Mycobacterial peritonitis: difference between non-tuberculous mycobacteria and Mycobacterium tuberculosis
Abstract
Clin Microbiol Infect 2012; 18: 246–252
Unlike tuberculous peritonitis, peritonitis due to non-tuberculous mycobacteria (NTM) has unclear clinical manifestations. This study aimed to clarify the clinical manifestations and laboratory results of NTM peritonitis and compare it to tuberculous peritonitis. This retrospective study was conducted from 2000 to 2008 in a medical centre in Taiwan. Patients with mycobacteria isolated from ascites were identified and compared according to causative pathogens (Mycobacterium tuberculosis or NTM). Those with NTM peritonitis were further classified into the ‘probable’ and ‘possible’ groups based on diagnostic evidence. Twenty-five patients with NTM peritonitis and 65 with tuberculous peritonitis were reviewed. Mycobacterium avium complex was the most common NTM pathogen (52%). There was no obvious difference between the ‘probable’ and ‘possible’ NTM peritonitis groups regarding age and laboratory data. Patients with NTM peritonitis and those with tuberculous peritonitis had no differences in age or gender but varied in symptoms and serum laboratory data. NTM peritonitis was 100% associated with underlying co-morbidities and had lower proportions of lymphocytes and albumin level in ascites. Twelve (48%) NTM peritonitis and 21 (32%) tuberculous peritonitis patients died during the 6-month follow-up. Anti-mycobacterial treatment, but not mycobacterial species, was correlated with better 6-month survival. In Taiwan, NTM is responsible for 28% of mycobacterial peritonitis cases, which have a poor prognosis if untreated. There are some differences in clinical manifestations between NTM and tuberculous peritonitis. NTM peritonitis should be considered in patients with peritonitis but without causative microorganisms identified other than NTM.
Introduction
Diagnosis of extrapulmonary mycobacterial infections is complicated due to variable manifestations and difficulty in collecting clinical samples [1]. One such infection is mycobacterial peritonitis. Though uncommon, mycobacterial peritonitis has a high mortality rate, especially in immunocompromised hosts [1]. Most reported cases are caused by Mycobacterium tuberculosis. However, due to ageing, increasing numbers of immunocompromised individuals, and advances in non-tuberculous mycobacteria (NTM) isolation [2–5], the overall incidence of NTM disease has increased in recent years [5–8]. From a clinical standpoint, it is important to know the relative proportions of tuberculosis (TB) and NTM disease in patients with mycobacterial peritonitis.
The clinical characteristics of NTM peritonitis remain unclear. Ding et al. reported 11 cases of abdominal NTM infection with a mortality rate of 73% [9]. In their series, 55% had liver cirrhosis and only 18% received anti-NTM treatment. Liver cirrhosis further increases the difficulty of early diagnosis of mycobacterial peritonitis because the transudative ascites may mask the peritoneal inflammation while the bleeding tendency prevents invasive diagnostic procedures such as peritoneal biopsy.
Because anti-NTM treatment can improve survival in NTM lung disease [10], familiarity with the presentations of NTM peritonitis is of practical importance for early diagnosis and prompt treatment. This retrospective study aimed to clarify the clinical manifestations and laboratory results of NTM peritonitis and compare them with TB peritonitis.
Materials and Methods
This study was conducted in a tertiary referral centre in northern Taiwan and the Institutional Review Board of the Research Ethics Committee approved the study design (No. 201002023R). Medical records and the mycobacterial laboratory registry database were reviewed. In our hospital, mycobacteriological study is routinely performed for the first ascites sample of every patient and for multiple ascites samples of those whose ascites has no definite aetiology, is exudative, or responds poorly to treatment [11]. Mycobacterial culture and identification were performed as previously described [12,13] and quality control assessment of the mycobacterial laboratory was periodically performed by the National Reference Laboratory of the Centres for Disease Control of Taiwan.
All of the patients with ascites samples sent for mycobacterial culture from January 2000 to December 2008 were eligible. In order to know the whole picture of culture-confirmed mycobacterial peritonitis, all of the patients whose samples yielded mycobacteria were recruited and those with concomitant bacterial or cancerous peritonitis were not excluded. The first positive ascites sample was defined as the index sample. The recruited patients were classified into TB and NTM peritonitis groups. Because NTM was ubiquitous in the environment and laboratory contamination was possible, NTM patients were further classified into two groups. Those with additional diagnostic evidences of NTM, including (i) more than one specimen of ascites yielding the same NTM, (ii) having the same NTM species growing in specimens other than ascites or (iii) presence of tissue pathology comparable for mycobacterial infection, were defined as the ‘probable group’. Those with single ascites culture positive for NTM were defined as the ‘possible group’.
