43.6 Cy | + | <40 | <40 | <40 | <40 | Recovered | ||
| B9 | Mie | 2 August 2007 | 62 | M | Yes | Yes | Yes | − (Sc) | + (Sc) 40.2 Cy | <40 | <40 | Recovered | |||||
- Group A = 31 seropositive cases and one autopsy case (A1–A32), Group B = 9 seronegative cases (B1–B9).
- Es, eschar; Er, eruption; Sc, scab; Au, autopsy; Cy, cycles, representing cycle threshold values.
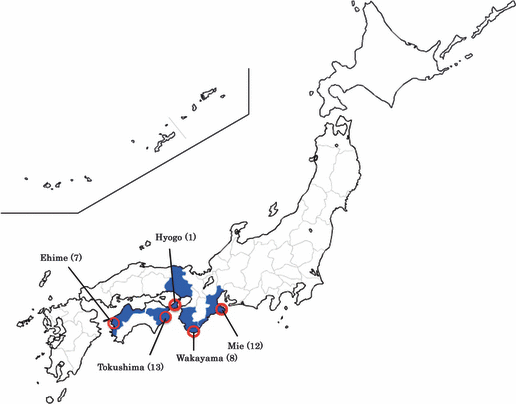
Distribution of JSF in the present study (n = 41). Cases of JSF are seen in the south-western part of Japan, facing the Ocean. Blue shows the involved prefectures, and red circles indicate the endemic spots.
Nine (18%) of 50 cases were judged as non-JSF disorders (group C, detailed data not shown), based on clinical follow-up features, negative serology and negative IHC/real-time PCR findings in 11 biopsies (eschar 8, eruptions 2 and scab 1). Final clinical diagnoses included tick bite fever of unknown nature (n = 4), Tsutsugamushi disease (Irie/Kawasaki type), streptococcosis, pneumonia, herpes and allergy (n = 1, respectively).
IHC and RT-PCR
Fifty skin biopsy specimens (eschar 34, eruptions 10 and scabs 6) were obtained from group A (n = 38) and group B (n = 12). Rickettsial antigens were immunolocalized as coarse dots in the cytoplasm of endothelial cells and macrophages in the lesions. Both monoclonals consistently gave comparable results. Representative IHC features are demonstrated in Fig. 2. Autopsy tissues (spleen, liver, intestine, salivary gland, kidney and testis) showed positivity of the rickettsial antigens and DNA [11].
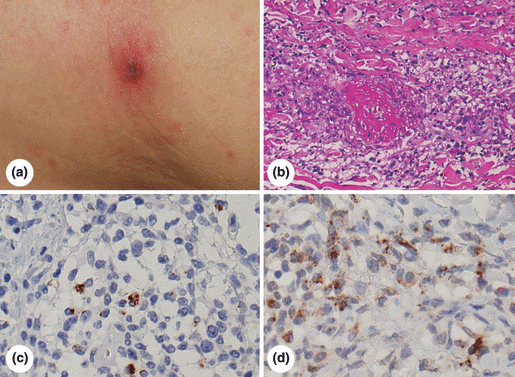
Immunohistochemical diagnosis of JSF in a representative case (A18). (a) Gross appearance of eschar, (b) histopathology of eschar, haematoxylin and eosin staining, (c, d) immunostaining for SFG rickettsial antigens using monoclonal antibodies S3 (c) and X1 (d). Eschar is covered with scab, and associated with haloed redness. Small eruptions are scattered in the surrounding skin (a). Histologically, perivascular infiltration of lymphocytes and macrophages is evident in the dermis (b). Immunohistochemically, the cytoplasm of endothelial cells and macrophages shows coarse dotted positivity (coloured brown) with both monoclonal antibodies (c, d).
Positivity rates of IHC and RT-PCR were 94% (32/34) and 62% (21/34) for eschar, 80% (8/10) and 30% (3/10) for eruptions, and 33% (2/6) and 50% (3/6) for scabs. For IHC, eschar was most suitable, while scabs were insufficient. For scab samples, RT-PCR functioned better than IHC.