A specially designed reporting form was used to collect data on the clinical characteristics, laboratory findings, treatment course and outcomes. Patients were followed-up for at least 6 months after the index sample or until death or lost to follow-up. The end of follow-up date was defined as the last-visit date for the last group. Mycobacteria-related mortality was considered sepsis complicated by multi-organ failure with no evidence of pathogens other than mycobacteria.
Proper anti-tuberculous and anti-NTM regimens were defined according to the TB treatment guidelines established by the American Thoracic Society [14,15]. Gastrointestinal symptoms included vomiting, haematemesis, tarry stool and haematochezia. The disease was considered disseminated if samples other than ascites yielded the same mycobacteria [15]. The duration from presentation to diagnosis of mycobacterial peritonitis was considered prolonged if it was >6 weeks [16]. Alcoholism was diagnosed when a patient had alcohol abuse or dependence (with history of alcohol withdrawal or tolerance) [17].
Three histological findings from peritoneal tissues were considered typical for mycobacterial infection: (i) granulomatous inflammation, (ii) caseous necrosis or (iii) the presence of acid-fast bacilli [18]. Child–Pugh classification for liver cirrhosis was scored according to a previous report [19]. The estimated glomerular filtration rate was obtained using the Modification of Diet in Renal Disease Study equation [20]. Severe chronic renal disease was defined as an estimated glomerular filtration rate of ≤30 mL/min/1.73 m2.
Statistical analysis
Inter-group differences were compared using the Student t-test or one-way ANOVA for numerical variables, where appropriate, while the chi-square test was used for categorical variables. Six-month survival curves for each variable were generated using the Kaplan–Meier method and compared using the log-rank test. Variables with a significant difference in univariate analysis were entered into the Cox proportional hazard regression analysis. A two-sided p <0.05 was considered statistically significant. All analyses were performed with the SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA).
Results
During the study period, 10 781 ascites samples from 5298 patients were sent for mycobacterial study, and 65 patients with TB peritonitis and 25 with NTM peritonitis were identified. MAC was the most common NTM species (n = 13), followed by rapidly growing mycobacteria (n = 7). Of the NTM group, 8 (32%) and 17 (68%) patients were classified into the ‘probable’ and ‘possible’ groups, respectively (Tables 1 and 2). In the ‘probable group’, eight had additional culture evidence and three had comparable pathology of mycobacterial infection. Co-bacterial peritonitis with Salmonella sp. was noted in one patient with MAC peritonitis and of three patients with TB peritonitis, two patients had Escherichia coli and one had Candida albicans. Cytology-proven cancerous peritonitis was noted in one with MAC peritonitis and another with M. abscessus peritonitis.
| Patient no. | Ascites | Underlying co-morbidity | Other positive samples for NTM | NTM comparable pathology | ||
|---|---|---|---|---|---|---|
| Culture result | No. of positive cultures | Specimens | Findings | |||
| 1 | MAC | 3 | AIDS | Blood, PE, stool | Bone marrow | AFB |
| 2 | MAC | 3 | AIDS | Blood | Intra-abdominal LN | GI and AFB |
| 3 | MAC | 2 | AIDS, alcoholism | − | Liver | AFB |
| 4 | MAC | 2 | HCC, LC | − | − | − |
| 5 | MAC | 1 | AIDS, alcoholism | Sputum | − | − |
| 6 | MAC | 1 | AIDS | Colon tissuea | − | − |
| 7 | M. chelonae | 2 | Breast cancer | − | − | − |
| 8 | M. abscessus | 1 | Thyroid cancer, DM | BAL | − | − |
- AFB, acid-fast bacilli; AIDS, acquired immunodeficiency syndrome; BAL, bronchio-alveolar lavage; DM, diabetes mellitus; GI, granulomatous inflammation; HCC, hepatocellular carcinoma; LC, liver cirrhosis; LN, lymph node; MAC, Mycobacterium avium complex; PE, pleural effusion.
- aSampled by colonoscopy biopsy.
| Patient no. | Ascites culture result | Underlying co-morbidity |
|---|---|---|
| 1 | MAC | Intra-abdominal MUO |
| 2 | MAC | Rectal cancer |
| 3 | MAC | HCC, LC, alcoholism |
| 4 | MAC | Severe CKD |
| 5 | MAC | Ovarian cancer |
| 6 | MAC | HCC, LC |
| 7 | MAC | Ovarian cancer |
| 8 | M. fortuitum | HCC, LC |
| 19 | M. fortuitum | HCC, LC |
| 10 | M. chelonae | LC, DM |
| 11 | M. chelonae | HCC, LC |
| 12 | M. abscessus | Prostate cancer |
| 13 | M. kansasii | ESRD |
| 14 | M. kansasii | Intra-abdominal MUO |
| 15 | Undifferentiated species | Ovarian cancer |
| 16 | Undifferentiated species | Gastric cancer |
| 17 | Undifferentiated species | HCC |
- DM, diabetes mellitus; ESRD, end-stage renal disease; HCC, hepatocellular carcinoma; LC, liver cirrhosis; MAC, Mycobacterium avium complex; MUO, malignancy with unknown origin.
Clinical characteristics and laboratory and imaging studies
All patients in the NTM group had underlying co-morbidities, with malignancy as the most common co-morbidity (Table 3). In contrast, liver cirrhosis was the leading underlying disease in TB peritonitis. Fever and gastrointestinal/abdominal symptoms were the most common presentations, which were not significantly different between the TB and NTM groups. Those with NTM peritonitis had lower percentages of lymphocytes (p 0.004) and lower albumin levels (p 0.020) in ascites (Table 4).
| NTM (n = 25) | Tuberculosis (n = 65) | p-value | |
|---|---|---|---|
| Age >65 years | 7 (28%) | 30 (46%) | 0.117 |
| Male gender | 16 (64%) | 32 (49%) | 0.208 |
| Underlying co-morbiditya | 25 (100%) | 53 (82%) | 0.021 |
| Malignancy | 17 (68%) | 16 (25%) | <0.001 |
| Liver cirrhosis | 7 (28%)b | 31 (48%)c | 0.090 |
| Acquired immunodeficiency syndrome | 5 (20%) | 3 (5%) | 0.022 |
| Alcoholism | 3 (12%) | 6 (9%) | 0.695 |
| Severe chronic kidney disease | 2 (8%) | 13 (20%) | 0.171 |
| Receiving peritoneal dialysis | 1 (4%) | 10 (15%) | 0.140 |
| Diabetes mellitus | 2 (8%) | 12 (18%) | 0.220 |
| Autoimmune disease | 0 | 4 (6%) | 0.204 |
| Initial presentation | |||
| Abdominal pain/distension | 14 (56%) | 25 (38%) | 0.187 |
| Gastrointestinal symptomsd | 4 (16%) | 7 (11%) | 0.559 |
| Fever | 2 (8%) | 8 (12%) | 0.507 |
| Duration of symptoms (days) | 10.5 [13.2] | 20.0 [32.5] | 0.182 |
| Disseminated | 4 (16%) | 21 (32%) | 0.122 |
| Treatment | 5 (20%) | 42 (65%) | <0.001 |
- Data are either no. (%) or mean [SD] unless otherwise indicated.
- NTM, non-tuberculous mycobacteria.
- aMultiple underlying co-morbidities were noted in 29 patients with TB peritonitis (three had three co-morbidities and 26 had two) and ten patients with NTM peritonitis (three co-morbidities in one and two in 9).
- bOne had Child-Pugh class A liver cirrhosis, three class B, and three class C.
- cTwo had Child-Pugh class A liver cirrhosis, 11 class B, and 18 class C.
- dGastrointestinal symptoms included vomiting (n = 2), haematemesis (n = 2), tarry stool (n = 4) and haematochezia (n = 3).
| NTM (n = 25) | Tuberculosis (n = 65) | p-value | |
|---|---|---|---|
| Laboratory: Ascites | |||
| Leukocyte count (/μL) | 10 008 [42 530] | 1241 [1743] | 0.357 |
| Lymphocyte (%) | 41 [31] | 64 [29] | 0.004 |
| Neutrophil (%) | 42 [36] | 24 [27] | 0.064 |
| Mesothelial cells (%) | 17 [21] | 12 [18] | 0.377 |
| Lactate dehydrogenase (U/L) | 3025 [9879] | 528 [669] | 0.328 |
| Albumin (g/dL) | 1.0 [0.8] | 1.6 [1.0] | 0.020 |
| Glucose (mg/dL) | 134 [71] | 143 [92] | 0.750 |
| Laboratory: Serum | |||
| Leukocyte count (/μL) | 8481 [3826] | 8836 [6583] | 0.815 |
| Haemoglobin (g/dL) | 10.4 [1.7] | 9.9 [1.9] | 0.340 |
| Creatinine (mg/dL) | 1.5 [2.5] | 2.5 [2.5] | 0.129 |
| Total bilirubin (mg/dL) | 4.9 [8.1] | 3.8 [6.3] | 0.528 |
| Albumin (g/dL) | 2.9 [0.7] | 2.8 [0.7] | 0.307 |
- Data are mean [SD] unless otherwise indicated.