Unexpectedly, 18 biopsies sampled in Mahara Clinic happened to be fixed in 100% formalin, and this evidently lowered the detection rate of RT-PCR, whereas IHC was tolerant of such harsh conditions. In samples fixed in 10% formalin, RT-PCR gave positive results in 73% (16/22) for eschar and in 60% (3/5) for eruptions, while for the samples fixed in 100% formalin, the rates were 42% (5/12) for eschar and 0% (0/5) for eruptions (Table 2).
| Formalin concentration | 10% | 100% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site of biopsy | Specimen | Group A (seropositive)a | Group B (seronegative) | Group A (seropositive) | Group B (seronegative) | ||||
| IHC | Real-time PCR | IHC | Real-time PCR | IHC | Real-time PCR | IHC | Real-time PCR | ||
| Eschar | 34 | 94% (17/18) | 83% (15/18) | 75% (3/4) | 25% (1/4) | 100% (9/9) | 44% (4/9) | 100% (3/3) | 33% (1/3) |
| Mean CN (range) | 39.1 (30.5–48.0) | 33.6 | 39.4 (37.1–41.3) | 40.0 | |||||
| Eruptions | 10 | 100% (3/3) | 67% (2/3) | 100% (2/2) | 50% (1/2) | 75% (3/4) | 0% (0/4) | 0% (0/1) | 0% (0/1) |
| Mean CN (range) | 42.6 (40.5–44.7) | 38.7 | |||||||
| Scabs | 6 | 50% (2/4) | 25% (1/4) | 0% (0/1) | 100% (1/1) | – | – | 0% (0/1) | 100% (1/1) |
| Mean CN (range) | 42.7 | 40.2 | 43.6 | ||||||
| Total | 50 | 88% (22/25) | 72% (18/25) | 71% (5/7) | 43% (3/7) | 92% (12/13) | 31% (4/13) | 60% (3/5) | 40% (2/5) |
- CN, cycle number representing cycle threshold values in real-time PCR.
- aResult of non-skin lesions in one autopsy case tentatively included in the eschar.
Both IHC and RT-PCR were concordantly positive in 51% (26/51) of 50 skin biopsies plus one autopsy sample. IHC was positive but RT-PCR negative in 33% (17/51). Two (4%) scab samples were IHC negative and RT-PCR positive. In six (12%), both methods gave negative results. On a patient basis, only three (7%) of 41 cases in groups A and B were negative in both methods, and in two of them, only tiny scabs were submitted (cases A27 and A28). In 26 (84%) of 31 seropositive cases, the diagnosis of JSF was made earlier by IHC and/or RT-PCR than by serology.
Cell blocks of Rj-infected L929 cells served as positive controls for both IHC and RT-PCR. Positive signals were also obtained from R. conorii-infected monkey Vero cells with both methods. No positivity of IHC and RT-PCR was seen in uninfected or Ot-infected cells and in normal skin. Human/monkey or mouse β2m DNA was consistently amplified from all DNA samples. Cycle threshold values varied from sample to sample (Tables 1 and 2). The mean cycle values were 26.4 for Rj-infected L929 cells, 36.8 for regularly fixed eschar (n = 15; median, 35.6; range, 30.5–48.0), and 42.6 (n = 3; range, 40.5–44.7) for regularly fixed eruptions and scabs. Eschar fixed in 100% formalin required 39.4 cycles (n = 4; range, 37.1–41.3).
Sequencing analysis
An exactly homologous sequence of the 114 bp stretch in the Rj 17k genus common antigen gene was confirmed in the positive control cells and five skin biopsy specimens. As expected (GenBank M28480), the DNA sequence of R. conorii showed three bases difference in the amplified fragment.
Discussion
Registered JSF cases are gradually increasing in number, and endemic areas are spreading in southwest Japan [4,5,20]. Rj has also been isolated in Korea and Thailand [21,22]. The mortality rate of JSF is calculated to be 1.9%, but this figure may be underestimated due to early antibiotic treatment. The reported mortality rates of other SFG rickettsioses are 2.5%, 7% and 30% for Mediterranean, Rocky Mountain and Brazilian spotted fevers, respectively [5,23–25].
We evaluated diagnostic methodology, IHC and RT-PCR, for detecting Rj with monoclonal antibodies and primers directed toward SFG rickettsiae in FFPE specimens. JSF is the only SFG rickettsiosis in southwestern Japan. This is the first study where Rj was detected in FFPE skin biopsies and the detectability was compared between both methods. Sequencing analysis using five representative specimens of eschar and scab confirmed the specificity of our approach. Handling and transfer of rickettsia-infected skin tissue may be biohazardous. This is one of the reasons why we chose FFPE samples as the target of study. Once established, our methodology is applicable to histopathological diagnosis.