- NTM, non-tuberculous mycobacteria.
Compared with patients in the ‘possible’ NTM group, those in the ‘probable’ NTM group were more likely to have acquired immunodeficiency syndrome (AIDS) (63% vs. 0%, p <0.001), fever (25% vs. 0%, p 0.021) and a lower serum albumin level (2.4 vs. 3.1 g/dL, p 0.030). Patients in the ‘probable’ group were also more likely to receive treatment (63% vs. 0%, p <0.001), but less likely to have malignancy (38% vs. 82%, p 0.025) (Supporting Information).
Abdominal computed tomography (CT) was performed in 31 (48%) TB and 10 (40%) NTM peritonitis patients. Of them, 13 TB and three NTM peritonitis patients had massive amounts of ascites. The ascites was complex and septated in one TB patient, while two TB patients and one NTM patient had nodules in the omentum. One TB patient and two NTM patients had intra-abdominal lymphadenopathy.
Seven patients with NTM peritonitis received colonoscopy, which showed non-specific colitis in three. Of the TB peritonitis patients, eight underwent colonoscopy and three received endoscopic biopsy, which showed chronic colitis in two and non-specific colitis in one.
Histopathology findings
Ten patients with TB peritonitis received peritoneal biopsy by either laparoscopy (n = 6), laparotomy (n = 3), or image-guidance (n = 1). Except for the image-guided biopsy showing chronic inflammation, the others reported typical pathological findings, including granulomatous inflammation in nine, acid-fast bacilli in six, and caseous necrosis in four. Another three TB patients received intra-abdominal biopsy (one each from the ovary, lymph node and small intestine) and all had typical pathological findings. One NTM patient had an intra-abdominal lymph node biopsy, which showed granulomatous inflammation with acid-fast bacilli. Another NTM patient had a liver biopsy, which revealed inflammation with acid-fast bacilli.
Survival analysis
Within the 6-month follow-up, 12 (48%) NTM and 21 (32%) TB peritonitis patients died of multiple organ failure without evidence of aetiologies or pathogens other than mycobacteria. Among them, 10 (83%) NTM and 16 (76%) TB peritonitis patients died before the results of the ascites culture became available. In the NTM group, the 6-month mortality rate was similar in those with malignancy (53%), AIDS (40%), liver cirrhosis (57%) and diabetes mellitus (50%). NTM patients with malignancy had an insignificantly higher mortality rate than TB patients with malignancy (9 (53%) vs. 4 (25%), p 0.101). Similar findings were noted in patients with AIDS (2 (40%) vs. 0, p 0.206), liver cirrhosis (4 (57%) vs. 14 (45%), p 0.566), and DM (1 (50%) vs. 5 (42%), p 0.825).
Six-month survival was not significantly different between NTM and TB peritonitis (Fig. 1a) and between the ‘probable’ and the ‘possible’ NTM groups (Fig. 1b). Cox regression analysis revealed that lack of anti-mycobacterial treatment was the independent poor prognostic factor (Fig. 1c and Table 5; HR, 5.83; 95% CI, 2.10–16.17).
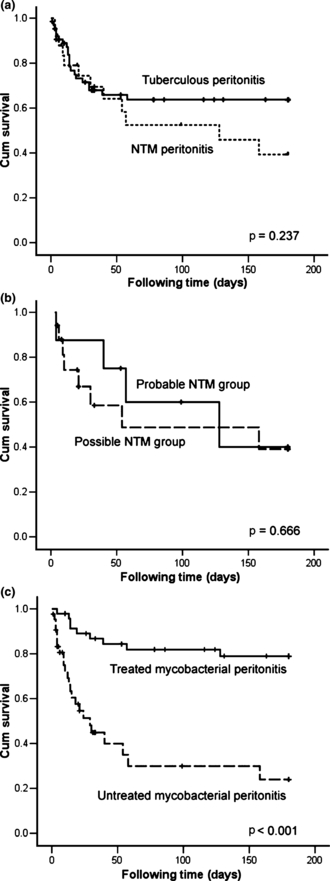
Survival curves of patients with mycobacterial peritonitis were plotted using the Kaplan–Meier method and compared using the log-rank test according to (a) causative pathogen (non-tuberculous mycobacterial (NTM) or Mycobacterium tuberculosis), (b) ‘probable’ or ‘possible’ NTM group, and (c) treatment status. Black dots represent patients still alive at the end of the study.
| Characteristics | p-value | |
|---|---|---|
| Univariate | Multivariate | |
| Age: ≥65 vs. <65 years | 0.842 | |
| Sex: male vs. female | 0.834 | |
| Malignancy: presence vs. absence | 0.652 | |
| AIDS: presence vs. absence | 0.470 | |
| DM: presence vs. absence | 0.713 | |
| Liver cirrhosis: presence vs. absence | 0.018 | 0.401 |
| Severe CKD: presence vs. absence | 0.319 | |
| Serum albumin level: <3.5 vs. ≥3.5 g/dL | 0.248 | |
| Serum total bilirubin: >1.5 vs. ≤1.5 mg/dL | <0.001 | 0.228 |
| Ascites leukocyte count: ≤500 vs. >500/μL | <0.001 | 0.088 |
| Ascites lymphocyte ratio: >50 vs. ≤50% | 0.062 | |
| Ascites LDH: >460 vs. ≤460 IU/L | 0.145 | |
| Ascites albumin level: ≤1.3 vs. >1.3 g/dL | 0.001 | 0.081 |
| Presentation to diagnosis: >6 vs. ≤6 weeks | 0.047 | 0.073 |
| Biopsya: yes vs. no | 0.209 | |
| Causing pathogens: NTM vs. M. tuberculosis | 0.237 | |
| Anti-mycobacterial treatment: no vs. yes | <0.001 | 0.001b |
- AIDS, acquired immunodeficiency syndrome; CKD, chronic kidney disease; DM, diabetes mellitus; LDH, lactate dehydrogenase; NTM, non-tuberculous mycobacteria.
- aIncluding the peritoneum or intra-abdominal organ.
- bHR, 5.83; 95% CI, 2.10–16.17.
Comparison of peritonitis from different NTM species
Patients with MAC peritonitis were younger, more likely to have AIDS, and less likely to have cancer, diabetes mellitus or liver cirrhosis (Table 6). The laboratory findings, 6-month mortality rate and 6-month survival (p 0.821, log-rank test) were not significantly different among the three subgroups.
| MAC (n = 13) | RGMa (n = 7) | Other speciesb (n = 5) | p-value | |
|---|---|---|---|---|
| Age >65 years | 1 (8%) | 3 (43%) | 3 (60%) | 0.060 |
| Male gender | 8 (62%) | 4 (57%) | 4 (80%) | 0.520 |
| Underlying co-morbidity | 13 (100%) | 7 (100%) | 5 (100%) | 0.149 |
| Malignancy | 7 (54%) | 6 (86%) | 4 (80%) | 0.001 |
| Liver cirrhosis | 3 (23%) | 4 (57%) | 0 | 0.071 |
| Diabetes mellitus | 0 | 0 | 2 (40%) | 0.013 |
| Alcoholism | 3 (23%) | 0 | 0 | 0.207 |
| Acquired immunodeficiency syndrome | 5 (38%) | 0 | 0 | 0.001 |
| Severe chronic kidney disease | 1 (8%) | 0 | 1 (20%) | 0.438 |
| Disseminated | 3 (23%) | 1 (14%) | 0 | 0.339 |
| Treatment | 5 (38%) | 0 | 0 | <0.001 |
| Six-month mortality | 7 (54%) | 3 (43%) | 2 (40%) | 0.508 |
- Data are no. (%) unless otherwise indicated.
- MAC, Mycobacterium avium complex; RGM, rapidly growing mycobacteria.
- aIncluded three patients infected by M. chelonae, two by M. abscessus, and two by M. fortuitum.
- bOther species included Mycobacterium kansasii in two patients and undifferentiated species in three.
Discussion
Mycobacterial peritonitis has a high mortality rate and is commonly caused by M. tuberculosis [18]. In the current study conducted in an endemic area of TB (incidence: 62.0 persons per 100 000 populations in 2008) [21], NTM accounted for 28% of all cases of mycobacterial peritonitis and had a similar 6-month mortality rate as TB peritonitis. Although analysis suggested that proper treatment could improve survival, few NTM patients received treatment and many died before their culture results became available. Unfamiliarity with the clinical manifestations of NTM peritonitis might be a key reason for the high mortality rate. The impact is even more serious in Taiwan because liver cirrhosis, an important risk factor for tuberculosis and tuberculous peritonitis, is common due to the high prevalence of hepatitis B and hepatitis C virus infection [18,22,23].