PCR detection of SFG rickettsiae such as R. conorii and R. prowazekii using skin biopsy specimens has been described [26,27]. Mahara documented that all of 53 JSF cases showed skin eruptions and 94% eschar [5]. In the present study, biopsy from eschar was most suitable for diagnostic testing of JSF.
Of 31 seropositive JSF cases plus one autopsy case (group A) and nine seronegative JSF cases (group B), all but three (93%) were positive by IHC and/or RT-PCR. Under our present conditions using FFPE sections, IHC was more effective than RT-PCR in diagnosing JSF. In two of three false-negative cases, only scab samples were submitted. Scabs were insufficient for IHC. Two other scab samples were IHC negative and RT-PCR positive. Low detectability by RT-PCR was partly due to improper fixation in this series. Eighteen samples happened to be fixed in 100% formalin, and high formaldehyde concentration might have caused alteration of DNA structure, as reported previously [28], while the antigenicity was tolerant of such conditions. In fact, cycle threshold values were larger for 100% formalin-fixed specimens than for regularly fixed specimens. Cycle threshold values, smaller for eschar than for eruptions or scabs, may reflect the number of pathogens within the lesion.
Because of DNA fragmentation by formalin fixation and/or paraffin embedding, short PCR products are needed to increase detection sensitivity [29]. In the present study, primers to yield short products (114 bp length) were designed, but obstacles to formalin fixation were still inevitable. β2m DNA fragments of 86 and 152 bp length were reproducibly amplified from human/monkey and mouse samples, respectively.
Among 31 seropositive patients, the diagnosis of JSF was made earlier in 26 (84%) patients by IHC and/or RT-PCR than serology. Manifesting trias (eschar, rash and high fever >38°C) empirically lead clinicians in endemic areas to Minocyclin treatment. The trias are common to JSF and Tsutsugamushi disease [4,5]. New quinolones can be added to Minocyclin in JSF, but are ineffective for Tsutsugamushi disease [5]. In fulminant cases, early diagnosis indicated change in therapeutic regimen to save the patient’s life.
Serology is widely used for diagnosing rickettsioses [30]. Seronegativity was observed in nine biopsy cases and one autopsy case (representing 24% of 41 JSF patients), and this might be related to therapeutic eradication of pathogens in the early stages of infection. Long-term serological follow-up is needed.
Acknowledgements
The excellent technical assistance given by Ms Mika Maeshima and Ms Hisayo Ban, Department of Pathology, Fujita Health University School of Medicine, Toyoake, is cordially appreciated. The authors also deeply thank Dr Hiromi Fujita, Ohara Research Laboratory, Fukushima, for providing us with strains of R. japonica and O. tsutsugamushi and also for performing serological assays. Dr Yosaburo Oikawa, Department of Parasitology, Kanazawa Medical University, Kanazawa, kindly supplied us with mouse monoclonal antibodies S3 and X1. Professor Shigeru Akimoto, Department of Microbiology, Wakayama Medical University, Wakayama, allowed us to use the biosafety level 3 facility. Professor Yasuo Chinzei, Faculty of Medical Engineering, Suzuka University of Medical Science, Suzuka, Mie, Dr Yuji Morita, Myojin Clinic, Kozagawa, Wakayama, and Dr Naoki Yakushiji, Department of Dermatology, Uwajima Municipal Hospital, Uwajima, Ehime, kindly sent us clinical samples. Dr Hidehisa Horiguchi, Pathology Division, Hyogo Prefectural Awaji Hospital, Sumoto, Hyogo, provided us with an autopsy case. Dr Toshio Kishimoto, Okayama Prefectural Institute for Environmental Science and Public Health, Okayama, cooperatively gave us valuable advice and suggestions. Dr Olivier Aoun, Bégin Military Hospital, Saint-Mandé, France, critically reviewed the manuscript. This work was supported by the Research Grant (#19590460 to F.M.) and Open Research Center Project (#30131 to Y.T.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and also by the Research Grant for Emerging and Re-emerging Infections from the Ministry of Health and Welfare, Japan (#H18-Shinko-14, H21-Shinko-6 to Y.T.). The Research Grant from Fujita Health University (2007–2010 to K.I.) also in part supported this work.
Transparency Declaration
Nothing to declare.