Mycobacterial peritonitis, especially NTM peritonitis, is frequently associated with underlying co-morbidities, which compromise either local or systemic immunity with frequent bacterial translocation [15,24,25]. Early suspicion of mycobacterial peritonitis is difficult because of the non-specific presentation, which is further influenced by complicated co-morbidities. Unlike patients with TB peritonitis, those with NTM peritonitis have relatively short disease courses (about 1 week). With low lymphocyte percentages and albumin levels, their ascites can mimic spontaneous bacterial peritonitis [26]. These findings are similar to previous observations in patients with NTM pleurisy [27].
Few patients here received treatment for NTM peritonitis. The low treatment rate has three possible reasons. First, ten patients had already died before the results of the ascites culture became available. Second, fever and other toxic signs of infection are not common in the study patients, possibly due to old age and the high prevalence of underlying co-morbidity. Third, 17 (68%) of the NTM group had only one positive ascites sample. Clinicians may consider this as laboratory contamination and decide to keep observing the patients until the diagnosis of NTM peritonitis is confirmed. Nonetheless, the clinical characteristics and outcomes are similar in the ‘probable’ and the ‘possible’ NTM groups. In addition, because prognosis is poor if left untreated, invasive diagnostic procedures, along with histopathological examinations and bacteriological studies, may be justified, especially if the bleeding tendency can, at least partially, be corrected [16,18,27].
Mycobacterium avium complex remains the most common pathogen in NTM peritonitis, pulmonary NTM infection (43%) and extra-pulmonary NTM infection in Taiwan [27–29]. Because MAC is a well-documented pathogen of opportunistic infection in AIDS patients, those with MAC peritonitis receive treatment more frequently than those with other NTM species. However, the 6-month mortality rate and survival are similar in MAC and in other NTM peritonitis. This finding has two possible explanations. First, none of the patients with peritonitis due to other NTM had HIV infection. Second, infections caused by NTM species other than MAC might have a less virulent course [15]. These findings further emphasize the importance of maintaining a high index of suspicion of mycobacterial peritonitis and prompt treatment for compromised hosts in endemic areas.
This study has several limitations. First, and most important, the diagnostic procedures and treatment protocols are not standardized in the retrospective study. The incidence of mycobacterial peritonitis is very likely underestimated. The values of the ascites adenosine deaminase, nucleic acid amplification test and interferon-gamma release assay for the early diagnosis of mycobacterial peritonitis are not evaluated. Second, the possibility of co-bacterial infection may be also underestimated because the yield rate of ascites culture for bacteria is only 40–50% [30]. Third, because the study was conducted in a medical centre and many patients with severe underlying co-morbidities were included, the mortality rate may have been overestimated. However, this should not be a serious bias because only compromised hosts acquire mycobacterial peritonitis, especially due to NTM. Finally, the small number of patients limits the statistical power of inter-group comparisons. More studies are warranted to further explore differences among disease subgroups.
Conclusions
In Taiwan, an endemic area for TB, NTM accounted for 28% of all mycobacterial peritonitis cases and has worse survival if left untreated. MAC is the most common NTM species. Unlike those of TB peritonitis, the clinical manifestations of NTM peritonitis mimic spontaneous bacterial peritonitis, with a short disease course (1–2 weeks) and relatively low lymphocyte percentage in ascites. Thus, NTM peritonitis should be kept in mind when evaluating peritonitis with no other identified microorganism except NTM and when the clinical infection does not improve under broad-spectrum empirical antibiotic treatment.
Acknowledgement
This study was supported by the Institute for Biotechnology and Medicine Industry, Taiwan.
Authors’ Contributions
Dr Jann-Yuan Wang and Professor Chong-Jen Yu designed the study; Dr Chin-Chung Shu was involved in the manuscript writing and clinical data collection/analysis; Dr Jann-Tay Wang participated in the data analysis; and Prof Li-Na Lee was the director responsible for the general organization and coordination.
Transparency Declaration
The authors have no conflict of interest to declare.
Ethics committee approval
This retrospective study was approved by the institutional review board of National Taiwan University Hospital (No. 201002023R).




